Abstract
Mild cognitive impairment (MCI) is a clinical syndrome operationalized for early diagnosis and treatment of Alzheimer’s disease (AD). Many individuals with MCI are at the prodromal stage of AD or other dementia. Various quantitative MR techniques that measure the anatomic, biochemical, microstructural, functional, and blood-flow changes are being evaluated as possible surrogate measures for early diagnosis and disease progression in AD. The pathology underlying MCI heterogenous, dominated by AD, cerebrovascular disease, Lewy body disease, or a mixture of these pathologies in community-based autopsy cohorts. Proton MRS metabolite markers may help identify and track etiologies that typically underlie MCI in the elderly. The role of proton MRS will especially be critical for pathophysiological processes for which a reliable biomarker does not exist such as glial and microglial activation in neurodegenerative dementia.
Keywords: Mild cognitive impairment, magnetic resonance spectroscopy, dementia, Alzheimer’s disease
The incidence and prevalence of Alzheimer’s disease (AD) increase significantly with aging. With improvements in healthcare and increasing life expectancy, AD has become a significant public health problem of this century (1). There are no proven treatments for AD pathology, however current efforts to arrest or slow disease progression generate the prospect for preventive interventions (2). There is considerable interest in early diagnosis by identifying individuals with cognitive difficulties who eventually progress to dementia, from those who are aging with normal cognitive function (3). Mild cognitive impairment (MCI) was established on clinical grounds in order to identify symptomatic individuals who do not meet the criteria for dementia (4). A majority of these individuals develop dementia in the future. This article reviews the proton magnetic resonance spectroscopy (1H MRS) findings in MCI and the value of 1H MRS for early diagnosis and disease progression in dementia. First, 1H MRS findings in dementia syndromes that are commonly preceded by MCI will be reviewed in order to interpret the role of 1H MRS in MCI.
1H MRS Findings in Dementia
1H MRS is unique among diagnostic imaging modalities, because the signals from several different metabolites that are sensitive to a different aspect of the neurodegenerative disease processes are measured within a single acquisition period. Each metabolite may be a surrogate marker to in-vivo pathologic processes at the molecular or cellular level. The potential utilities of 1H MRS as a biomarker in MCI and dementia include early diagnosis, differential diagnosis, and monitoring of pathological progression (5).
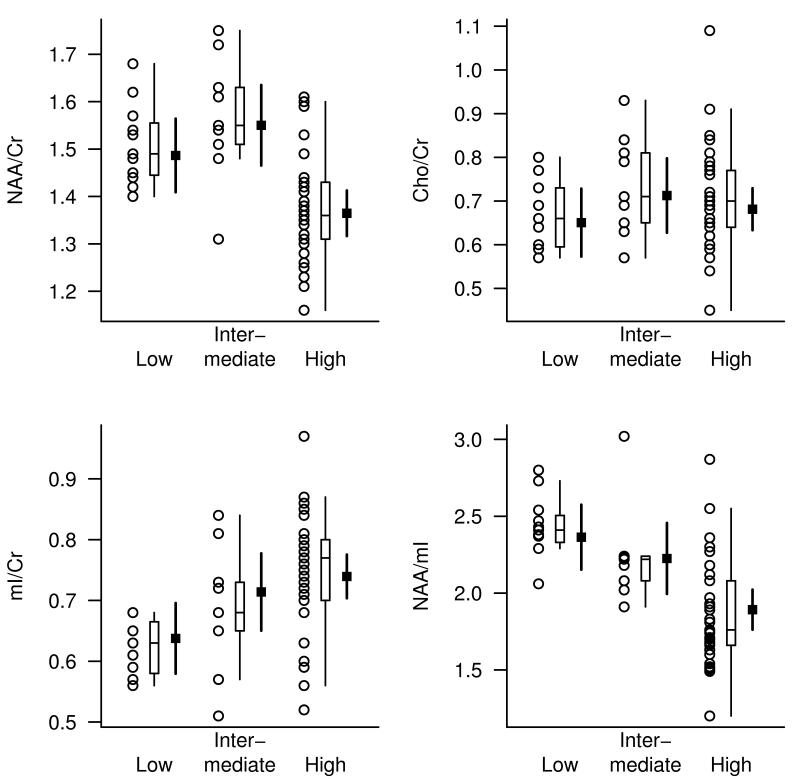
1H MRS metabolite markers of AD have been investigated in clinical cohorts for more than two decades and the neuronal metabolite NAA is consistently found to be lower and the glial metabolite mI is found to be higher in the 1H MR spectra of patients with AD than cognitively normal elderly(6-15). There are conflicting reports on the membrane integrity marker choline (Cho) levels. Some studies identified elevated Cho levels (10,13,16) in people with AD, and some did not (14,17-20). Creatine (Cr) levels are typically stable in AD compared to age matched controls (14,15,18,19,21-23). For this reason, Cr peak is generally used as an internal reference to adjust for atrophy and acquisition related variability. Postmortem MRS analyses of perchloric acid extracts of AD brains show correlations between MRS metabolites and density of NFT and senile plaques in the tissues (9,24). Furthemore, 1H MRS in studies in transgenic mouse models of AD consistently showed in vivo metabolite alterations with increasing age (25-27). Recently, we validated these findings in a clinical cohort by demonstrating that antemortem posterior cingulate gyrus NAA/Cr, mI/Cr and NAA/mI on MRS correlate with the likelihood of postmortem Alzheimer type pathology at autopsy (28) (Figure 1).
