Abstract
The post-translational citrullination (deimination) process is mediated by peptidylarginine deiminases (PADs), which convert peptidylarginine into peptidylcitrulline in the presence of high calcium concentrations. Over the past decade, PADs and protein citrullination have been commonly implicated as abnormal pathological features in neurodegeneration and inflammatory responses associated with diseases such as multiple sclerosis, Alzheimer disease and rheumatoid arthritis. Based on this evidence, we investigated the roles of PADs and citrullination in the pathogenesis of prion diseases. Prion diseases (also known as transmissible spongiform encephalopathies) are fatal neurodegenerative diseases that are pathologically well characterized as the accumulation of disease-associated misfolded prion proteins, spongiform changes, glial cell activation and neuronal loss. We previously demonstrated that the upregulation of PAD2, mainly found in reactive astrocytes of infected brains, leads to excessive citrullination, which is correlated with disease progression. Further, we demonstrated that various cytoskeletal and energy metabolism-associated proteins are particularly vulnerable to citrullination. Our recent in vivo and in vitro studies elicited altered functions of enolase as the result of citrullination; these altered functions included reduced enzyme activity, increased protease sensitivity and enhanced plasminogen-binding affinity. These findings suggest that PAD2 and citrullinated proteins may play a key role in the brain pathology of prion diseases. By extension, we believe that abnormal increases in protein citrullination may be strong evidence of neurodegeneration.
Keywords: peptidylarginine deiminase, citrullination, prion, enolase, neurodegeneration
Introduction
Prion
Prions are unique unconventional infectious pathogens propagated by alternatively folded cellular prion proteins (PrPC) that give rise to a devastating neurodegenerative disease in humans and animals. The disease-associated misfolded form of prion proteins (termed PrPSc) is characterized by β-sheet enrichment and proteinase-K resistance relative to PrPC. In particular, PrPSc forms are transmissible particles that can be transmitted directly between animals and humans.
In humans, prion diseases (also known as transmissible spongiform encephalopathies) include the following: autosomal dominant inherited fatal familial insomnia (FFI); Gerstmann-Sträussler-Scheinker syndrome (GSS); familial Creutzfeldt-Jakob disease (fCJD); sporadic CJD (sCJD), which is caused by an unknown etiological factor; variant CJD (vCJD), which is caused by transmission material from cases of bovine spongiform encephalopathies; iatrogenic CJD (iCJD), which is caused by prion-contaminated surgical instrument or gonadotropin preparations; and kuru, which is transmitted by ritualistic anthropophagy.1,2 Genetic mutation-derived prion diseases are caused by a point mutation in a codon of the PrP gene (PRNP) located on chromosome 20 in humans. In addition, the PRNP polymorphism at position 129 of PrP, which may be either methionine or valine, contributes to the onset, progression and symptoms of the disease.3 The most common type of human prion disease is sCJD, which accounts for more than 90% of human prion cases. The annual incidence of all prion diseases is approximately 1 per every 1 million individuals.3
Prion infections affect the central nervous system (CNS), resulting in spongiform changes, neuronal cell loss, microglia activation, reactive astrocytosis and the accumulation of PrPSc. Recent studies have demonstrated that accumulated citrullinated proteins and abnormal activation of peptidylarginine deiminase 2 (PAD2) may play a role in the pathogenesis of prion diseases.4,5 In addition, citrullinated proteins including glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), and several newly identified proteins were found in prion disease and other neurodegenerative diseases.4-6
PADs and citrullination
The post-translational citrullination process is defined as the modification of an arginine residue to a citrulline residue in proteins, and this process is mediated by calcium (Ca2+)-dependent PAD enzymes (Fig. 1). This process causes changes in target proteins in a variety of ways that affect structure and/or function (an irreversible modification): the changes include loss of positive charge, a conformational change, altered protease susceptibility and affinity to protein-protein interaction. One of the fascinating facts is that Ca2+, an essential prerequisite for PAD activation, requires approximately 100-fold higher level than the normal cytosolic Ca2+ concentration (~10−8–10−6M). Moreover, in vitro, PAD2 is known to be activated at millimolar Ca2+ concentrations.7
