Abstract
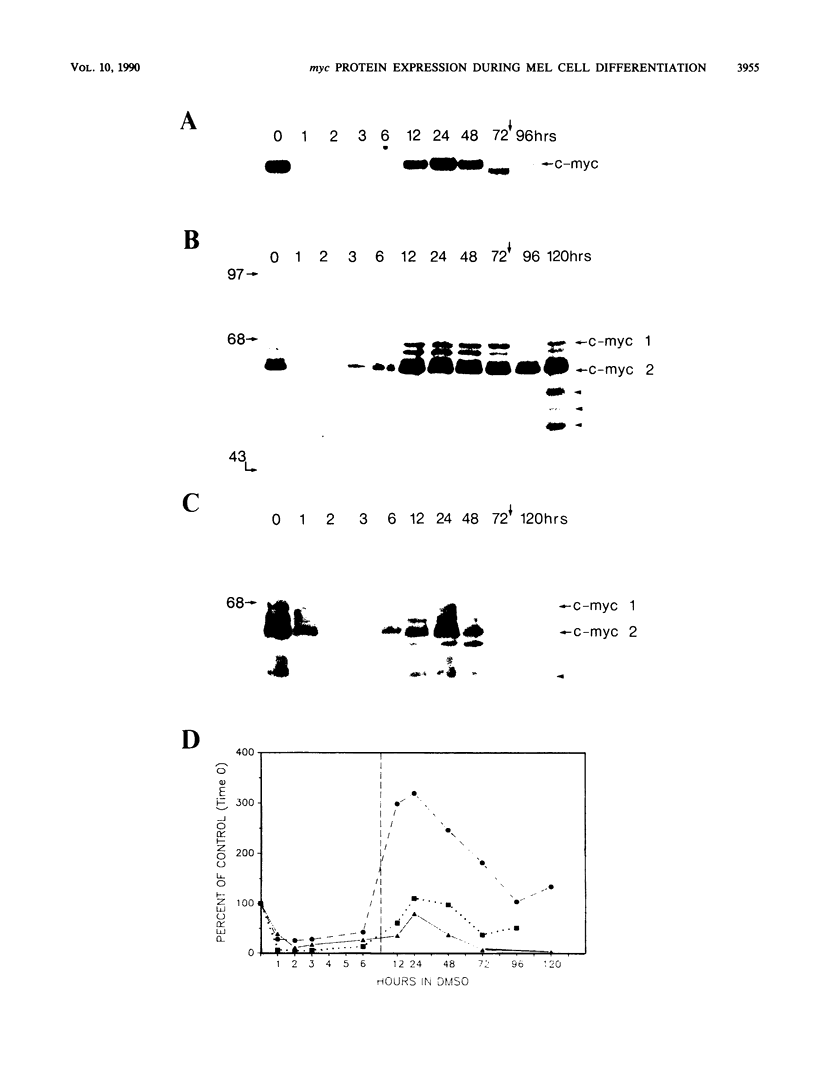
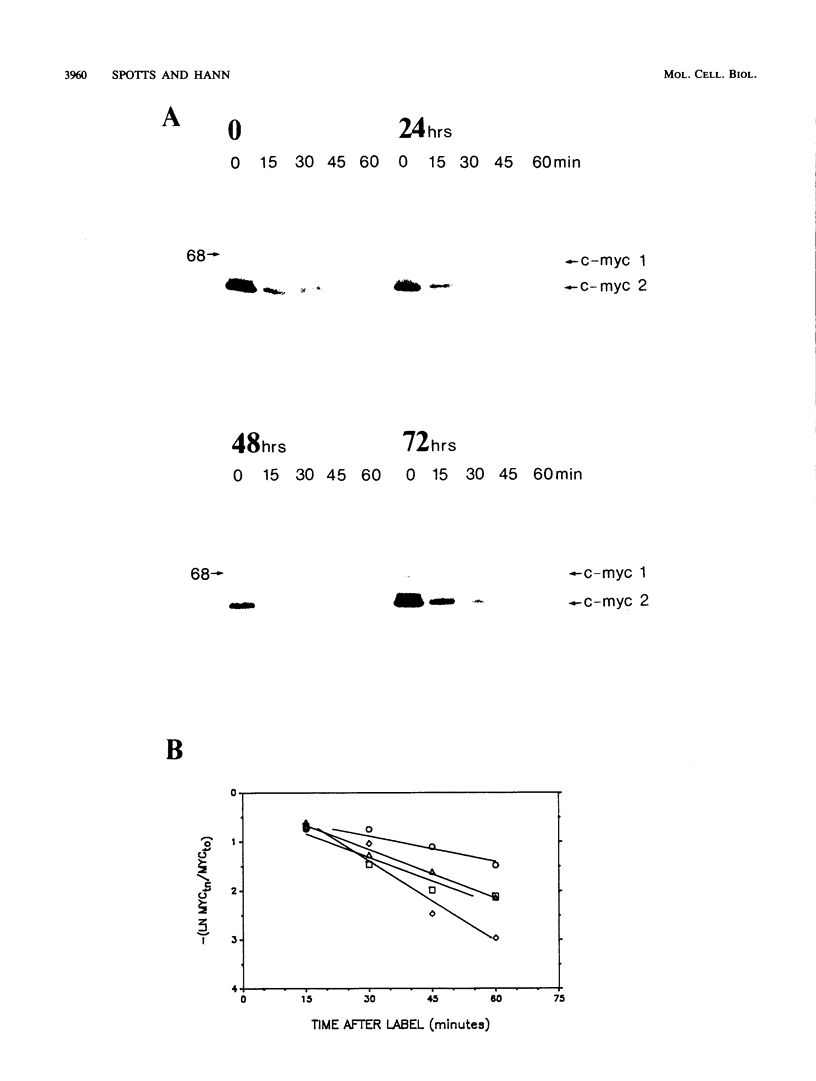
To determine whether regulation of c-myc proteins occurs during the differentiation of murine erythroleukemia cells, we examined c-myc protein synthesis and accumulation throughout dimethyl sulfoxide (DMSO)- or hypoxanthine-induced differentiation. c-myc protein expression exhibited an overall biphasic reduction, with an initial concomitant decrease in c-myc RNA, protein synthesis, and protein accumulation early during the commitment phase. However, as the mRNA and protein levels recovered, c-myc protein synthesis levels dissociated from the levels of c-myc mRNA and protein accumulation. This dissociation appears to result from unusual translational and posttranslational regulation during differentiation. Translational enhancement was suggested by the observation that relatively high levels of c-myc proteins were synthesized from relatively moderate levels of c-myc RNA. This translational enhancement was not observed with c-myb. Under certain culture conditions, we also observed a change in the relative synthesis ratio of the two independently initiated c-myc proteins. Posttranslational regulation was evidenced by a dramatic postcommitment decrease in the accumulated c-myc protein levels despite relatively high levels of c-myc protein synthesis. This decrease corresponded with a twofold increase in the turnover of c-myc proteins. The consequence of this regulation was that the most substantial decrease in c-myc protein accumulation occurred during the postcommitment phase of differentiation. This result supports the hypothesis that the reduction in c-myc at relatively late times is most important for completion of murine erythroleukemia cell terminal differentiation.