Figure 1. Posterior cingulate gyrus voxel 1H MRS findings by pathological diagnosis of AD.
The pathological diagnosis of AD is classified as low, intermediate and low likelihood of AD. For each pathological diagnosis, the plot shows individual values, a box plot of the distribution, and the estimated mean and 95% CI for the mean. The strongest association was observed with the NAA/mI ratio (RN2=0.40) (28). With permission from Radiology.
Although AD is the most common etiology of MCI, Lewy body related pathology, ischemic vascular disease and infarctions are other pathophysiological processes that are common along with early AD pathology in people with MCI (29-32). 1H MRS findings in vascular dementia is characterized by a reduction in NAA and NAA/Cr levels even in cortical regions remote from the infarction (16). White matter NAA/Cr is lower in patients with vascular dementia than in patients with AD, (33,34). NAA levels were also lower in patients with stroke who had cognitive impairment compared to those who were cognitively normal (35). Because reduction in NAA was observed in regions remote from the infarction, it is thought that the reduction in NAA/Cr is associated with neuronal dysfunction (35). Cortical mI/Cr levels on the other hand are normal in patients with vascular dementia (16,36). Because mI/Cr is elevated in patients with AD, mI/Cr may help identify the presence of AD in a demented patient with cerebrovascular disease or in a patient with MCI.
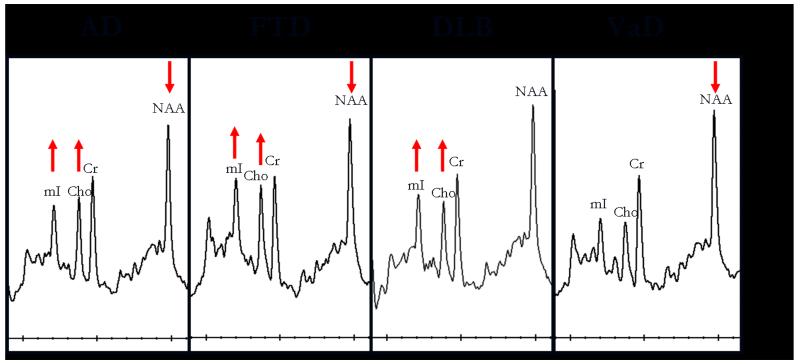
Another common dementia pathology identified in people with MCI is Lewy body pathology. Lewy body pathology by itself is less common than the mixed (AD and Lewy body) type (37). In our 1H MRS series, patients clinically diagnosed as dementia with Lewy bodies have normal NAA/Cr levels, whereas patients with AD and vascular dementia have lower NAA/Cr levels than normal, in the posterior cingulate gyri(16). Patients with dementia with Lewy bodies have preserved neuronal numbers at autopsy (37). Likewise, normal NAA/Cr levels suggest integrity of neurons in the posterior cingulate gyri, which may be useful in distinguishing patients with dementia with Lewy bodies from AD or vascular dementia. White matter NAA/Cr however was significantly reduced in patients with DLB compared to the control group (38). It is possible that NAA/Cr is decreased in other cortical regions of people with dementia with Lewy bodies, that have not yet been studied with 1H MRS. On the other hand, Cho/Cr ratios are elevated in patients with dementia with Lewy bodies compared to normal. Elevation of Cho in dementia with Lewy bodies and AD may be the consequence of increased membrane turnover due to dying back of the neuropil. Another explanation however is the down regulation of choline acetyltransferase activity which may be responsible for this change in both AD and dementia with Lewy bodies. Both AD and dementia with Lewy bodies are characterized by cholinergic dysfunction, although cholinergic dysfunction may be more severe in dementia with Lewy bodies than in AD (39). The finding that Cho/Cr levels decrease with cholinergic agonist treatment in AD (40), raises the possibility that Cho/Cr levels may be a useful marker of cholinergic dysfunction associated with both AD and Lewy body related pathology (Figure 2)
Figure 2. Posterior cingulate gyrus voxel 1H MRS findings in common dementia syndromes.