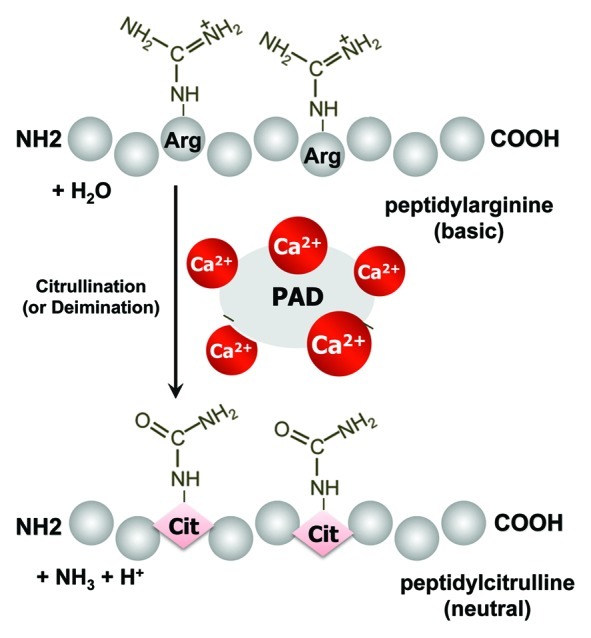
Figure 1. An outline of the protein citrullination (deimination) process. Calcium-dependent peptidylarginine deiminases (PADs) convert peptidylarginine into peptidylcitrulline, resulting in altered protein function.
The five PAD genes (types 1–4 and 6) in humans, mice and rats are located in a single gene cluster on chromosomes 1p36.1, 4E1 and 5q36, respectively. The five isotypes in mammals display 70–95% amino acid identity.7 The five Ca2+-binding sites (non-EF-hand motif) exist in PAD4 and are conserved in other isoforms,8,9 hence, all PADs are thought to contain five Ca2+-binding sites.9 PADs are distinct with regard to substrate and tissue specificity. PAD1 is expressed in the epidermis, uterus and hair follicles.10,11 It is detected in the entire epidermis with an increasing gradient from the basal layer to the granular layer. Keratins K1 and K10 are known substrates of PAD1 during keratinocyte terminal differentiation. Keratin citrullination is essential for the normal cornification process of the epidermis. PAD2 is widely distributed in mammals; it exists in the pancreas, skeletal muscle, breast, colon, eyes, spleen, kidneys, uterus, macrophages and CNS.7 During the development of normal mice, the expression of PAD2 protein is gradually increased from 18-d embryos to 2 mo of age, and is decreased to low levels from 3 mo onward.12,13 In the CNS, the dynamic Ca2+ signaling to which astrocytes are exposed may foster PAD2 activation and protein citrullination. Astrocyte-specific GFAP is one of the most susceptible substrates of PAD2, thereby citrullinated forms of GFAP are abundantly detected in patients with multiple sclerosis (MS), Alzheimer disease (AD) and prion diseases.4-6,14 Citrullinated GFAP has been shown to induce the disassembly of the intermediate filament.15 PAD2-transgenic mice develop astrocyte and microglial activation, MBP citrullination and myelin loss.16 PAD3, along with PAD1 and PAD2, is expressed in the epidermis and hair follicles, where it citrullinates filaggrin, keratin K1 and K10.7 In progenitor and neural cells during development, regulated PAD3 expression is responsible for protein citrullination in response to spinal cord injury, and it is therefore suggested that PAD3 contributes to the loss of regenerative ability.17 Distinctively, PAD4 (human PAD5 is the human homolog of mouse PAD4) primarily acts in the nucleus because it has a classical monopartite nuclear localization signal (56-PPAKKKST-63) at the N-terminal domain,8 resulting in localization in cell nuclei, where the enzyme citrullinates histones.18,19 In the nucleus, one of the important functions of PAD4 is to citrullinate histones which regulate gene transcription, this includes not only the arginine residues but also monomethylated arginines. Therefore, PAD4 counteracts the functions of protein Arg methyltransferases. In addition, PAD4 is highly expressed in various cancer cells20 and it is associated with the inhibition of p53 target gene, repressing its expression,21-24 this suggests that PAD4 is a strategic target for cancer therapy. Interestingly, histone citrullination by PAD4 mediates histone decondensation and neutrophil extracellular trap formation.25 PAD4−/− mice are susceptible to bacterial infection, suggesting that PAD4 has a role in antibacterial innate immunity.26 Finally, PAD6 is expressed only in male and female germ cells.27 It participates in oocyte cytoskeletal sheet formation and female fertility,28 is a critical factor for cytoplasmic lattice formation in growing oocytes,29 and regulates microtubule-mediated organelle positioning and movement.30
PADs and citrullination in human diseases
Increased PAD expression and protein citrullination are commonly observed in several neurodegenerative diseases, including MS, AD, optic glaucoma and Parkinson disease, and in psoriasis, rheumatoid arthritis (RA) and cancer.4-7,20,31-34 In AD patients, the abnormal accumulation of citrullinated proteins and increased PAD2 expression are seen in the hippocampus, where vimentin and GFAP have been identified as PAD substrates.6 Citrullinated proteins have also been observed in the cytoplasm of the substantia nigra dopamine neurons in Parkinson disease patients.35 Nevertheless, the role of PAD and citrullination are poorly understood in these diseases, despite the fact that presence of PAD and citrullination have been studied extensively, particularly in RA and MS.
