Abstract
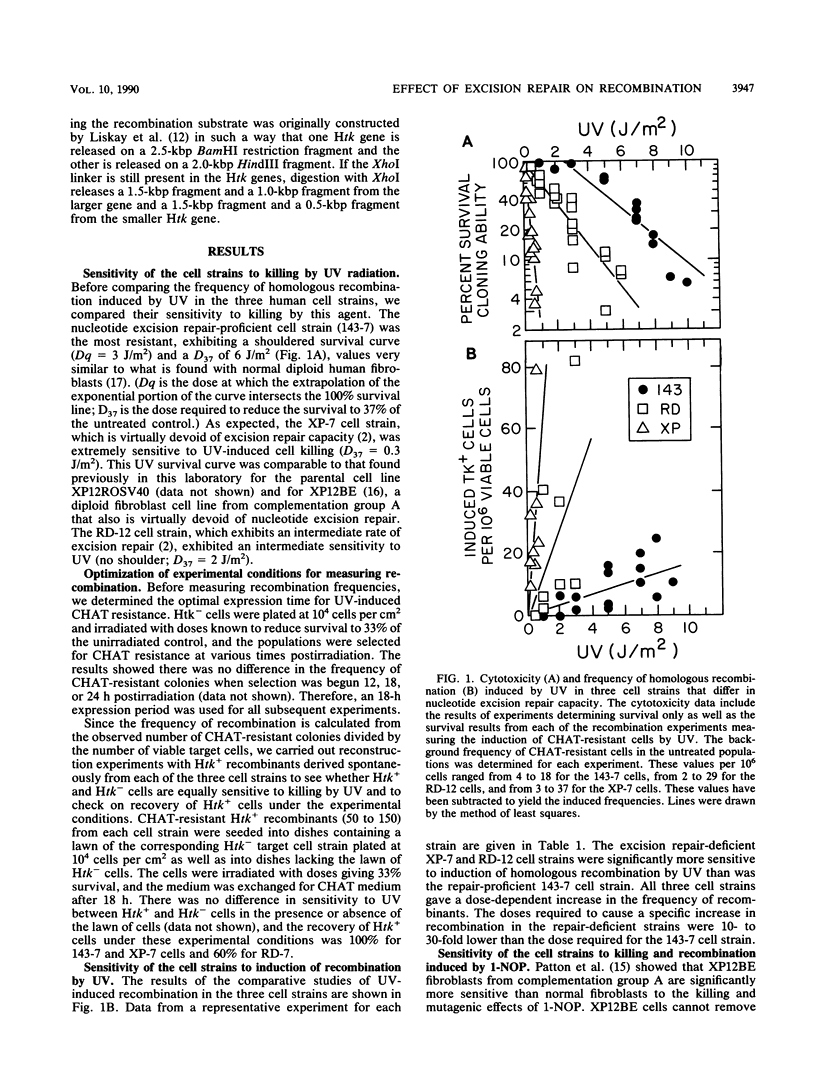
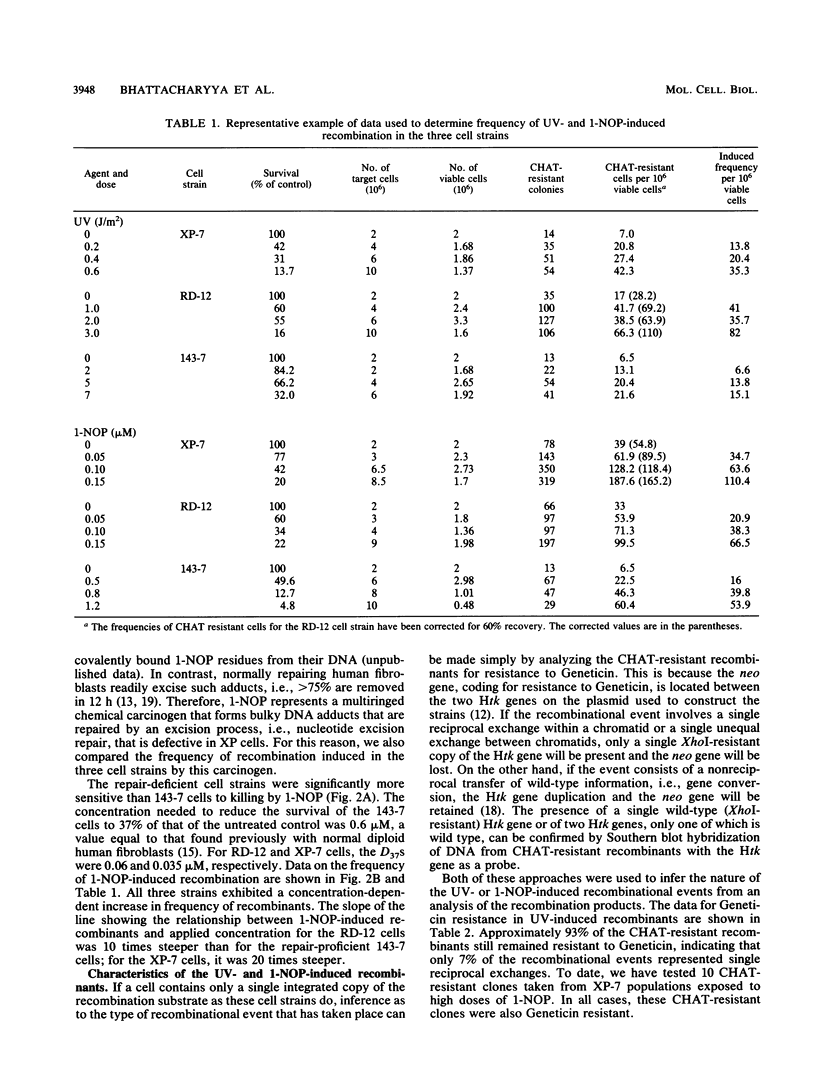
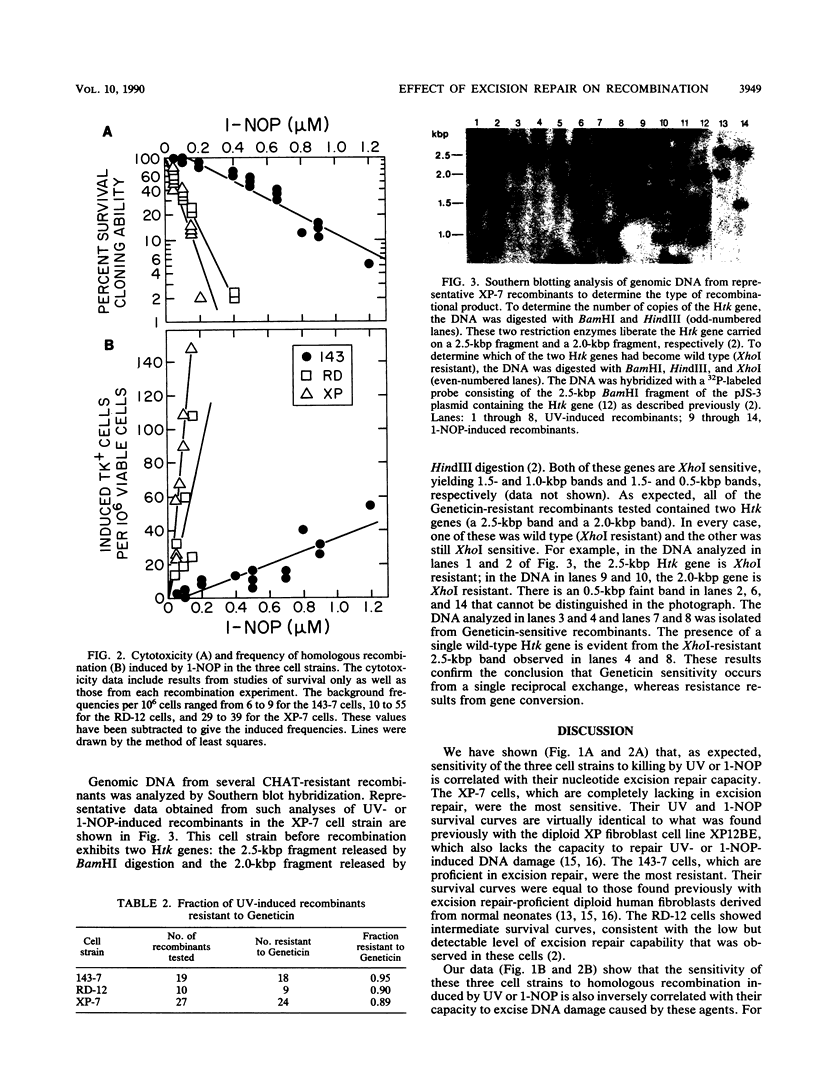
To study the role of nucleotide excision repair in the induction of intrachromosomal homologous recombination in mammalian cells, we introduced a plasmid containing a substrate for recombination into three human cell lines that differ in their repair capacity and compared the frequency of recombination induced by UV radiation and by 1-nitrosopyrene. One strain had a normal capacity for nucleotide excision repair, the second exhibited an intermediate rate of repair, and the third, derived from a patient with xeroderma pigmentosum, had no ability to repair UV- or 1-nitrosopyrene-induced DNA damage. The endogenous thymidine kinase genes in these cell strains had been inactivated, and the cells contained an integrated copy of a plasmid carrying duplicated copies of the herpes simplex virus type 1 thymidine kinase (Htk) gene, each inactivated by an 8-base-pair XhoI site inserted at a unique site. A functional tk gene can only be generated by a productive recombination event between the two Htk genes. In all three stains, UV and 1-nitrosopyrene induced dose-dependent increases in the frequency of recombinants. However, the doses required to cause a specific increase in recombination in the repair-deficient strains were 10 to 30 times lower than the dose required for the cell strain with a normal capacity for repair. These results strongly suggest that unexcised DNA lesions, rather than excision repair per se, stimulate intrachromosomal homologous recombination. Southern blot analysis of DNA from representative recombinants indicated that in all cases one of the two Htk genes had become wild type (XhoI resistant). The majority (90%) retained the Htk duplication, consistent with nonreciprocal transfer of genetic information (gene conversion).