Alzheimer’s disease (AD), frontotemporal dementia (FTD), dementia with Lewy bodies (DLB), vascular dementia (VaD).
The Heterogeneity of Mild Cognitive Impairment
The progression of AD pathophysiological processes start decades before the clinical diagnosis of AD and the earliest cognitive impairments occur in the memory domain (41). The syndrome of amnestic MCI represents this prodromal phase in the progression of AD (4). More recently, the construct of MCI has been broadened to include individuals with impairments in non-amnestic cognitive domains such as attention/executive, language or visual-spatial processing domains (42). The clinical presentation of this broadened definition of MCI is heterogeneous.. Both the amnestic and non-amnestic subtypes of MCI may present with involvement of a single cognitive domain or multiple cognitive domains. It is clear from several independent studies that most people with the amnestic form of MCI who progress to dementia in the future, develop AD(43-49). People with non-amnestic MCI on average have more vascular comorbidity and infarctions as well as a higher prevalence of extra pyramidal features, mood disorders, and behavioral symptoms than people with amnestic MCI (50,51). The ethiology of MCI is also heterogenous. A variety of early stage dementia-associated pathophysiological processes such as AD, cerebrovascular disease and Lewy body pathology have been identified in patients with MCI at autopsy (29,30,31,32). Many of these pathologies co-exist in MCI (29) and require different therapeutic strategies.
Furthermore, all patients with MCI do not develop dementia at a similar rate (52,53). The heterogeneity of MCI warrants development of non-invasive biomarkers that can predict the rate of future progression to different dementias, for early diagnosis and treatment with potential disease-specific preventive interventions.
1H MRS Findings in MCI
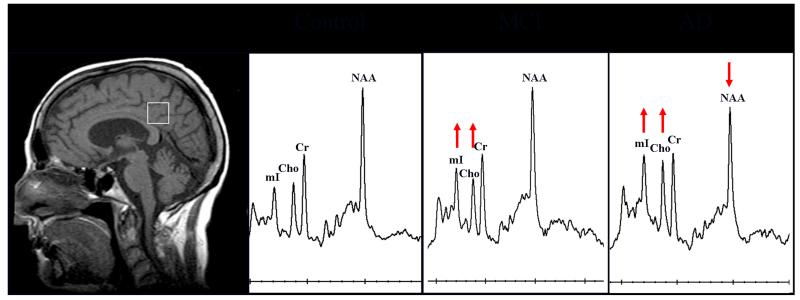
Early 1H MRS studies in MCI included individuals who had impairments in memory function (i.e. amnestic MCI) (8,54-56). A majority of patients with amnestic MCI develop AD in the future, and many of these individuals have early AD pathology (46). In keeping with this, the 1H MRS findings in amnestic MCI are similar to but milder than the findings in AD (8,55,56) (Figure 3). However there are distinct group wise differences in MRI and 1H MRS findings between amnestic MCI and non-amnestic MCI subtypes. Patients with amnestic MCI tend to have smaller hippocampal volumes and elevated mI/Cr ratios compared to patients with non-amnestic MCI and cognitively normal controls. On the other hand, non-amnestic MCI patients have normal hippocampal volumes and normal mI/Cr ratios, but a greater proportion of these patients had cortical infarctions compared to the amnestic MCI patients (50). Both hippocampal atrophy and elevated mI/Cr are sensitive markers of early AD pathology, and the severity of these abnormalities correlate with the pathologic severity of AD (28,57-62). For this reason, hippocampal atrophy and elevated mI/Cr most likely represent a higher frequency of early AD pathology in patients with amnestic MCI compared to non-amnestic MCI. On the contrary, normal hippocampal volumes and mI/Cr ratios in the non-amnestic MCI subtype suggest that other pathologies in addition to AD underlie non-amnestic MCI. Higher prevalence of cortical infarctions on MRI, history of TIA and stroke in non-amnestic MCI patients suggest that cerebrovascular disease is one of the pathological contributors to non-amnestic MCI.
Figure 3. Posterior cingulate gyrus voxel 1H MRS findings in patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) compared to a cognitively normal subject.