RA is a chronic inflammatory disorder that is characterized by progressive articular damage affecting synovial-lined diarthrodial joints.40 In this disease, upregulated expression of PAD2 and PAD4 as well as increased citrullinated proteins are key aspects of the abnormalities seen in the synovial tissue.41-43 Over the past 10 years, many research groups have demonstrated that antibodies against citrullinated proteins (ACPAs) are present in ~70% of RA patients and that the presence of ACPAs is highly specific for RA. In addition, ACPA-positive patients were shown to have a more severe disease course than RA patients without ACPAs.34 A recent study suggests that autoantibody production in response to citrullinated vimentin directly induces bone loss by increasing osteoclastogenesis.44
The 18.5 kDa isoform of MBP, which is a major component of the myelin sheath, generally undergoes the citrullination of 6 of its 19 total arginine residues. While the citrullinated form normally accounts for approximately 20% of the total MBP in a healthy human brain, in MS patients, the proportion has been found to be increased to 45%.33 In the case of fulminant MS (Marburg variant), more than 80% of the MBP containing 18 citrulline residues was citrullinated.36 However, in other neurological disorders, including AD, Parkinson disease, Huntington disease and amyotrophic lateral sclerosis, the citrullinated form remained at 20% of the total.33 A biochemical study revealed that the rates of digestion of human MBPs containing 6 citrulline residues and 18 citrulline residues by cathepsin D were 4-fold and 45-fold, respectively, more rapid than the rate of digestion of non-citrullinated MBP.37 Increasing the number of citrulline residues in MBP results in a more open conformation, decreases the charge and allows better access of the Phe-Phe linkages to cathepsin D,38 thus, yielding much less compact protein-lipid complexes leading to a looser structure of the myelin sheath.39 It is currently hypothesized that citrullinated MBP is involved in an important novel pathway in the pathogenesis of MS.37
PADs and Citrullination in Prion Diseases
As mentioned above, increased PAD expression and activation and an abnormal increase in citrullination are commonly involved in disease pathophysiology in a number of human diseases. Therefore, we postulated that this phenomenon also contributes to the pathogenesis of prion diseases. To assess the concept, we investigated for the first time the PAD2 expression and protein citrullination in the brains of scrapie-infected mice, a prion disease model. PAD2 expression was shown to be increased in several brain regions including cerebral cortex, hippocampus, striatum and brain stem (but not in cerebellum) of scrapie-infected mice compared with controls. In addition, PAD activity gradually elevated in tandem with the progression of disease status.4 In a manner dependent on the upregulation of PAD2 expression and activity, brain proteins were markedly citrullinated in all brain regions of scrapie-infected mice,4 and a large number of citrullinated proteins were detected in the membrane fractions including microsomes and mitochondria, although PAD2 is mainly present in cytosol.31 This result suggests that PAD2 and citrullination are involved in abnormal pathological phenotypes in prion diseases similar to findings in other neurodegenerative diseases. In the case of humans, most results were shown to be parallel to those obtained in this animal model: PAD2 expression and protein citrullination were elevated in frontal cortex of CJD patients, and PAD enzyme activity in CJD patients was higher than in non-CJD patients.5 These results support our finding that the upregulated expression and activation of PAD2 and abnormally increased citrullination occurred in mice affected by prions. Interestingly, the amount of citrullinated proteins is displayed as random patterns in each brain region. Some cases showed very high levels of citrullination in thalamus and midbrain compared with frontal cortex, although PAD2 expression is similar or lower in these two brain regions compared with frontal cortex (unpublished data). This result suggests that excessive citrullination