Abstract
Prion diseases are subacute neurodegenerative diseases that affect humans and a range of domestic and free-ranging animal species. These diseases are characterized by the accumulation of PrPSc, an abnormally folded isoform of the cellular prion protein (PrPC), in affected tissues. The pathology during prion disease appears to occur almost exclusively within the central nervous system. The extensive neurodegeneration which occurs ultimately leads to the death of the host. An intriguing feature of the prion diseases, when compared with other protein-misfolding diseases, is their transmissibility. Following peripheral exposure, some prion diseases accumulate to high levels within lymphoid tissues. The replication of prions within lymphoid tissue has been shown to be important for the efficient spread of disease to the brain. This article describes recent progress in our understanding of the cellular mechanisms that influence the propagation of prions from peripheral sites of exposure (such as the lumen of the intestine) to the brain. A thorough understanding of these events will lead to the identification of important targets for therapeutic intervention, or alternatively, reveal additional processes that influence disease susceptibility to peripherally-acquired prion diseases.
Keywords: prion transmission, prion protein, secondary lymphoid tissue, follicular dendritic cells, mononuclear phagocytes, M cells
Prion diseases (transmissible spongiform encephalopathies) are sub-acute neurodegenerative diseases that affect both humans and animals. During prion disease aggregations of PrPSc, an abnormally folded isoform of the cellular prion protein (PrPC), accumulate in affected tissues. Prion infectivity co-purifies with PrPSc and is considered by some to constitute the major component of the infectious agent.1,2 Many prion diseases, including natural sheep scrapie, bovine spongiform encephalopathy (BSE), chronic wasting disease (CWD) in cervids, and variant Creutzfeldt-Jakob disease (vCJD) in humans, are acquired peripherally such as by oral exposure. The transmissible nature of many prions diseases is an intriguing feature, when compared with other protein-misfolding diseases. However, recent data have started to challenge the opinion that this is restricted to prion diseases. Peripherally applied β-amyloid has been reported to induce cerebral β-amyloidosis in recipient APP23 transgenic mice.3 Similarly, pathological α-synuclein aggregates from the brain of a patient with Lewy body disease when injected into A53T α-synuclein transgenic mice induced the aggregation of endogenous α-synuclein in their enteric nervous systems.4
After exposure, many prion diseases first replicate within the secondary lymphoid organs (SLO) as they make their journey from the site of infection to the central nervous system (CNS) (a process termed, neuroinvasion).5-9 Prion replication within SLO is critical for efficient neuroinvasion.5-7,10 From lymphoid tissues, prions appear to subsequently invade the CNS by spreading throughout the peripheral nervous system,11 although hematogenous spread cannot be entirely excluded.
Due to the availability of an ever increasing battery of immunodeficient and transgenic mouse, much attention in recent years has focused on how prions are initially transported from the site of infection to the SLO, and onwards into the CNS. This article therefore focuses on the pathogenesis of the acquired prion diseases, and describes recent progress in our understanding of the cellular mechanisms that influence prion neuroinvasion from peripheral sites of exposure.
Identification of the Cellular Sites of Prion Replication in SLO
The stromal cells within SLO are a heterogenous group of non-migratory cells with diverse functions including the organization and compartmentalization of SLO, the formation of the extracellular matrix, the guidance and survival of immune cells, and the formation of conduits through which small lymph-borne antigens may be delivered to the lymph node parenchyma.12-15 Follicular dendritic cells (FDC) are an important subset of these stromal cells and are situated within the primary B-cell follicles and germinal centers of SLO. FDC should not be confused with the bone-marrow-derived classical dendritic cells (DC),16 as they are an entirely distinct, non-phagocytic, and non-migratory stromal cell lineage.17,18 In the SLO of some sheep with natural scrapie, cervids with CWD and patients with vCJD, prions accumulate first upon FDC as they make their journey from the site of infection to the CNS (a process termed, neuroinvasion).8,9,19 Many studies in mice have shown that prion accumulation upon FDC is important for efficient neuroinvasion after peripheral exposure. Furthermore, prion accumulation in the spleen and subsequent neuroinvasion are both impeded in immunodeficient mice that specifically lack FDC,7,20,21 or following their temporary de-differentiation.5,22,23
Although prion neuroinvasion from peripheral sites of exposure is dependent upon the presence of FDC in lymphoid tissues, it was uncertain whether these cells actually express PrPC and replicate prions. Expression cellular of PrPC is mandatory to sustain prion infection,24,25 and FDC appear to express high levels on their cell surfaces.26,27 Some studies have exploited the mesenchymal and non-hematopoietic-origin of FDC in order to attempt to address their role in prion pathogenesis. In these studies, mismatches were created in Prnp expression (which encodes PrPc) between the FDC-containing stromal and lymphocyte- and leukocyte-containing hematopoietic compartments by grafting bone marrow cells from PrPC-deficient (Prnp−/−) mice into PrPC-expressing wild-type mice, and vice versa.26,27 Using this approach FDC and all other stromal cells were derived from the recipient, whereas lymphocytes and other hematopoietic lineages were derived from the donor cells. Following peripheral exposure prion accumulation upon FDC was only detected in the spleens of mice with a Prnp-expressing stromal compartment.