An 8 cm3 (2×2×2cm) voxel includes the right and left posterior cingulate gyri and inferior precuneate gyri. The anterior border of splenium, the superior border of corpus callosum and the cingulate sulcus are used as the anatomical landmarks for voxel placement.
MRS as a Biomarker in for Early Diagnosis of Dementia in MCI
The pathologic and clinical heterogeneity of MCI require multimodality imaging markers that are sensitive to the various dementia related pathophysiological processes for early diagnosis in patients with MCI. The most common dementia-related pathologies observed in MCI include AD, cerebrovascular disease and Lewy body disease (29-31). Lesions that are associated with cerebrovascular disease on MRI include infarctions and white matter hyperintensities on T2 weighted images. These cerebrovascular lesions are more common in patients with MCI compared to cognitively normal older adults (50). An MR marker that is highly sensitive to the pathophysiological processes of AD specifically the neurofibrillary tangle pathology-associated neurodegeneration early in the disease course is hippocampal atrophy (60,62). Both the presence of cortical infarctions (63) and hippocampal atrophy (64) are predictors of dementia risk in MCI.
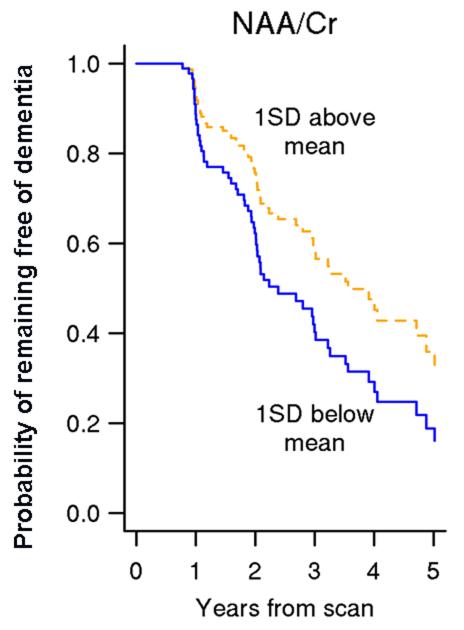
There is evidence that MRS is sensitive to the pathophysiological processes associated with dementia in patients with MCI (65,66). Decreased NAA/Cr ratio in the posterior cingulate gyrus voxel is associated with an increased risk of dementia in patients with MCI (63) (Figure 4). Furthermore, posterior cingulate gyrus voxel NAA/Cr levels decline over time in patients with MCI who progress to AD diagnosis (67). MRS is complementary in predicting future progression to dementia in MCI when considered with other strong predictors of dementia risk in MCI such as hippocampal volumes and cortical infarctions. Decreased posterior cingulate gyrus NAA/Cr increases the risk of progression to dementia in MCI patients with hippocampal atrophy and the risk of dementia increases even further when cortical infarctions are present in a patient with MCI (63) (Figure 5). This is consistent with cross-sectional studies showing the added value of 1H MRS and hippocampal volumes for discriminating cognitively impaired but non-demented individuals from cognitively normal subjects (56). Furthermore, hippocampal volumes and NAA/Cr levels are independent and complementary predictors of verbal memory on neuropsychometric testing in non-demented older adults, demonstrating that verbal memory depends on both structural and metabolic integrity of the hippocampus (68).
Figure 4. Lower NAA/Cr increases the risk of dementia in MCI.
Estimates of the probability of remaining free of dementia over time based on age, gender, and education -adjusted Cox models with NAA/Cr as the predictor. Estimates assume a 76 year old patient with 14.6 years of education and represent a weighted average of the curves for men and women where the weights are the proportion of men and women in our sample (63). With permission from Neurology.