is more directly relevant to the activation status of PAD enzyme rather than the expression level of PAD. In a proteomic analysis to identify citrullinated proteins in prion diseases, various cytoskeletal and energy metabolism-associated proteins were identified as citrullinated candidates including GFAP, MBP, vimentin, enolase, aldolase and phosphoglycerate kinase.4,5 We believe that PAD isotypes have distinct substrate specificities,45-47 which results in the prevalent citrullination of certain protein groups. In accordance with the results from scrapie-infected mice, increased PAD2 expression, abundant protein citrullination and reactive astrocytes were associated with CJD patients.5
PAD activation requires a high Ca2+ concentration in vitro. Previous findings, including our own, demonstrated that the levels of PAD2 and citrullinated proteins are markedly increased in reactive astrocytes. Physiologically, astrocytes are the predominant cell type in the brain, and reactive astrocytes increase in number dramatically during CNS injuries. The elevation of intracellular Ca2+ in astrocytes depends largely on a release from the internal store. This release can be a response to neuronal activity, and can also be propagated through Ca2+ signaling from neighboring astrocytes through various mechanisms involving a number of channels and receptors such as voltage-gated Ca2+channels, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, N-methyl-d-aspartate receptors, and ionotropic glutamate and purine receptors.48-50 Astrocyte-released gliotransmitters connect astrocytes with neurons and other astrocytes for neuronal communication and are typically induced by Ca2+ signaling. Moreover, Ca2+ may play a regulatory role in PAD2 transcription.51 Therefore, upregulated PAD2 expression, increased PAD2 activation, and increased protein citrullination may be inevitable consequences of astrocyte excitation associated with neurodegeneration.
An altered glycolytic energy metabolism balance is one of the main signs of neurodegeneration. In a recent study, we evaluated for the first time whether PADs can regulate energy metabolism and change the biochemical properties of enolase 1 (ENO1) and neuron-specific enolase (NSE). Enolases participate in glycolysis and gluconeogenesis and are known as multifunctional proteins.52 Furthermore, citrullinated forms of enolase are identified consistently in the CNS during neurodegeneration and in the synovial tissue of RA patients. We began the study of enolase citrullination by developing three types of mouse IgG1 monoclonal antibodies with specificity to ENO1Cit9 (anti-CE1 antibody), NSECit429 (anti-CE2 antibody) and both ENO1Cit9 and NSECit9 (anti-CE1/2 antibody).53 Using these antibodies, we demonstrated that the levels of citrullinated ENO1 and NSE were increased in the frontal cortex of CJD and AD patients regardless of the expression levels of these proteins. In a biochemical study, the glycolytic enzyme activities of ENO1 and NSE were found to be inhibited by citrullination, and enolase was more inactive in CJD and AD patients than in controls.53 The data indicate that citrullination by PADs can lead to the irreversible inactivation of enolase activity, thereby downregulating the energy metabolism balance in neurodegenerative diseases. Additionally, we found that citrullinated enolase undergoes calpain-1-mediated degradation. Calpain is a Ca2+-dependent cysteine protease whose activation is associated with impaired intracellular Ca2+ homeostasis and is provoked in the brains of patients with AD and prion diseases.54 Obviously, Ca2+ is correlated with PAD and calpain activation; therefore, citrullination and protein degradation may coincide. A well-known function of enolase is the binding of plasminogen, which enhances the activation of plasminogen55 and facilitates the invasion and dissemination of pathogens into host cells.56 In addition, plasminogen has been implicated in wound healing and inflammation through its involvement in cell proliferation and migration, and microglial plasminogen has a neurotrophic effect on neurons.52 Interestingly, we found that citrullination effectively enhanced the enolase-plasminogen binding affinity. Therefore we hypothesize that citrullinated enolase regulates neurotrophic activity with or without plasminogen in neurodegenerative diseases. Our findings suggest that citrullination modulates the abnormal physiological role of enolase under degenerative conditions.