While these studies clearly show that the presence of FDC is important for prion accumulation in the spleen, it was not possible to dissociate the Prnp expression status of FDC from that of the nervous system and all the other stromal and non-hematopoietic host-cell populations.26,27 The precise identification of the cell populations that facilitate prion replication in SLO is important for a number of reasons. Prion infection can occur within an inflammatory PrPC-expressing stromal cell population at sites of chronic inflammation that is phenotypically distinct from FDC.28 Most evidence indicates that FDC do not derive from hematopoietic precursors.18,29 However, the detection of donor hematopoietic cell-derived antigens on the surface of FDC in recipient mice was originally considered evidence of an FDC precursor cell population within the bone marrow.30 With hindsight these observations were most likely due to the FDC’s efficient ability to acquire exosome-associated antigens from neighboring cells.31 These microvesicles enable FDC to passively acquire and display proteins on their surfaces that they do not express at the mRNA level.31 Both PrPC and PrPSc can be released from cells in association with exosomes.32 This suggests that FDC may likewise passively acquire PrPC and prions after their release in exosomes from other infected cells. The efficient ability of FDC to acquire exosome-associated proteins on their surfaces was recently highlighted in a detailed ultrastructural study of mouse Peyer’s patches.33 The intestinal epithelium-specific gene Gpa33 is expressed highly by enteroctyes throughout the intestine and not by Peyer’s patch FDC. However, IHC analysis of Peyer’s patches shows FDC accumulate high levels of enterocyte-derived Gpa33 protein on their surfaces after being shed from the intestinal epithelium in association with exosomes.33
To determine whether FDC also passively acquire PrPC embryonic day 15 spleen tissue from PrPC-deficient (Prnp−/−) mice or wild-type (WT) controls was transplanted under the kidney capsule of WT recipient mice.34 Within the grafted spleens, the stromal FDC are of donor Prnp-genotype, whereas the majority of the hematopoietically-derived cells within the graft (lymphocytes, leukocytes etc.) and all cell lineages within the rest of the mouse are of host Prnp-genotype. If FDC do acquire PrPC from other cell lineages one would expect to detect PrPC upon the surfaces of the FDC within the PrPC-deficient spleens grafted into WT mice (Prnp−/−→WT). Grafted spleen tissues within the recipient kidneys were analyzed by IHC 6 weeks after transplant and PrPC-expressing FDC were detected, as anticipated in the B cell follicles of the WT donor spleens grafted into WT recipients (WT→WT). However, the FDC within the Prnp−/− spleens grafted into WT mice (Prnp−/−→WT) lacked PrPC expression indicating that FDC do not simply acquire PrPC from other cell populations (Fig. 1).34
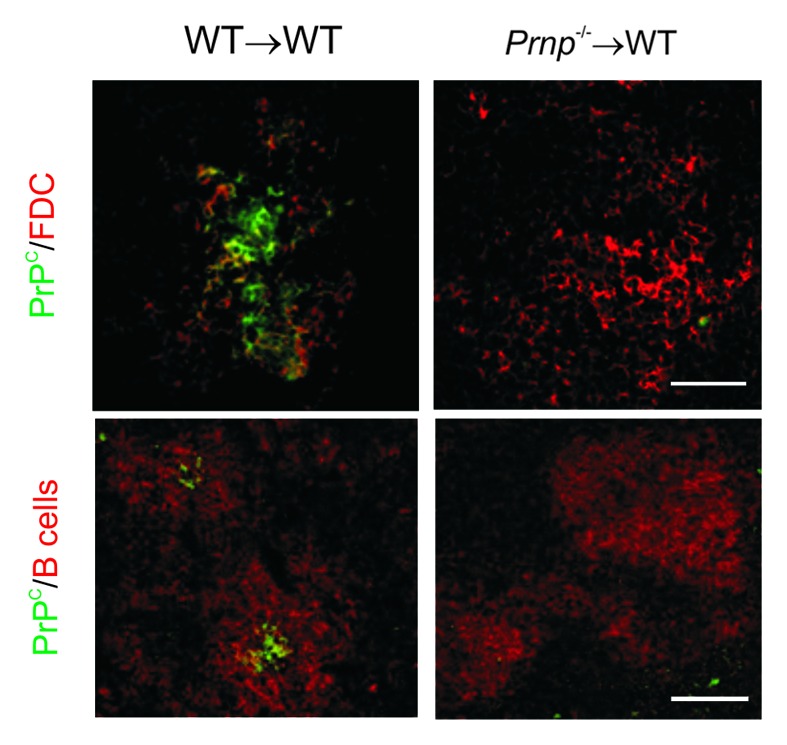
Figure 1. FDC do not passively acquire PrPC from other cell types. Whole embryonic day 15 spleen tissue from Prnp−/− mice or wild-type (WT) controls was transplanted under the kidney capsule of WT recipient mice. Tissues were analyzed 6 weeks after transplant. FDC expressing high levels of PrPC were detected in the B cell follicles of the WT donor spleens grafted into WT recipients (WT→WT; left-hand panels). However, no PrPC was detected upon FDC within the Prnp−/− spleens grafted into WT mice (Prnp−/−→WT; right-hand panels). Upper panels, scale bar = 200 µm. Lower panels, scale bar = 50 µm. Adapted from Brown et al.34
FDC characteristically trap and retain native antigen on their surfaces for long periods in the form of immune complexes, consisting of antigen-antibody and/or complement components. FDC themselves express negligible levels of complement component C4 at the mRNA level, but the detection of large quantities of activated C4 protein on their surfaces by IHC illustrates their capacity to capture and retention of immune complexes.35 Prions also appear to be acquired by FDC as complement-opsonized complexes.36-39 Thus FDC may simply act as concentrating depots for prion-containing, complement-opsonized complexes.
FDC and mature B cells express high levels of Cr2 which encodes the complement receptors CR2/CR1 (CD21/35).39,40 Victoratos and colleagues illustrated how CD21-cre mice could be used to study FDC-specific gene function.40 Using this approach we recently created unique compound transgenic mice in which PrPC expression could be specifically “switched on” or “switched off” only on FDC.10 These mice where then used to establish whether FDC amplify prions, or simply acquire them from other infected host cells. In the first mouse line, PrPC-expression was switched on only on FDC. In the SLO of these mice only the FDC had the potential to replicate prions since they were the only cells in the body which expressed PrPC. Our data showed that the expression of PrPC only on FDC was sufficient to sustain high levels of prion replication upon FDC after peripheral exposure. These data confirm that FDC are the critical sites of prion replication in SLO.
We also created a second compound transgenic mouse model in which PrPC expression was specifically “switched off” only on FDC. If FDC are the critical sites of prion replication in the spleen, then one would also expect this to be blocked when PrPC expression was ablated only on FDC. Our data confirmed this to be the case (Fig. 2). As PrPC expression in all other host cells (eg: nerves) in these mice was unaffected, these data clearly show that FDC do not simply acquire prions following release from other infected host cells, even in mice displaying clinical signs of prion disease in the brain.10
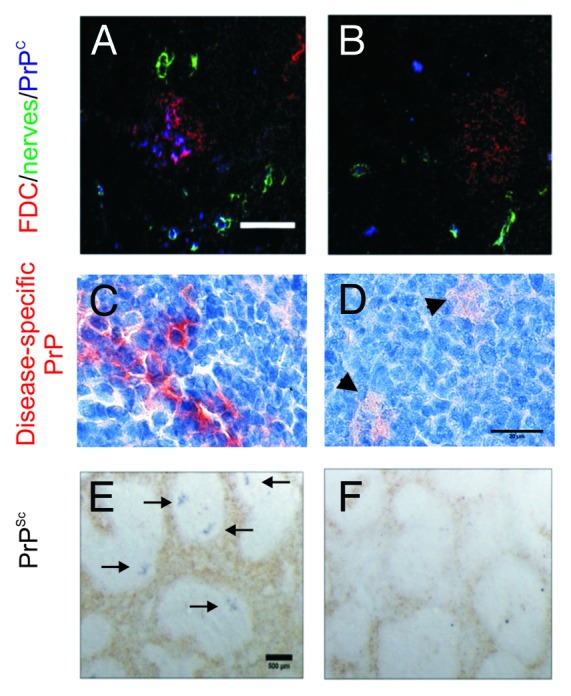
Figure 2. The specific ablation of PrPC expression only on FDC blocks prion replication in the spleen. CD21-cre mice can be used to study FDC-specific gene function.40 (A and B), Using this approach we recently created a compound transgenic mouse in which PrPC expression could be specifically “switched off” only on FDC.10 (C&E), Following peripheral prion exposure high levels of PrPSc accumulate upon the surfaces of FDC in the spleens of control mice. (D&F) However, prion replication is blocked in the spleens of mice in which PrPC expression was specifically “switched off” only on FDC. Arrow heads in D show scavenged PrP within TBM. Arrows in E show PrPSc-positive FDC in the spleens of control mice. A&B, scale bar = 100 µm; C&D, scale bar = 20 µm; E&F, scale bar = 500 µm. Adapted from McCulloch et al.10
Ultrastructural analysis of the cellular compartments within which PrPSc localizes upon/within FDC has failed to detect any intracellular accumulation. Instead the PrPSc appears to accumulate along the surfaces of their dendrites, increasing in density as the infection proceeds.33 This suggests that de novo PrPSc conversion occurs upon the FDC surface.
The accumulation of high levels of PrPSc within the brain ultimately leads to the development of neuropathology. Antigens trapped on the surface of FDC are considered to promote the development of high affinity antibody responses and to maintain immunological memory.41-45 Despite the detection of high levels PrPSc upon the surfaces of FDC within SLO throughout almost the entire duration of the disease, no gross immunological defect has been reported. However, ultrastructural analysis of prion-affected SLO has revealed evidence of disease-associated morphological changes to FDC. These include adversely affected maturation cycles, abnormal dendritic folding and exacerbated accumulation of immune complexes between the FDC dendrites.46 Further studies are necessary to determine whether these defects lead to subtle effects on FDC-dependent immune function such as impairments to antibody affinity maturation.