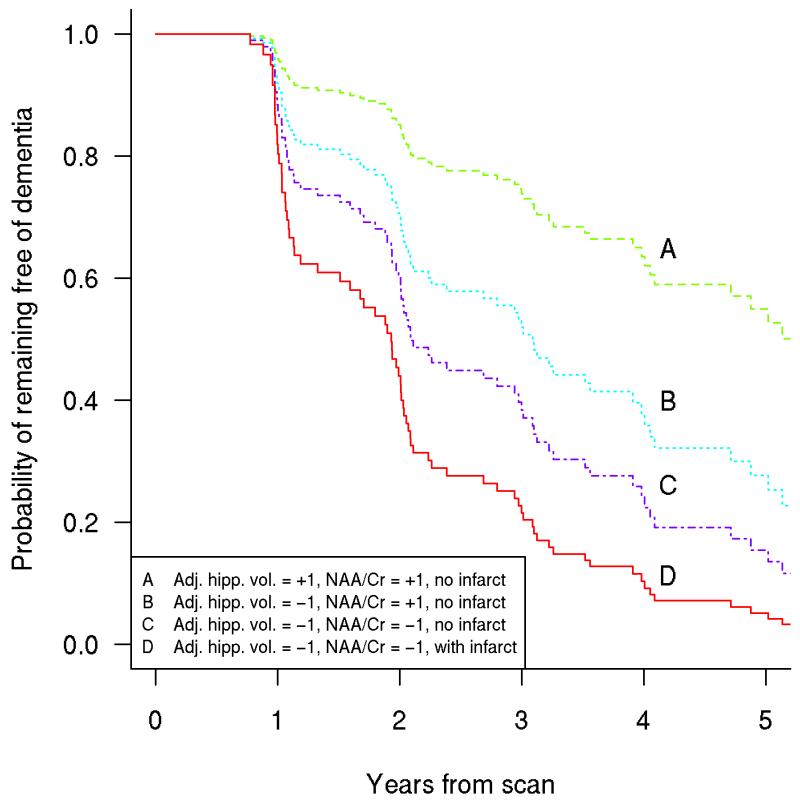
Figure 5. Multiple MR markers of underlying dementia pathologies improve the ability to identify patients with prodromal dementia over a single MR marker.
Estimates of the probability of remaining free of dementia for four patient groups with increasingly negative prognoses. Group A has adjusted hippocampal volume and NAA/Cr 1 SD above MCI average and no cortical infarctions. Group B has adjusted hippocampal volume 1 SD below MCI average with NAA/Cr 1 SD above MCI average and no cortical infarctions. Group C has adjusted hippocampal volume and NAA/Cr both 1 SD below MCI average and no cortical infarctions. Group D has adjusted hippocampal volume and NAA/Cr both 1 SD below MCI average and cortical infarctions (63). With permission from Neurology.
1H MRS in Preclinical AD
Recently, the diagnostic criteria for AD and MCI were revised by two separate workgroups charged by the National Institute on Aging and Alzheimer’s Association (69-71). A third work group was charged to define the preclinical stage of AD in light of the evidence that the pathophysiological process of AD begins decades before the diagnosis of clinical dementia (41). The change in most well validated imaging biomarkers have been modeled for the three clinical stages of AD (i.e. preclinical AD, MCI and AD). According to this hypothecial model, the change in β-amyloid (Aβ) pathology imaged with PET amyloid ligands precede the change in imaging markers of neurodegeneration associated with the neurofibrillary tangle pathology of AD such as hippocampal atrophy on MRI (72). The model that emerged from evidence on well validated imaging biomarkers will be critical for tracking disease progression and for assessment of primary and secondary preventive interventions in individuals at the preclinical and MCI stage of AD.
Several well validated imaging biomarkers exist for various pathological features of the early AD pathology such as increased Aβ load on PET, atrophy on structural MRI or glucose metabolic reductions on PET. However there are other features of AD pathology for which a well valid biomarker does not exist. For example there is no widely accepted biomarker for glial or microglial activation. The glial metabolite mI quantified with 1H MRS may potentially be useful as a biomarker for glial activation in neurodegenerative diseases including AD.
Cross-sectional studies indicate that mI/Cr is elevated in MCI and mild AD even in the absence of a decrease in NAA/Cr (8,54,73). We recently validiated this finding in a pathology-confirmed sample of older adults with a range of AD pathology showing that the mI/Cr elevation is associated with intermediate likelihood (i.e. an earlier stage) AD pathology whereas the decrease in NAA/Cr is associated with higher likelihood (i.e. a later stage) AD pathology (28) (Figure 1). Furthermore, mI/ Cr levels increase in the predementia phase of Down’s syndrome(6,74) and in presymptomatic individuals with familial dementia (75,76) even in the absence of structural MRI and NAA/Cr changes(76). The mI peak consists of glial metabolites that are responsible for osmoregulation (77,78). MI levels correlate with glial proliferation in inflammatory CNS demyelination (79). Because the dense cored amyloid deposits in AD are surrounded by clusters of microglia and astrocytes (80), it is thought that the elevation of the mI peak is related to glial proliferation and microglial activation in AD (81,82). A significant correlation between mI/Cr levels and amyloid load measured with Pittsburgh Compound-B PET imaging was found in a population-based sample of 311 cognitively normal older adults (83). It is possible that the mI/Cr levels are associated with the microglial and glial activation that surround the senile amyloid plaques. However a 1H MRS - histology correlation study in a mouse model of spinocerebellar ataxia type 1 found elevated mI/Cr levels even in the absence of gliosis (84). Based on limited evidence, it is not be possible to attribute elevation in mI solely to glial activation in neurodegenerative diseases. Although there is evidence that mI/Cr elevation is an early marker in sporadic AD, familial AD and frontotemporal lobar degeneration even before cognitive impairment, loss of neuronal integrity and atrophy, histological confirmation is needed to better understand the biological basis of mI/Cr elevation in MCI.