It is currently unknown whether prion proteins (either PrPC or PrPSc) are a substrate of PADs in either normal or disease states. Young et al.57 reported that, in vitro, PrP citrullination (ovine PrP) resulted in an increase in β-sheet secondary structures, a higher proportion of amyloid structures, greater resistance to trypsin digestion, and greater PK resistance in the presence of copper relative to non-citrullinated PrP. The templating ability of citrullinated PrP confers a conformational change and high PK resistance to non-citrullinated PrP. Although this study was performed in vitro, it is sufficient for understanding the modification effects of PrP by PADs and for proposing a potential role of PADs in prion diseases.
Concluding Remarks
Disrupting Ca2+ homeostasis leads to excessive posttranslational citrullination of proteins by activated PADs. The imbalance caused by irreversible citrullination may herald cell injury or death and may contribute to inflammatory and neurodegenerative disorders. In addition, abnormally increased levels of citrullination are reflected by acute and/or progressive diseases. Our findings and other research data suggest that citrullination may participate in the initiation and progression of degenerative diseases, including prion diseases. Although 14-3-3 and tau proteins are the most reliable diagnostic measures of prion diseases in cerebrospinal fluid, these markers have low specificity for the diagnosis of prion diseases.3 Therefore, specific antibodies against citrullinated proteins will be helpful for diagnosing the abnormal status of various neurodegenerative diseases, including prion group. We conclude that PAD-mediated citrullination is an undeniable factor associated with neurodegeneration, and this knowledge would be helpful for the development of diagnostic/therapeutic strategies in dealing with these diseases.
Acknowledgments
This work was supported by by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-619-E0001) and supported under the framework of international cooperation program managed by the National Research Foundation of Korea (H00002).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/22380
References
- 1.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 3.Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11:618–28. doi: 10.1016/S1474-4422(12)70063-7. [DOI] [PubMed] [Google Scholar]
- 4.Jang B, Kim E, Choi JK, Jin JK, Kim JI, Ishigami A, et al. Accumulation of citrullinated proteins by up-regulated peptidylarginine deiminase 2 in brains of scrapie-infected mice: a possible role in pathogenesis. Am J Pathol. 2008;173:1129–42. doi: 10.2353/ajpath.2008.080388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang B, Jin JK, Jeon YC, Cho HJ, Ishigami A, Choi KC, et al. Involvement of peptidylarginine deiminase-mediated post-translational citrullination in pathogenesis of sporadic Creutzfeldt-Jakob disease. Acta Neuropathol. 2010;119:199–210. doi: 10.1007/s00401-009-0625-x. [DOI] [PubMed] [Google Scholar]
- 6.Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, et al. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J Neurosci Res. 2005;80:120–8. doi: 10.1002/jnr.20431. [DOI] [PubMed] [Google Scholar]
- 7.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–18. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 8.Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11:777–83. doi: 10.1038/nsmb799. [DOI] [PubMed] [Google Scholar]
- 9.Méchin MC, Sebbag M, Arnaud J, Nachat R, Foulquier C, Adoue V, et al. Update on peptidylarginine deiminases and deimination in skin physiology and severe human diseases. Int J Cosmet Sci. 2007;29:147–68. doi: 10.1111/j.1467-2494.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 10.Nachat R, Méchin MC, Charveron M, Serre G, Constans J, Simon M. Peptidylarginine deiminase isoforms are differentially expressed in the anagen hair follicles and other human skin appendages. J Invest Dermatol. 2005;125:34–41. doi: 10.1111/j.0022-202X.2005.23763.x. a. [DOI] [PubMed] [Google Scholar]
- 11.Nachat R, Méchin MC, Takahara H, Chavanas S, Charveron M, Serre G, et al. Peptidylarginine deiminase isoforms 1-3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol. 2005;124:384–93. doi: 10.1111/j.0022-202X.2004.23568.x. b. [DOI] [PubMed] [Google Scholar]