Prions Spread from SLO to the Brain via the Peripheral Nervous System
The SLO are highly innervated with sympathetic neurones,47 and following replication upon FDC prions subsequently infect the peripheral nervous system.33 The depletion of sympathetic nerves impedes prion neuroinvasion,11 indicating that following replication upon FDC, translocation to the CNS occurs via the peripheral nervous system.11,48 While the relative positioning of FDC to peripheral nerves has been shown to influence the rate of neuroinvasion,49 little is known of how the neuroinvasion actually occurs. Studies have also suggested that mononuclear phagocytes such as classical DC may transfer prions directly to the nervous system.50-52 However, whether these cells do play an important role in the transfer of prions from the immune to the peripheral nervous system in vivo,50 is uncertain since subsequent data suggested this was unlikely.53
M Cells: Portals for Prions across the Intestinal Epithelium
The gut-associated lymphoid tissues (GALT) include the appendix, tonsils, Peyer’s patches, colonic and cecal patches and isolated lymphoid follicles (ILF). In combination with the mesenteric lymph nodes, these tissues help protect the host from gastro-intestinal infections. However, within days of oral prion exposure early replication occurs upon FDC in Peyer’s patches,33 and is obligatory for efficient neuroinvasion.6,7 Data also show that FDC within tertiary lymphoid tissues, such as mature ILF, are also potentially important sites of prion replication and neuroinvasion in the intestine.7 To be able to replicate upon FDC in Peyer’s patches and ILF, the prions must first cross the follicle-associated epithelium (FAE) which overlies the mucosal surface of these tissues. Many studies have attempted to address how prions cross this specialized, single layer of epithelial cells bound by tight-junctions, and survive intact in sufficient quantities to infect the host. Scattered within the FAE of Peyer’s patches are a unique subset of epithelial cells termed microfold cells (M cells). These highly transcytotic cells enable the host’s immune system to sample the intestinal lumen and, where necessary, elicit an appropriate immune response.54,55 However, the ability of M cells to mediate the transepithelial transfer of particulate antigens has been exploited by a range of pathogens, including bacteria and viruses, to enable them to gain entry into mucosal tissues.56-60
Data from studies in which prions were immunohistologically traced after oral exposure,61,62 or using an in vitro system with “M cell-like” transcytotic properties,63 suggest that M cells are the initial sites of prion transcytosis across the intestinal epithelium. However, other studies using similar approaches suggested that this translocation occurred instead via enterocytes, independently of M cells.64,65 A further study showed that while some uptake of PrPSc was detectable within M cells, the majority appeared to localize to, and be transcytosed by, FAE-associated enterocytes.33 The significance of these observations for prion neuroinvasion from the intestine was uncertain.
The tumor necrosis factor (TNF) superfamily member receptor activator of NF-κB ligand (RANKL) has been shown to be a critical factor that induces the differentiation of M cells in the gut epithelium and maintains them in their differentiated state.54 Within Peyer’s patches RANKL is expressed by subepithelial stromal cells beneath the FAE and signals via its receptor RANK (receptor activator of NF-κB) which is expressed by epithelial cells throughout the intestine. M cells can be specifically depleted in vivo by RANKL neutralization, and are absent in RANKL-deficient mice.54 Data from recent experiments utilizing RANKL neutralization to specifically deplete M cells show that both the early prion accumulation upon FDC in Peyer’s patches and neuroinvasion were blocked in their absence at the time of oral exposure (Fig. 3). These data suggest that M cells are the important sites of prion uptake from the gut lumen into Peyer’s patches.
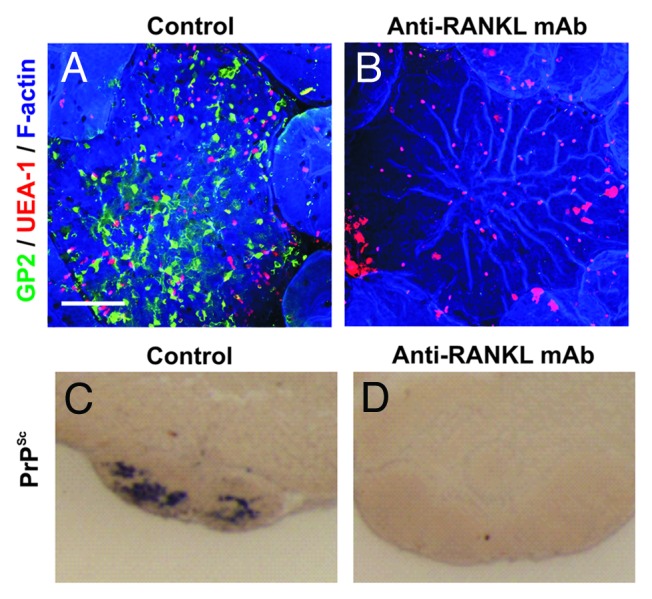
Figure 3. The specific ablation of M cells blocks oral prion pathogenesis. (A&B), Whole-mount immunostaining shows that treatment of mice with anti-RANKL-mAb blocks RANKL-RANKL signaling and specifically depletes M cells (GP2+ UEA-1+ cells) in the FAE of Peyer’s patches. (C&D), In the specific absence of M cells at the time of oral exposure prion accumulation (PrPSc, black) upon FDC in Peyer’s patches is blocked. Adapted from Donaldson et al.66
Whether prions are specifically acquired by M cells is uncertain. Their transcytosis could occur non-specifically via the bulk uptake of particulate antigen. However, molecules such as Gp2 which are highly and specifically expressed by M cells can be utilized by certain types of bacteria to aid their uptake.67 M cells also express high level of cellular PrPC on their surfaces highlighting a potential mechanism by which prions might be specifically acquired.55 However, data from the immunohistological tracing studies indicate that PrPC-deficiency throughout the intestinal epithelium does not impair PrPSc uptake from the gut lumen.33,61 Further studies are necessary to determine whether other molecules on the apical surface of M cells specifically bind prions, or whether PrPC acts as a transcytotic receptor for other pathogenic microorganisms as suggested for the uptake of Brucella by macrophages.68
CD11c+ Cells Aid the Delivery of Prions to FDC in the GALT
Once antigens have been trancytosed by M cells they exit into the intraepithelial pocket beneath the basolateral membrane where they are processed by the lymphocytes and mononuclear phagocytes (MNP, a heterogeneous population of macrophages and classical dendritic cells)69 within it or immediately below in the sub-epithelial dome.59 Migratory bone marrow-derived classical DC are centrally involved in the transport of antigens both within Peyer’s patches and on into the mesenteric lymph nodes.70,71 Classical DC characteristically internalize antigens and process them into short peptides which they present to naïve T cells. Unlike macrophages, classical DC appear to be equipped with both degradative and nondegradative antigen uptake pathways to facilitate antigen presentation to both T and B cells.72,73 The ability of classical DC to capture and retain unprocessed antigen,74,75 and migrate into B-cell follicles suggested classical DC are plausible candidates for the propagation of prions to and within SLO.
Expression of the integrin α X (Itgax, CD11c) is commonly used to discriminate classical DC from macrophages. The use of transgenic mice in which CD11c+ cells can be specifically depleted has significantly extended our in vivo understanding of the immunobiology of classical DC.76 The depletion of CD11c+ cells prior to prion exposure impedes replication upon FDC in the draining SLO and delays neuroinvasion from the intestine,77 peritoneal cavity,78 and skin.79 These data imply that following their transcytosis across the FAE by M cells,66 prions are subsequently propagated toward the FDC-containing B-cell follicles by classical DC.77 However, further studies are necessary to test this hypothesis. Our recent data illustrate how there is a distinct lack of absolutely unique cellular markers in the MNP system, and CD11c-expression alone does not specifically identify classical DC.69,80 Thus, whether the CD11c+ cells that influence prion pathogenesis are classical DC, or another MNP population within the Peyer’s patches, likewise remains to be determined.
Tunnelling nanotubes (TNT) have been proposed as a novel intercellular conduit through which prions and other pathogens such as HIV, may disseminate intracellularly between cells.51,81 Data also suggest that HIV may further utilize TNT to evade virus-specific antibody responses by shuttling virus-derived immunosuppressive factors from infected MNP to B cells.82 The protein M-Sec (encoded by Tnfaip2) is expressed at high levels by M cells and MNP and functions as a key regulator of TNT formation.83 Most M cells within the FAE of Peyer’s patches appear to have a one-to-one association with MNP which extend their dendritic processes into the basolateral pockets of M cells.84 This tight association raises the suggestion that prions may also be transferred intracellularly between M cells and MNP via TNT. It is also plausible that prions subsequently disseminate onwards from infected MNP to other cells via TNT.52
Mononuclear Phagocytes: A Double-edged Sword during Prion Pathogenesis
While the nature of the CD11c+ cells implicated in prion propagation remains to be determined, it is clear from current data that depending on the context, MNP may act as a double-edged sword during prion pathogenesis. As described above, some MNP, perhaps those typical of classical DC, might behave as “Trojan horses” and provide a safe haven in which prions may be propagated to and within SLO. In contrast, highly phagocytic macrophages may facilitate their destruction.