Conclusions
MCI is a clinically and pathologically heterogeneous disorder. Current data indicate that a single imaging marker will be insufficient for determining the underlying pathophysiological processes that contribute to cognitive impairment in MCI. 1H MRS may potentially provide information on the underlying pathologies in patients with MCI that is not available from other imaging biomarkers. Data from cognitively normal older adults and cognitively normal adults at risk for familial dementia suggest that 1H MRS may be useful as a biomarker for preclinical pathological processes and potentially assessing the response to preventive interventions. Although significant progress has been made on improving the acquisition and analysis techniques in 1H MRS, translation of these technical developments to clinical practice have not been effective. The main reasons for ineffective translation of technology to clinical practice or patient-oreinted research are two fold: 1) Lack of standardization for multi-site applications and normative data 2) Insufficient understanding the pathological basis of 1H MRS metabolite changes. Advances on these grounds would further increase the impact of 1H MRS as biomarker for the early pathological involvement in neurodegenerative diseases and in turn increase the use 1H MRS in clinical practice.
Acknowledgement
The author thanks Denise A. Reyes for helping with the manuscript preparation.
Grant Support: Dr. Kantarci’s research program is supported by the R01 AG40042, P50 AG16574/Project1 and R21 NS066147.
References
- 1.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Jack CR, Jr., Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3(111):111cm133. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thal LJ, Kantarci K, Reiman EM, et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):6–15. doi: 10.1097/01.wad.0000191420.61260.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 5.Kantarci K. 1H magnetic resonance spectroscopy in dementia. Br J Radiol. 2007;80(Spec No 2):S146–152. doi: 10.1259/bjr/60346217. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Alexander GE, Daly EM, et al. High brain myo-inositol levels in the predementia phase of Alzheimer’s disease in adults with Down’s syndrome: a 1H MRS study. American Journal of Psychiatry. 1999;156(12):1879–1886. doi: 10.1176/ajp.156.12.1879. [DOI] [PubMed] [Google Scholar]
- 7.Jessen F, Block W, Traber F, et al. Proton MR spectroscopy detects a relative decrease of N-acetylaspartate in the medial temporal lobe of patients with AD. Neurology. 2000;55(5):684–688. doi: 10.1212/wnl.55.5.684. [DOI] [PubMed] [Google Scholar]
- 8.Kantarci K, Jack CR, Jr., Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klunk WE, Panchalingam K, Moossy J, McClure RJ, Pettegrew JW. N-acetyl-L-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance study. Neurology. 1992;42(8):1578–1585. doi: 10.1212/wnl.42.8.1578. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhoff DJ, MacKay S, Constans JM, et al. Axonal injury and membrane alterations in Alzheimer’s disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Annals of Neurology. 1994;36(1):40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 11.Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187(2):433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- 12.Parnetti L, Lowenthal DT, Presciutti O, et al. 1H-MRS, MRI-based hippocampal volumetry, and 99mTc-HMPAO-SPECT in normal aging, age-associated memory impairment, and probable Alzheimer’s disease. Journal of the American Geriatrics Society. 1996;44(2):133–138. doi: 10.1111/j.1532-5415.1996.tb02428.x. see comment. [DOI] [PubMed] [Google Scholar]
- 13.Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magnetic Resonance in Medicine. 1999;41(2):276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Schuff N, Amend D, Ezekiel F, et al. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49(6):1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- 15.Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195(1):65–72. doi: 10.1148/radiology.195.1.7892497. see comment. [DOI] [PubMed] [Google Scholar]
- 16.Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–1398. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan KR, Charles HC, Doraiswamy PM, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. American Journal of Psychiatry. 2003;160(11):2003–2011. doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 18.Moats RA, Ernst T, Shonk TK, Ross BD. Abnormal cerebral metabolite concentrations in patients with probable Alzheimer disease. Magnetic Resonance in Medicine. 1994;32(1):110–115. doi: 10.1002/mrm.1910320115. [DOI] [PubMed] [Google Scholar]
- 19.Parnetti L, Tarducci R, Presciutti O, et al. Proton magnetic resonance spectroscopy can differentiate Alzheimer’s disease from normal aging. Mechanisms of Ageing & Development. 1997;97(1):9–14. doi: 10.1016/s0047-6374(97)01877-0. [DOI] [PubMed] [Google Scholar]