- 12.Moscarello MA, Pritzker L, Mastronardi FG, Wood DD. Peptidylarginine deiminase: a candidate factor in demyelinating disease. J Neurochem. 2002;81:335–43. doi: 10.1046/j.1471-4159.2002.00834.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimada N, Handa S, Uchida Y, Fukuda M, Maruyama N, Asaga H, et al. Developmental and age-related changes of peptidylarginine deiminase 2 in the mouse brain. J Neurosci Res. 2010;88:798–806. doi: 10.1002/jnr.22255. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas AP, Sambandam T, Echols JD, Tourtellotte WW. Increased citrullinated glial fibrillary acidic protein in secondary progressive multiple sclerosis. J Comp Neurol. 2004;473:128–36. doi: 10.1002/cne.20102. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki M, Takahara H, Nishi Y, Sugawara K, Sato C. Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem. 1989;264:18119–27. [PubMed] [Google Scholar]
- 16.Musse AA, Li Z, Ackerley CA, Bienzle D, Lei H, Poma R, et al. Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system. Dis Model Mech. 2008;1:229–40. doi: 10.1242/dmm.000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange S, Gögel S, Leung KY, Vernay B, Nicholas AP, Causey CP, et al. Protein deiminases: new players in the developmentally regulated loss of neural regenerative ability. Dev Biol. 2011;355:205–14. doi: 10.1016/j.ydbio.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, et al. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545–53. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–83. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 20.Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer. 2009;9:40. doi: 10.1186/1471-2407-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao H, Li P, Venters BJ, Zheng S, Thompson PR, Pugh BF, et al. Histone Arg modifications and p53 regulate the expression of OKL38, a mediator of apoptosis. J Biol Chem. 2008;283:20060–8. doi: 10.1074/jbc.M802940200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Yao H, Zhang Z, Li M, Luo Y, Thompson PR, et al. Regulation of p53 target gene expression by peptidylarginine deiminase 4. Mol Cell Biol. 2008;28:4745–58. doi: 10.1128/MCB.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Wang D, Yao H, Doret P, Hao G, Shen Q, et al. Coordination of PAD4 and HDAC2 in the regulation of p53-target gene expression. Oncogene. 2010;29:3153–62. doi: 10.1038/onc.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanikawa C, Espinosa M, Suzuki A, Masuda K, Yamamoto K, Tsuchiya E, et al. Regulation of histone modification and chromatin structure by the p53-PADI4 pathway. Nat Commun. 2012;3:676. doi: 10.1038/ncomms1676. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright PW, Bolling LC, Calvert ME, Sarmento OF, Berkeley EV, Shea MC, et al. ePAD, an oocyte and early embryo-abundant peptidylarginine deiminase-like protein that localizes to egg cytoplasmic sheets. Dev Biol. 2003;256:73–88. doi: 10.1016/S0012-1606(02)00126-4. [DOI] [PubMed] [Google Scholar]
- 28.Esposito G, Vitale AM, Leijten FP, Strik AM, Koonen-Reemst AM, Yurttas P, et al. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol Cell Endocrinol. 2007;273:25–31. doi: 10.1016/j.mce.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Yurttas P, Vitale AM, Fitzhenry RJ, Cohen-Gould L, Wu W, Gossen JA, et al. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo. Development. 2008;135:2627–36. doi: 10.1242/dev.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kan R, Yurttas P, Kim B, Jin M, Wo L, Lee B, et al. Regulation of mouse oocyte microtubule and organelle dynamics by PADI6 and the cytoplasmic lattices. Dev Biol. 2011;350:311–22. doi: 10.1016/j.ydbio.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang B, Shin HY, Choi JK, Nguyen PT, Jeong BH, Ishigami A, et al. Subcellular localization of peptidylarginine deiminase 2 and citrullinated proteins in brains of scrapie-infected mice: nuclear localization of PAD2 and membrane fraction-enriched citrullinated proteins. J Neuropathol Exp Neurol. 2011;70:116–24. doi: 10.1097/NEN.0b013e318207559e. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya SK, Crabb JS, Bonilha VL, Gu X, Takahara H, Crabb JW. Proteomics implicates peptidyl arginine deiminase 2 and optic nerve citrullination in glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2006;47:2508–14. doi: 10.1167/iovs.05-1499. [DOI] [PubMed] [Google Scholar]