Tingible body macrophages (TBM) are a subset of large MNP specific to the germinal centers of SLO. TBM characteristically contain many remnants of phagocytised apoptotic lymphocytes in various states of degradation (termed tingible bodies). Increased numbers of disease-specific PrP-containing TBM are found within the B-cell follicles of prion-affected animals.33,46 The detection of PrPSc only within the endosomal compartments of TBM,33 suggests that these cells scavenge and degrade prions from infected FDC.10 High levels of prions rapidly accumulate within the SLO within weeks of peripheral exposure. Following infection of the CNS the accumulation/replication of prions appears to occur exponentially until the death of the animal. However, the magnitude of the prion accumulation within the spleen rapidly reaches a plateau level which is maintained for the duration of the disease.20,26 How this plateau is maintained is uncertain. This may be due to the establishment of a competitive state whereby FDC act to amplify prions above the threshold required to achieve neuroinvasion, whereas macrophages act to destroy them.85,86 The ability of MNP to provide a protective response against prions is further illustrated when signaling pathways which stimulate pro-inflammatory responses by these cells are blocked. Prion accumulation in the spleen is enhanced in mice deficient in interferon regulatory factor 3 (IRF3), a key transcription factor of the MyD88-independent type I interferon production pathway.87 Similarly, tumor necrosis factor receptor 1-deficiency facilitates replication of RML scrapie prions within macrophages within SLO.21
Recent data challenge the opinion that M cells are major site of antigen transcytosis in the FAE. Ultrastructural analysis shows FAE enterocytes may also exocytose the intravacuolar contents of their late endosomes into the extracellular space of the SED.33 This potentially represents a novel, previously unrecognized important component of the normal machinery for antigen presentation in the GALT. These data also raise the question of whether the route through which antigens are transcytosed across the FAE fundamentally influences the nature of the immune response induced to those antigens? Do antigens which are sampled via M cells induce protective immune responses, whereas a more tolerogenic response is induced after transfer via FAE enterocytes? After being exocytosed by FAE enterocytes PrPSc appeared to be subsequently detected within the endosomal compartments of more macrophage-like MNP cells rather than classical DC.33 In the absence of M cells after RANKL-neutralization, prion neuroinvasion from the GALT is blocked.66 Although possible effects of RANKL-neutralization on FAE enterocytes cannot be entirely excluded in the above study,66 it is tempting to speculate that following their transcytosis by M cells or FAE enterocytes the fate of the prions also differs: transfer via FAE enterocytes, leading to phagocytosis and destruction by MNP in the sub-epithelial dome; transfer via M cells, facilitating uptake and transport by classical DC toward FDC within the B-cell follicles.
Aging Dramatically Influences Prion Pathogenesis within SLO
The ingestion of BSE contaminated meat products is most likely the original source of vCJD in humans.88 During the UK BSE epidemic almost 500,000 infected cattle were likely to have been slaughtered for human consumption.89 Despite the probable widespread exposure of the UK human population to the BSE agent, most clinical cases of vCJD have occurred almost exclusively in young adults (median age at onset of disease = 26 y; median age at death = 28 y). These data starkly contrast those for sporadic CJD which have predominantly occurred in the elderly (median age at onset of disease = 67 y).90 The suggestion that this age-related incidence of vCJD was simply due to exposure to greater levels of BSE through dietary preference has not been substantiated.91 This indicated that other age-related factors were also influencing the susceptibility to some acquired prion diseases.
Host age significantly impairs immune function. This raised the suggestion that the effects of aging on the host’s immune system may also influence the pathogenesis of many acquired prion diseases such as natural sheep scrapie, CWD and vCJD which replicate in SLO prior to neuroinvasion. This hypothesis is consistent with data from a comparative study of Peyer’s patches from sheep, cattle and humans that suggested an age-related association between Peyer’s patch development and susceptibility to natural prion disease.92 In aged (600 d old) mice FDC are impaired, and in contrast to those in young adult spleens, appear atrophic and have limited capacity to trap and retain immune complexes. As a consequence, aged mice elicit poor antibody responses.93-96 Studies using aged mice that show that coincident with the effects of host age on FDC status, the early replication of ME7 scrapie prions upon FDC in the spleen was significantly impaired.97 Furthermore, following peripheral exposure (via oral and intraperitoneal routes), none of the aged mice developed clinical prion disease during their lifespans, although some displayed histopathological signs of disease in their brains. A similar effect of host age on susceptibility to peripheral infection with RML scrapie prions has also been reported.98 Data also show that the underdeveloped or reduced functional status of FDC in neonatal mice likewise coincides with impaired prion neuroinvasion following peripheral exposure.99,100 Whether a similar correlation is observed in natural prion infections remains to be determined?
Four cases of vCJD have also been reported in recipients of blood or blood products derived from vCJD-infected donors.101-104 Interestingly, three of these cases were reported in elderly patients: two cases preclinical;102,104 one case clinical.101 This raised the possibility that intravenous (i.v.) exposure may be more efficient in the elderly when compared with other peripheral routes of exposure. Using an aged mouse model, data show that, as in young adult mice, the i.v. route is more efficient than other peripheral routes of prion exposure.105 However, disease pathogenesis in i.v. exposed aged mice was significantly impaired when compared with young mice with most failing to develop clinical disease during their lifespans.
The effects of host age on the ability of FDC trap and retain immune complexes are not simply due to the reduced expression of complement receptors, or reduced levels of opsonising complement components in the serum, since these were unaffected in aged mice.34 In the spleen the marginal zone (MZ) forms a barrier around the lymphocyte-containing white pulp and plays an important role in the capture of blood-borne antigens and immune complexes, and facilitates their efficient transport to FDC.12,106 The B cells within the MZ continuously shuttle between the MZ and B-cell follicles in the underlying white pulp and play an important role in the capture and transport of blood-borne, complement-bound antigens to FDC.106 Aging dramatically disturbs the microarchitecture of the MZ (Fig. 4), which impedes the delivery of immune complexes to FDC.34 Since prions are likewise considered to be acquired by FDC as complement-bound complexes,36-39 these aging-related disturbances to the MZ appear to impede the shuttling of prion-containing complement-bound complexes to FDC. As a consequence, prion replication on FDC is reduced and neuroinvasion impeded.34,97
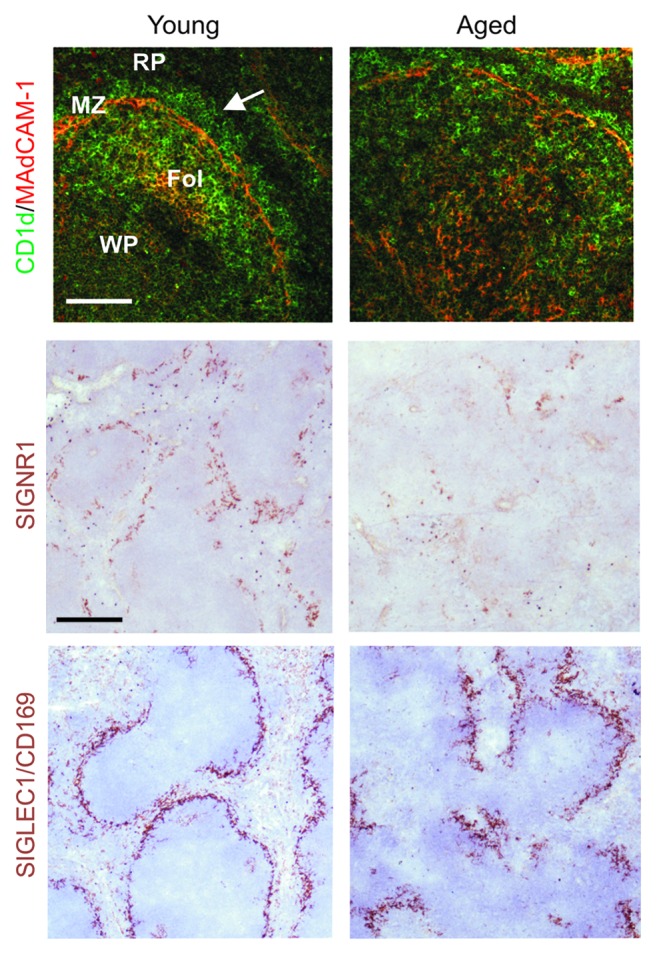
Figure 4. The microarchitecture of the splenic MZ is disrupted in the spleens of aged mice. Left-hand-panels; In the spleens of young mice MADCAM1-expressing sinus-lining cells form a distinct barrier between the MZ and the white pulp (WP, arrow). RP, red pulp; Fol, FDC-containing B cell follicle. Within the MZ abundant CD1d-expressing MZ B cells (upper panels, green) continually shuttle between MZ and B cell follicles as they transfer immune complexes to FDC. Two distinct populations of macrophages also reside in the MZ: SIGNR1-expressing MZ macrophages, and SIGLEC1/CD169-expressing MZ metallophilic macrophages (middle and lower panels, brown). In the spleens of aged mice the distribution and density of these cells are all severely disrupted when compared with young mice (right-hand panels). Upper panels, scale bar = 50 µm; middle and lower panels, scale bar = 100 µm. Adapted from Brown et al.34
The expression of PrPC by FDC in the spleens of aged mice is also dramatically reduced.97 The mechanism responsible for the downregulation of PrPC expression on aged FDC is uncertain. The expression of PrPC by FDC in the spleen can be stimulated by immune complex-trapping, and is blocked in the absence of complement.107 Whether the reduced expression of PrPC by aged FDC is a consequence of their reduced ability to trap complement-opsonised immune complexes remains to be determined.