- 20.Rose SE, de Zubicaray GI, Wang D, et al. A 1H MRS study of probable Alzheimer’s disease and normal aging: implications for longitudinal monitoring of dementia progression. Magnetic Resonance Imaging. 1999;17(2):291–299. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 21.Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203(3):829–836. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- 22.Mohanakrishnan P, Fowler AH, Vonsattel JP, et al. Regional metabolic alterations in Alzheimer’s disease: an in vitro 1H NMR study of the hippocampus and cerebellum. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1997;52(2):B111–117. doi: 10.1093/gerona/52a.2.b111. [DOI] [PubMed] [Google Scholar]
- 23.Schuff N, Capizzano AA, Du AT, et al. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58(6):928–935. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klunk WE, Panchalingam K, McClure RJ, Stanley JA, Pettegrew JW. Metabolic alterations in postmortem Alzheimer’s disease brain are exaggerated by Apo-E4. Neurobiology of Aging. 1998;19(6):511–515. doi: 10.1016/s0197-4580(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 25.Marjanska M, Curran GL, Wengenack TM, et al. Monitoring disease progression in transgenic mouse models of Alzheimer’s disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 2005;102(33):11906–11910. doi: 10.1073/pnas.0505513102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberg J, Spenger C, Wang FH, et al. Age related changes in brain metabolites observed by 1H MRS in APP/PS1 mice. Neurobiol Aging. 2008;29(9):1423–1433. doi: 10.1016/j.neurobiolaging.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Chen SQ, Wang PJ, Ten GJ, Zhan W, Li MH, Zang FC. Role of myo-inositol by magnetic resonance spectroscopy in early diagnosis of Alzheimer’s disease in APP/PS1 transgenic mice. Dement Geriatr Cogn Disord. 2009;28(6):558–566. doi: 10.1159/000261646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248(1):210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133(Pt 2):540–556. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 32.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 33.Kattapong VJ, Brooks WM, Wesley MH, Kodituwakku PW, Rosenberg GA. Proton magnetic resonance spectroscopy of vascular- and Alzheimer-type dementia. Archives of Neurology. 1996;53(7):678–680. doi: 10.1001/archneur.1996.00550070116019. [DOI] [PubMed] [Google Scholar]
- 34.MacKay S, Ezekiel F, Di Sclafani V, et al. Alzheimer disease and subcortical ischemic vascular dementia: evaluation by combining MR imaging segmentation and H-1 MR spectroscopic imaging. Radiology. 1996;198(2):537–545. doi: 10.1148/radiology.198.2.8596863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross AJ, Sachdev PS, Wen W, Valenzuela MJ, Brodaty H. 1H MRS in stroke patients with and without cognitive impairment. Neurobiol Aging. 2005;26(6):873–882. doi: 10.1016/j.neurobiolaging.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Waldman AD, Rai GS, McConnell JR, Chaudry M, Grant D. Clinical brain proton magnetic resonance spectroscopy for management of Alzheimer’s and sub-cortical ischemic vascular dementia in older people. Arch Gerontol Geriatr. 2002;35(2):137–142. doi: 10.1016/s0167-4943(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Isla T, Growdon WB, McNamara M, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53(9):2003–2009. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- 38.Molina JA, Garcia-Segura JM, Benito-Leon J, et al. Proton magnetic resonance spectroscopy in dementia with Lewy bodies. European Neurology. 2002;48(3):158–163. doi: 10.1159/000065520. [DOI] [PubMed] [Google Scholar]
- 39.Tiraboschi P, Hansen LA, Alford M, et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Archives of General Psychiatry. 2002;59(10):946–951. doi: 10.1001/archpsyc.59.10.946. [DOI] [PubMed] [Google Scholar]
- 40.Satlin A, Bodick N, Offen WW, Renshaw PF. Brain proton magnetic resonance spectroscopy (1H-MRS) in Alzheimer’s disease: changes after treatment with xanomeline, an M1 selective cholinergic agonist. American Journal of Psychiatry. 1997;154(10):1459–1461. doi: 10.1176/ajp.154.10.1459. [DOI] [PubMed] [Google Scholar]
- 41.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 43.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 44.Bowen J, Teri L, Kukull W, McCormick W, McCurry SM, Larson EB. Progression to dementia in patients with isolated memory loss. Lancet. 1997;349(9054):763–765. doi: 10.1016/S0140-6736(96)08256-6. [DOI] [PubMed] [Google Scholar]
- 45.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 46.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 47.Meyer JS, Xu G, Thornby J, Chowdhury MH, Quach M. Is mild cognitive impairment prodromal for vascular dementia like Alzheimer’s disease? Stroke. 2002;33(8):1981–1985. doi: 10.1161/01.str.0000024432.34557.10. [DOI] [PubMed] [Google Scholar]
- 48.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. see comment. [DOI] [PubMed] [Google Scholar]