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharyya N. P., Maher V. M., McCormick J. J. Ability of structurally related polycyclic aromatic carcinogens to induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mutat Res. 1989 Apr;211(2):205–214. doi: 10.1016/0027-5107(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya N. P., Maher V. M., McCormick J. J. Intrachromosomal homologous recombination in human cells which differ in nucleotide excision-repair capacity. Mutat Res. 1990 Feb;234(1):31–41. doi: 10.1016/0165-1161(90)90028-m. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Hanawalt P. C. Replicative intermediates in UV-irradiated simian virus 40. Mutat Res. 1984 Jul-Aug;132(1-2):1–14. doi: 10.1016/0167-8817(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Dasgupta U. B., Summers W. C. Genetic recombination of herpes simplex virus, the role of the host cell and UV-irradiation of the virus. Mol Gen Genet. 1980;178(3):617–623. doi: 10.1007/BF00337869. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr Recombination of parent and daughter strand DNA after UV-irradiation in mammalian cells. Nature. 1983 Aug 11;304(5926):552–554. doi: 10.1038/304552a0. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Haynes R. H. Phenomenology and genetic control of mitotic recombination in yeast. Annu Rev Genet. 1981;15:57–89. doi: 10.1146/annurev.ge.15.120181.000421. [DOI] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H., German J. Evidence for increased in vivo mutation and somatic recombination in Bloom's syndrome. Proc Natl Acad Sci U S A. 1989 Jan;86(2):670–674. doi: 10.1073/pnas.86.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L., Letsou A. Homologous recombination between repeated chromosomal sequences in mouse cells. Cold Spring Harb Symp Quant Biol. 1984;49:183–189. doi: 10.1101/sqb.1984.049.01.021. [DOI] [PubMed] [Google Scholar]