The Presence of Prions in SLO and Brain Does Not Always Correlate
No prion-specific preclinical diagnostics are currently available compounding the problems for the assessment of disease-incidence, treatment and eradication. The detection of prions in blood,108 or lymphoid tissue biopsy specimens such as tonsils, appendix,19,109 and rectal-associated lymphoid follicles,110-113 have each been considered as useful preclinical diagnostics. Using transgenic mice expressing the ovine or human form of PrPC, a recent study compared the ability of brain and SLO to replicate foreign, inefficiently transmitted prions. This might occur following the transmission of a prion disease between different species (termed the species barrier).114 The SLO in these transgenic mice were found to be consistently and markedly more permissive than the brain to cross-species infection with prions including CWD or BSE. These data illustrate how continued surveillance of SLO will be important to provide a realistic measure of the incidence of preclinical prion disease, or “silent carriers,” within populations.
Analysis of the spleens and brains of prion-exposed aged mice likewise revealed that there was considerable variation in the detection of prions in the spleens of the clinically-negative aged survivors.34,97 While host age may represent an important barrier to the efficient transmission of peripherally acquired prion disease, data from these studies also suggest that there may be significant levels of subclinical prion disease in the elderly population.34,97 Although many prion-exposed aged mice displayed histopathological signs of prion disease in their brains, prions were absent in the spleens of some mice. Thus, while the analysis of SLO will aid our understanding of the prevalence prion diseases,114 such tests may be much less sensitive when used on elderly patients and livestock.
Conclusions
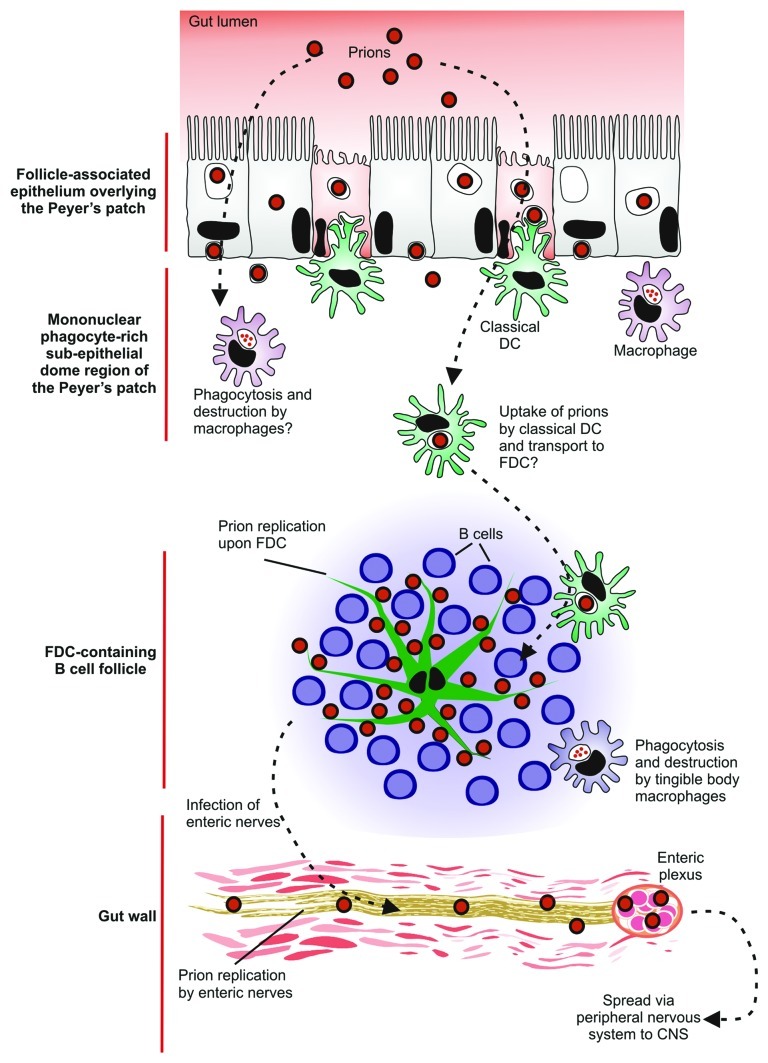
Recent advances in the study of the spread of prions to the brain suggest that after oral exposure neuroinvasion occurs via an elegant cellular relay (Fig. 5). After ingestion of a contaminated meal, prions appear to be actively transcytosed across the FAE from the gut lumen by M cells and FAE enterocytes.33 However, in the absence of M cells neuroinvasion is blocked.66 Once the prions have crossed the epithelium they are subsequently acquired by MNP (macrophages and classical DC). Current hypotheses suggest classical DC, in contrast to macrophages, act as ‘Trojan horses’ and carry the prions onwards to the FDC in the B-cell follicles.77-79 The prions then infect and replicate upon FDC which are considered to amplify the prions above the threshold level required for neuroinvasion.10 Following their expansion upon FDC, prions subsequently infect neighboring nerve fibers of the enteric nervous system.33 Prions are mainly considered to spread to the CNS via the peripheral nervous system,11,49 (sympathetic and parasympathetic) although it is plausible that under some circumstances the hematogenous route may provide a parallel pathway of neuroinvasion.
Figure 5. A potential cellular relay that mediates prion neuroinvasion after oral exposure. After ingestion of a contaminated meal, prions appear to be actively transcytosed into Peyer’s patches by M cells and enterocytes in the follicle-associated epithelium. In the absence of M cells neuroinvasion is blocked suggesting that M cells are the important sites of prion uptake from the gut lumen. The prions are subsequently acquired by mononuclear phagocytes (macrophages and classical DC) in the sub-epithelial dome of the Peyer’s patches. Current hypotheses suggest classical DC, in contrast to macrophages, act as ‘Trojan horses’ and carry the prions to the FDC in the B cell follicles. The prions then infect and replicate upon FDC. Following their expansion upon FDC, prions subsequently infect enteric nerves. The prions then spread to the CNS via the peripheral nervous system (both sympathetic and parasympathetic).
Multiple factors other than the prion protein genotype of the host have been shown to significantly influence prion disease susceptibility, such as the effects of aging on the microarchitecture of the SLO.34,97 Studies show chronic inflammation may enhance prion uptake or expand their tissue distribution.7,28,115 Bacterial colitis also influences oral prion susceptibility.116 Infection with certain pathogenic bacteria has been shown to affect the density of M cells within the gut epithelium.117,118 Therefore, it is plausible that an expansion of M cells induced by concurrent infection with an intestinal pathogen or inflammatory stimuli may also influence oral prion susceptibility.