- 49.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. erratum appears in Arch Neurol 1999 Jun;56(6):760. [DOI] [PubMed] [Google Scholar]
- 50.Kantarci K, Petersen RC, Przybelski SA, et al. Hippocampal volumes, proton magnetic resonance spectroscopy metabolites, and cerebrovascular disease in mild cognitive impairment subtypes. Arch Neurol. 2008;65(12):1621–1628. doi: 10.1001/archneur.65.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mariani E, Monastero R, Ercolani S, et al. Vascular Risk Factors in Mild Cognitive Impairment Subtypes. Findings from the ReGAl Project. Dement Geriatr Cogn Disord. 2007;24(6):448–456. doi: 10.1159/000110653. [DOI] [PubMed] [Google Scholar]
- 52.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 53.Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68(4):288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 54.Catani M, Cherubini A, Howard R, et al. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12(11):2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- 55.Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Research. 2004;1003(1-2):26–35. doi: 10.1016/j.brainres.2003.11.074. [DOI] [PubMed] [Google Scholar]
- 56.Chao LL, Schuff N, Kramer JH, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64(2):282–289. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barkhof F, Polvikoski TM, van Straaten EC, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology. 2007;69(15):1521–1527. doi: 10.1212/01.wnl.0000277459.83543.99. [DOI] [PubMed] [Google Scholar]
- 58.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 1997;56(4):414–420. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58(10):1476–1482. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- 60.Jack CR, Jr., Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarow C, Vinters HV, Ellis WG, et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57(6):896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63(1):72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72(17):1519–1525. doi: 10.1212/WNL.0b013e3181a2e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack CR, Jr., Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Bisbal MC, Arana E, Marti-Bonmati L, Molla E, Celda B. Cognitive impairment: classification by 1H magnetic resonance spectroscopy. Eur J Neurol. 2004;11(3):187–193. doi: 10.1046/j.1468-1331.2003.00746.x. [DOI] [PubMed] [Google Scholar]
- 66.Metastasio A, Rinaldi P, Tarducci R, et al. Conversion of MCI to dementia: Role of proton magnetic resonance spectroscopy. Neurobiol Aging. 2006;27(7):926–932. doi: 10.1016/j.neurobiolaging.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007;28(9):1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal neurochemistry, neuromorphometry, and verbal memory in nondemented older adults. Neurology. 2008;70(18):1594–1600. doi: 10.1212/01.wnl.0000306314.77311.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jack CR, Jr., Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang W, Alexander GE, Chang L, et al. Brain metabolite concentration and dementia severity in Alzheimer’s disease: a (1)H MRS study. Neurology. 2001;57(4):626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- 74.Shonk T, Ross BD. Role of increased cerebral myo-inositol in the dementia of Down syndrome. Magnetic Resonance in Medicine. 1995;33(6):858–861. doi: 10.1002/mrm.1910330619. [DOI] [PubMed] [Google Scholar]
- 75.Godbolt AK, Waldman AD, MacManus DG, et al. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology. 2006;66(5):718–722. doi: 10.1212/01.wnl.0000201237.05869.df. [DOI] [PubMed] [Google Scholar]
- 76.Kantarci K, Boeve BF, Wszolek ZK, et al. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology. 2010;75(9):771–778. doi: 10.1212/WNL.0b013e3181f073c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience. 1993;15(3-5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 78.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. Journal of Neuroscience. 1993;13(3):981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. American Journal of Neuroradiology. 1999;20(9):1619–1627. [PMC free article] [PubMed] [Google Scholar]
- 80.Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56(4):321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clinics of North America. 1998;8(4):809–822. [PubMed] [Google Scholar]
- 82.Sailasuta N, Harris K, Tran T, Ross B. Minimally invasive biomarker confirms glial activation present in Alzheimer’s disease: a preliminary study. Neuropsychiatr Dis Treat. 2011;7:495–499. doi: 10.2147/NDT.S23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kantarci K, Lowe V, Przybelski SA, et al. Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77(10):951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oz G, Nelson CD, Koski DM, et al. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010;30(10):3831–3838. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]