- 33.Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest. 1994;94:146–54. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klareskog L, Rönnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–75. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 35.Nicholas AP. Dual immunofluorescence study of citrullinated proteins in Parkinson diseased substantia nigra. Neurosci Lett. 2011;495:26–9. doi: 10.1016/j.neulet.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Wood DD, Bilbao JM, O’Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol. 1996;40:18–24. doi: 10.1002/ana.410400106. [DOI] [PubMed] [Google Scholar]
- 37.Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32:251–6. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39:5374–81. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- 39.Beniac DR, Wood DD, Palaniyar N, Ottensmeyer FP, Moscarello MA, Harauz G. Marburg’s variant of multiple sclerosis correlates with a less compact structure of myelin basic protein. Mol Cell Biol Res Commun. 1999;1:48–51. doi: 10.1006/mcbr.1999.0111. [DOI] [PubMed] [Google Scholar]
- 40.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 41.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Méchin MC, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007;56:3541–53. doi: 10.1002/art.22983. [DOI] [PubMed] [Google Scholar]
- 42.Chang X, Jian X, Yan X. Expression and citrullination of keratin in synovial tissue of rheumatoid arthritis. Rheumatol Int. 2009;29:1337–42. doi: 10.1007/s00296-009-0863-1. [DOI] [PubMed] [Google Scholar]
- 43.Chang X, Zhao Y, Sun S, Zhang Y, Zhu Y. The expression of PADI4 in synovium of rheumatoid arthritis. Rheumatol Int. 2009;29:1411–6. doi: 10.1007/s00296-009-0870-2. [DOI] [PubMed] [Google Scholar]
- 44.Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122:1791–802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Méchin MC, Enji M, Nachat R, Chavanas S, Charveron M, Ishida-Yamamoto A, et al. The peptidylarginine deiminases expressed in human epidermis differ in their substrate specificities and subcellular locations. Cell Mol Life Sci. 2005;62:1984–95. doi: 10.1007/s00018-005-5196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, et al. Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry. 2010;49:4852–63. doi: 10.1021/bi100363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis. 2012;71:92–8. doi: 10.1136/ard.2011.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–25. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiacco TA, McCarthy KD. Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia. 2006;54:676–90. doi: 10.1002/glia.20396. [DOI] [PubMed] [Google Scholar]
- 50.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong S, Kojima T, Shiraiwa M, Méchin MC, Chavanas S, Serre G, et al. Regulation of the expression of peptidylarginine deiminase type II gene (PADI2) in human keratinocytes involves Sp1 and Sp3 transcription factors. J Invest Dermatol. 2005;124:1026–33. doi: 10.1111/j.0022-202X.2005.23690.x. [DOI] [PubMed] [Google Scholar]
- 52.Butterfield DA, Lange ML. Multifunctional roles of enolase in Alzheimer’s disease brain: beyond altered glucose metabolism. J Neurochem. 2009;111:915–33. doi: 10.1111/j.1471-4159.2009.06397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang B, Jeon YC, Choi JK, Park M, Kim JI, Ishigami A, et al. Peptidylarginine deiminase modulates the physiological roles of enolase via citrullination: links between altered multifunction of enolase and neurodegenerative diseases. Biochem J. 2012;445:183–92. doi: 10.1042/BJ20120025. [DOI] [PubMed] [Google Scholar]
- 54.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227:407–15. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 56.Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, et al. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113:5588–98. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 57.Young DS, Meersman F, Oxley D, Webster J, Gill AC, Bronstein I, et al. Effect of enzymatic deimination on the conformation of recombinant prion protein. Biochim Biophys Acta. 2009;1794:1123–33. doi: 10.1016/j.bbapap.2009.03.013. [DOI] [PubMed] [Google Scholar]