Recently, exposure via the nasal cavity has been proposed as another potentially important route of prion transmission.119-121 Furthermore, high levels of prions have been reported in the olfactory sensory epithelium.122,123 Furthermore, prions appear to be shed from the olfactory mucosa of affected animals.122,123 Interestingly, despite the presence of FDC within the nasal-associated lymphoid tissues, prion neuroinvasion appears to occur independently of SLO after exposure via the tongue or nasal cavity, implying direct infection of the nervous system.120,124 Further studies are clearly necessary to determine whether inhalation represents a significant natural route of prion transmission in domestic and free-ranging ruminants.
Finally, although significant progress has been made in our understanding of how some prion diseases establish infection within the SLO, little is known of how they subsequently infect the peripheral nervous system. A thorough understanding of this key event in neuroinvasion may identify important targets for therapeutic intervention, or alternatively, reveal additional processes that influence disease susceptibility to peripherally-acquired prion diseases.
Acknowledgments
The author is supported by project and Institute Strategic Programme Grant funding from the Biotechnology and Biological Sciences Research Council, Medical Research Council, European Commission (FP7) and University of Edinburgh Development Fund.
Glossary
Abbreviations:
- BSE
bovine spongiform encephalopathy
- CNS
central nervous system
- CWD
chronic wasting disease, DC, classical dendritic cell
- FAE
follicle-associated epithelium
- FDC
follicular dendritic cell
- GALT
gut-associated lymphoid tissue
- MNP
mononuclear phagocyte
- M cell
microfold cell
- MZ
margninal zone
- PrP
Prion protein
- RANK
receptor activator of NF-κB
- RANKL
receptor activator of NF-κB ligand
- SLO
secondary lymphoid organ
- TBM
tingible body macrophage
- TNT
tunnelling nanotube
- vCJD
variant Creutzfeldt-Jakob disease
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/20676
References
- 1.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–11. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 2.Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, DeArmond SJ, et al. Synthetic mammalian prions. Science. 2004;305:673–6. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 3.Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–2. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HJ, Suk JE, Lee KW, Park SH, Blumbergs PC, Gai WP, et al. Transmission of Synucleiopathies in the Enteric Nervous System of A53T Alpha-Synuclein Transgenic Mice. Exp Neurobiol. 2011;20:181–8. doi: 10.5607/en.2011.20.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabbott NA, Young J, McConnell I, Bruce ME. Follicular dendritic cell dedifferentiation by treatment with an inhibitor of the lymphotoxin pathway dramatically reduces scrapie susceptibility. J Virol. 2003;77:6845–54. doi: 10.1128/JVI.77.12.6845-6854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prinz M, Huber G, Macpherson AJS, Heppner FL, Glatzel M, Eugster H-P, et al. Oral prion infection requires normal numbers of Peyer’s patches but not of enteric lymphocytes. Am J Pathol. 2003;162:1103–11. doi: 10.1016/S0002-9440(10)63907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaysher BR, Mabbott NA. Role of the GALT in scrapie agent neuroinvasion from the intestine. J Immunol. 2007;178:3757–66. doi: 10.4049/jimmunol.178.6.3757. [DOI] [PubMed] [Google Scholar]
- 8.Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, et al. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–26. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 9.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–64. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 10.McCulloch L, Brown KL, Bradford BM, Hopkins J, Bailey M, Rajewsky K, et al. Follicular dendritic cell-specific prion protein (PrP) expression alone is sufficient to sustain prion infection in the spleen. PLoS Pathog. 2011;7:e1002402. doi: 10.1371/journal.ppat.1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron. 2001;31:25–34. doi: 10.1016/S0896-6273(01)00331-2. [DOI] [PubMed] [Google Scholar]
- 12.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 13.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20:1483–7. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 14.Buettner M, Pabst R, Bode U. Stromal cell heterogeneity in lymphoid organs. Trends Immunol. 2010;31:80–6. doi: 10.1016/j.it.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 17.Imazeki N, Senoo A, Fuse Y. Is the follicular dendritic cell a primarily stationary cell? Immunology. 1992;76:508–10. [PMC free article] [PubMed] [Google Scholar]
- 18.Mabbott NA, Kenneth Baillie J, Kobayashi A, Donaldson DS, Ohmori H, Yoon S-O, et al. Expression of mesenchyme-specific gene signatures by follicular dendritic cells: insights from the meta-analysis of microarray data from multiple mouse cell populations. Immunology. 2011;133:482–98. doi: 10.1111/j.1365-2567.2011.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton DA, Fathers E, Edwards P, Ironside JW, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet. 1998;352:703–4. doi: 10.1016/S0140-6736(98)24035-9. [DOI] [PubMed] [Google Scholar]
- 20.Mabbott NA, Williams A, Farquhar CF, Pasparakis M, Kollias G, Bruce ME. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J Virol. 2000;74:3338–44. doi: 10.1128/JVI.74.7.3338-3344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prinz M, Montrasio F, Klein MA, Schwarz P, Priller J, Odermatt B, et al. Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc Natl Acad Sci U S A. 2002;99:919–24. doi: 10.1073/pnas.022626399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabbott NA, Mackay F, Minns F, Bruce ME. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med. 2000;6:719–20. doi: 10.1038/77401. [DOI] [PubMed] [Google Scholar]
- 23.Montrasio F, Frigg R, Glatzel M, Klein MA, Mackay F, Aguzzi A, et al. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science. 2000;288:1257–9. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- 24.Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–47. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 25.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–8. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 26.Brown KL, Stewart K, Ritchie DL, Mabbott NA, Williams A, Fraser H, et al. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat Med. 1999;5:1308–12. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 27.Klein MA, Frigg R, Raeber AJ, Flechsig E, Hegyi I, Zinkernagel RM, et al. PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat Med. 1998;4:1429–33. doi: 10.1038/4022. [DOI] [PubMed] [Google Scholar]
- 28.Heikenwalder M, Kurrer MO, Margalith I, Kranich J, Zeller N, Haybaeck J, et al. Lymphotoxin-dependent prion replication in inflammatory stromal cells of granulomas. Immunity. 2008;29:998–1008. doi: 10.1016/j.immuni.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Tkachuk M, Bolliger S, Ryffel B, Pluschke G, Banks TA, Herren S, et al. Crucial role of tumor necrosis factor receptor 1 expression on nonhematopoietic cells for B cell localization within the splenic white pulp. J Exp Med. 1998;187:469–77. doi: 10.1084/jem.187.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapasi ZF, Qin D, Kerr WG, Kosco-Vilbois MH, Shultz LD, Tew JG, et al. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998;160:1078–84. [PubMed] [Google Scholar]
- 31.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–65. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 32.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101:9683–8. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kujala P, Raymond CR, Romeijn M, Godsave SF, van Kasteren SI, Wille H, et al. Prion uptake in the gut: identification of the first uptake and replication sites. PLoS Pathog. 2011;7:e1002449. doi: 10.1371/journal.ppat.1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown KL, Gossner A, Mok S, Mabbott NA. The effects of host age on the transport of complement-bound complexes to the spleen and the pathogenesis of intravenous scrapie infection. J Virol. 2012;86:25–35. doi: 10.1128/JVI.05581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor PR, Pickering MC, Kosco-Vilbois MH, Walport MJ, Botto M, Gordon S, et al. The follicular dendritic cell restricted epitope, FDC-M2, is complement C4; localization of immune complexes in mouse tissues. Eur J Immunol. 2002;32:1888–96. doi: 10.1002/1521-4141(200207)32:7<1883::AID-IMMU1888>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, et al. Complement facilitates early prion pathogenesis. Nat Med. 2001;7:488–92. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- 37.Mabbott NA, Bruce ME, Botto M, Walport MJ, Pepys MB. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat Med. 2001;7:485–7. doi: 10.1038/86562. [DOI] [PubMed] [Google Scholar]