- Nairn R. S., Humphrey R. M., Adair G. M. Transformation depending on intermolecular homologous recombination is stimulated by UV damage in transfected DNA. Mutat Res. 1988 Jul;208(3-4):137–141. doi: 10.1016/0165-7992(88)90049-8. [DOI] [PubMed] [Google Scholar]
- Patton J. D., Maher V. M., McCormick J. J. Cytotoxic and mutagenic effects of 1-nitropyrene and 1-nitrosopyrene in diploid human fibroblasts. Carcinogenesis. 1986 Jan;7(1):89–93. doi: 10.1093/carcin/7.1.89. [DOI] [PubMed] [Google Scholar]
- Patton J. D., Rowan L. A., Mendrala A. L., Howell J. N., Maher V. M., McCormick J. J. Xeroderma pigmentosum fibroblasts including cells from XP variants are abnormally sensitive to the mutagenic and cytotoxic action of broad spectrum simulated sunlight. Photochem Photobiol. 1984 Jan;39(1):37–42. doi: 10.1111/j.1751-1097.1984.tb03401.x. [DOI] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Effect of DNA damage on stable transformation of mammalian cells with integrative and episomal plasmids. Mutat Res. 1989 Mar-May;220(2-3):205–220. doi: 10.1016/0165-1110(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y., Maher V. M., Liskay R. M., McCormick J. J. Carcinogens can induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mol Cell Biol. 1988 Jan;8(1):196–202. doi: 10.1128/mcb.8.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds and spectrum of mutations induced by 1-nitrosopyrene adducts during plasmid replication in human cells. Mol Cell Biol. 1988 Aug;8(8):3364–3372. doi: 10.1128/mcb.8.8.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]