- 38.Mabbott NA, Bruce ME. Complement component C5 is not involved in scrapie pathogenesis. Immunobiology. 2004;209:545–9. doi: 10.1016/j.imbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Zabel MD, Heikenwalder M, Prinz M, Arrighi I, Schwarz P, Kranich J, et al. Stromal complement receptor CD21/35 facilitates lymphoid prion colonization and pathogenesis. J Immunol. 2007;179:6144–52. doi: 10.4049/jimmunol.179.9.6144. [DOI] [PubMed] [Google Scholar]
- 40.Victoratos P, Lagnel J, Tzima S, Alimzhanov MB, Rajewsky K, Pasparakis M, et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006;24:65–77. doi: 10.1016/j.immuni.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Fu Y-X, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-α (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J Exp Med. 1997;185:2111–20. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α-dependent fashion. J Exp Med. 1998;187:1009–18. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endres R, Alimzhanov MB, Plitz T, Fütterer A, Kosco-Vilbois MH, Nedospasov SA, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin β receptor by radioresistant stromal cells and of lymphotoxin β and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–68. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y-X, Huang G, Wang Y, Chaplin DD. Lymphotoxin-α-dependent spleen microenvironment supports the generation of memory B cells and is required for their subsequent antigen-induced activation. J Immunol. 2000;164:2508–14. doi: 10.4049/jimmunol.164.5.2508. [DOI] [PubMed] [Google Scholar]
- 45.Aydar Y, Sukumar S, Szakal AK, Tew JG. The influence of immune complex-bearing follicular dendritic cells on the IgM response, Ig class switching, and production of high affinity IgG. J Immunol. 2005;174:5358–66. doi: 10.4049/jimmunol.174.9.5358. [DOI] [PubMed] [Google Scholar]
- 46.McGovern G, Mabbott NA, Jeffrey M. Scrapie affects the maturation cycle and immune complex trapping by follicular dendritic cells in mice. PLoS One. 2009;4:e8186. doi: 10.1371/journal.pone.0008186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felten SY, Felten DL. Innervation of Lymphoid Tissue. Psychoneuroimmunology: Academic Press Inc, 1991:27-69. [Google Scholar]
- 48.Beekes M, McBride PA. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274:588–605. doi: 10.1111/j.1742-4658.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- 49.Prinz M, Heikenwalder M, Junt T, Schwarz P, Glatzel M, Heppner FL, et al. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 2003;425:957–62. doi: 10.1038/nature02072. [DOI] [PubMed] [Google Scholar]
- 50.Aucouturier P, Geissmann F, Damotte D, Saborio GP, Meeker HC, Kascsak R, et al. Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J Clin Invest. 2001;108:703–8. doi: 10.1172/JCI13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11:328–36. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 52.Langevin C, Gousset K, Costanzo M, Richard-Le Goff O, Zurzolo C. Characterization of the role of dendritic cells in prion transfer to primary neurons. Biochem J. 2010;431:189–98. doi: 10.1042/BJ20100698. [DOI] [PubMed] [Google Scholar]
- 53.Raymond CR, Mabbott NA. Assessing the involvement of migratory dendritic cells in the transfer of the scrapie agent from the immune to peripheral nervous systems. J Neuroimmunol. 2007;187:114–25. doi: 10.1016/j.jneuroim.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–47. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakato G, Fukuda S, Hase K, Goitsuka R, Cooper MD, Ohno H. New approach for m-cell-specific molecules screening by comprehensive transcriptome analysis. DNA Res. 2009;16:227–35. doi: 10.1093/dnares/dsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohbata S, Yokoyama H, Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer’s patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30:1225–37. doi: 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- 57.Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4:760–5. [PubMed] [Google Scholar]
- 58.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–8. doi: 10.1016/S0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 60.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11:193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 61.Takakura I, Miyazawa K, Kanaya T, Itani W, Watanabe K, Ohwada S, et al. Orally administered prion protein is incorporated by m cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice. Am J Pathol. 2011;179:1301–9. doi: 10.1016/j.ajpath.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foster N, Macpherson GG. Murine cecal patch M cells transport infectious prions in vivo. J Infect Dis. 2010;202:1916–9. doi: 10.1086/657415. [DOI] [PubMed] [Google Scholar]
- 63.Heppner FL, Christ AD, Klein MA, Prinz M, Fried M, Kraehenbuhl J-P, et al. Transepithelial prion transport by M cells. Nat Med. 2001;7:976–7. doi: 10.1038/nm0901-976. [DOI] [PubMed] [Google Scholar]
- 64.Jeffrey M, González L, Espenes A, Press CM, Martin S, Chaplin M, et al. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol. 2006;209:4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 65.Mishra RS, Basu S, Gu Y, Luo X, Zou W-Q, Mishra R, et al. Protease-resistant human prion protein and ferritin are cotransported across Caco-2 epithelial cells: implications for species barrier in prion uptake from the intestine. J Neurosci. 2004;24:11280–90. doi: 10.1523/JNEUROSCI.2864-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donaldson DS, Kobayashi A, Ohno H, Yagita H, Williams IR, Mabbott NA. M cell-depletion blocks oral prion disease pathogenesis. Mucosal Immunol. 2012;5:216–25. doi: 10.1038/mi.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–30. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 68.Watarai M, Kim S, Erdenebaatar J, Makino S-I, Horiuchi M, Shirahata T, et al. Cellular prion protein promotes Brucella infection into macrophages. J Exp Med. 2003;198:5–17. doi: 10.1084/jem.20021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bradford BM, Sester DP, Hume DA, Mabbott NA. Defining the anatomical localisation of subsets of the murine mononuclear phagocyte system using integrin alpha X (Itgax, CD11c) and colony stimulating factor 1 receptor (Csf1r, CD115) expression fails to discriminate dendritic cells from macrophages. Immunobiology. 2011;216:1228–37. doi: 10.1016/j.imbio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 71.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 73.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–14. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–9. [PubMed] [Google Scholar]
- 75.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 76.Jung S, Unutmaz D, Wong P, Sano G-I, De los Santos K, Sparwasser T, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raymond CR, Aucouturier P, Mabbott NA. In vivo depletion of CD11c+ cells impairs scrapie agent neuroinvasion from the intestine. J Immunol. 2007;179:7758–66. doi: 10.4049/jimmunol.179.11.7758. [DOI] [PubMed] [Google Scholar]
- 78.Cordier-Dirikoc S, Chabry J. Temporary depletion of CD11c+ dendritic cells delays lymphoinvasion after intraperitonal scrapie infection. J Virol. 2008;82:8933–6. doi: 10.1128/JVI.02440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wathne GJ, Kissenpfennig A, Malissen B, Zurzolo C, Mabbott NA. Determining the role of mononuclear phagocytes in prion neuroinvasion from the skin. J Leukoc Biol. 2012;91:817–28. doi: 10.1189/jlb.1211633. [DOI] [PubMed] [Google Scholar]
- 80.Mabbott NA, Kenneth Baillie JC, Hume DA, Freeman TC. Meta-analysis of lineage-specific gene expression signatures in mouse leukocyte populations. Immunobiology. 2010;215:724–36. doi: 10.1016/j.imbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–8. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–17. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427–32. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Gusti V, Saraswati A, Lo DD. Convergent and divergent development among M cell lineages in mouse mucosal epithelium. J Immunol. 2011;187:5277–85. doi: 10.4049/jimmunol.1102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carp RI, Callahan SM. Effect of mouse peritoneal macrophages on scrapie infectivity during extended in vitro incubation. Intervirology. 1982;17:201–7. doi: 10.1159/000149289. [DOI] [PubMed] [Google Scholar]
- 86.Maignien T, Shakweh M, Calvo P, Marcé D, Salès N, Fattal E, et al. Role of gut macrophages in mice orally contaminated with scrapie or BSE. Int J Pharm. 2005;298:293–304. doi: 10.1016/j.ijpharm.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 87.Ishibashi D, Atarashi R, Fuse T, Nakagaki T, Yamaguchi N, Satoh K, et al. Protective role of interferon regulatory factor 3-mediated signaling against prion infection. J Virol. 2012;86:4947–55. doi: 10.1128/JVI.06326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 89.Valleron A-J, Boelle P-Y, Will R, Cesbron J-Y. Estimation of epidemic size and incubation time based on age characteristics of vCJD in the United Kingdom. Science. 2001;294:1726–8. doi: 10.1126/science.1066838. [DOI] [PubMed] [Google Scholar]
- 90.Unit NCS. Seventeenth Annual Report 2008 Creutzfeldt-Jakob Disease Surveillance in the UK. 2009.
- 91.Boëlle PY, Cesbron JY, Valleron AJ. Epidemiological evidence of higher susceptibility to vCJD in the young. BMC Infect Dis. 2004;4:26. doi: 10.1186/1471-2334-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.St Rose SG, Hunter N, Matthews L, Foster JD, Chase-Topping ME, Kruuk LEB, et al. Comparative evidence for a link between Peyer’s patch development and susceptibility to transmissible spongiform encephalopathies. BMC Infect Dis. 2006;6:5. doi: 10.1186/1471-2334-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szakal AK, Taylor JK, Smith JP, Kosco MH, Burton GF, Tew JJ. Kinetics of germinal center development in lymph nodes of young and aging immune mice. Anat Rec. 1990;227:475–85. doi: 10.1002/ar.1092270411. [DOI] [PubMed] [Google Scholar]
- 94.Aydar Y, Balogh P, Tew JG, Szakal AK. Age-related depression of FDC accessory functions and CD21 ligand-mediated repair of co-stimulation. Eur J Immunol. 2002;32:2817–26. doi: 10.1002/1521-4141(2002010)32:10<2817::AID-IMMU2817>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 95.Aydar Y, Balogh P, Tew JG, Szakal AK. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J Immunol. 2003;171:5975–87. doi: 10.4049/jimmunol.171.11.5975. [DOI] [PubMed] [Google Scholar]
- 96.Aydar Y, Balogh P, Tew JG, Szakal AK. Follicular dendritic cells in aging, a “bottle-neck” in the humoral immune response. Ageing Res Rev. 2004;3:15–29. doi: 10.1016/j.arr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Brown KL, Wathne GJ, Sales J, Bruce ME, Mabbott NA. The effects of host age on follicular dendritic cell status dramatically impair scrapie agent neuroinvasion in aged mice. J Immunol. 2009;183:5199–207. doi: 10.4049/jimmunol.0802695. [DOI] [PubMed] [Google Scholar]
- 98.Avrahami D, Gabizon R. Age-related alterations affect the susceptibility of mice to prion infection. Neurobiol Aging. 2011;32:2006–15. doi: 10.1016/j.neurobiolaging.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 99.Outram GW, Dickinson AG, Fraser H. Developmental maturation of susceptibility to scrapie in mice. Nature. 1973;241:536–7. doi: 10.1038/241536a0. [DOI] [PubMed] [Google Scholar]
- 100.Ierna MI, Farquhar CF, Outram GW, Bruce ME. Resistance of neonatal mice to scrapie is associated with inefficient infection of the immature spleen. J Virol. 2006;80:474–82. doi: 10.1128/JVI.80.1.474-482.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Llewelyn CA, Hewitt PE, Knight RSG, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–21. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 102.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364:527–9. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 103.Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet. 2006;368:2061–7. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 104.Health PA. vCJD abnormal protein found in a patient with haemophilia at post mortem. 2009.
- 105.Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: effect of route of inoculation on infectivity titres and dose-response curves. J Comp Pathol. 1978;88:39–47. doi: 10.1016/0021-9975(78)90059-2. [DOI] [PubMed] [Google Scholar]
- 106.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr., Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lötscher M, Recher M, Hunziker L, Klein MA. Immunologically induced, complement-dependent up-regulation of the prion protein in the mouse spleen: follicular dendritic cells versus capsule and trabeculae. J Immunol. 2003;170:6040–7. doi: 10.4049/jimmunol.170.12.6040. [DOI] [PubMed] [Google Scholar]
- 108.Castilla J, Saá P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–5. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 109.Ironside JW, Hilton DA, Ghani A, Johnston NJ, Conyers L, McCardle LM, et al. Retrospective study of prion-protein accumulation in tonsil and appendix tissues. Lancet. 2000;355:1693–4. doi: 10.1016/S0140-6736(00)02243-1. [DOI] [PubMed] [Google Scholar]
- 110.González L, Dagleish MP, Bellworthy SJ, Sisó S, Stack MJ, Chaplin MJ, et al. Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrP in their rectal mucosa. Vet Rec. 2006;158:325–31. doi: 10.1136/vr.158.10.325. [DOI] [PubMed] [Google Scholar]
- 111.Espenes A, Press CM, Landsverk T, Tranulis MA, Aleksandersen M, Gunnes G, et al. Detection of PrP(Sc) in rectal biopsy and necropsy samples from sheep with experimental scrapie. J Comp Pathol. 2006;134:115–25. doi: 10.1016/j.jcpa.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Wolfe LL, Spraker TR, González L, Dagleish MP, Sirochman TM, Brown JC, et al. PrPCWD in rectal lymphoid tissue of deer (Odocoileus spp.) J Gen Virol. 2007;88:2078–82. doi: 10.1099/vir.0.82342-0. [DOI] [PubMed] [Google Scholar]
- 113.Spraker TR, Gidlewski TL, Balachandran A, VerCauteren KC, Creekmore L, Munger RD. Detection of PrP(CWD) in postmortem rectal lymphoid tissues in Rocky Mountain elk (Cervus elaphus nelsoni) infected with chronic wasting disease. J Vet Diagn Invest. 2006;18:553–7. doi: 10.1177/104063870601800605. [DOI] [PubMed] [Google Scholar]
- 114.Béringue V, Herzog L, Jaumain E, Reine F, Sibille P, Le Dur A, et al. Facilitated cross-species transmission of prions in extraneural tissue. Science. 2012;335:472–5. doi: 10.1126/science.1215659. [DOI] [PubMed] [Google Scholar]
- 115.Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, et al. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–6. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 116.Sigurdson CJ, Heikenwalder M, Manco G, Barthel M, Schwarz P, Stecher B, et al. Bacterial colitis increases susceptibility to oral prion disease. J Infect Dis. 2009;199:243–52. doi: 10.1086/595791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Borghesi C, Taussig MJ, Nicoletti C. Rapid appearance of M cells after microbial challenge is restricted at the periphery of the follicle-associated epithelium of Peyer’s patch. Lab Invest. 1999;79:1393–401. [PubMed] [Google Scholar]
- 118.Meynell HM, Thomas NW, James PS, Holland J, Taussig MJ, Nicoletti C. Up-regulation of microsphere transport across the follicle-associated epithelium of Peyer’s patch by exposure to Streptococcus pneumoniae R36a. FASEB J. 1999;13:611–9. doi: 10.1096/fasebj.13.6.611. [DOI] [PubMed] [Google Scholar]
- 119.Kincaid AE, Bartz JC. The nasal cavity is a route for prion infection in hamsters. J Virol. 2007;81:4482–91. doi: 10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bessen RA, Martinka S, Kelly J, Gonzalez D. Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. J Virol. 2009;83:6435–45. doi: 10.1128/JVI.00018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Denkers ND, Seelig DM, Telling GC, Hoover EA. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J Gen Virol. 2010;91:1651–8. doi: 10.1099/vir.0.017335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bessen RA, Shearin H, Martinka S, Boharski R, Lowe D, Wilham JM, et al. Prion shedding from olfactory neurons into nasal secretions. PLoS Pathog. 2010;6:e1000837. doi: 10.1371/journal.ppat.1000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bessen RA, Wilham JM, Lowe D, Watschke CP, Shearin H, Martinka S, et al. Accelerated shedding of prions following damage to the olfactory epithelium. J Virol. 2012;86:1777–88. doi: 10.1128/JVI.06626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haybaeck J, Heikenwalder M, Klevenz B, Schwarz P, Margalith I, Bridel C, et al. Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathog. 2011;7:e1001257. doi: 10.1371/journal.ppat.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]