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle W. J., Lipsick J. S., Baluda M. A. Antibodies to the evolutionarily conserved amino-terminal region of the v-myb-encoded protein detect the c-myb protein in widely divergent metazoan species. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4685–4689. doi: 10.1073/pnas.83.13.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Parker J. M., Schuler G. D., Cole M. D. Continued withdrawal from the cell cycle and regulation of cellular genes in mouse erythroleukemia cells blocked in differentiation by the c-myc oncogene. Mol Cell Biol. 1989 Apr;9(4):1714–1720. doi: 10.1128/mcb.9.4.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling D., Tavassoli M., Linskens M. H., Farzaneh F. DMSO induced modulation of c-myc steady-state RNA levels in a variety of different cell lines. Oncogene. 1989 Feb;4(2):175–179. [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Human alpha-globin gene expression following chromosomal dependent gene transfer into mouse erythroleukemia cells. Cell. 1978 Sep;15(1):55–63. doi: 10.1016/0092-8674(78)90082-x. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fibach E., Reuben R. C., Rifkind R. A., Marks P. A. Effect of hexamethylene bisacetamide on the commitment to differentiation of murine erythroleukemia cells. Cancer Res. 1977 Feb;37(2):440–444. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Abrams H. D., Rohrschneider L. R., Eisenman R. N. Proteins encoded by v-myc and c-myc oncogenes: identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell. 1983 Oct;34(3):789–798. doi: 10.1016/0092-8674(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Eisenman R. N. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984 Nov;4(11):2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Kirsch I. R., Bertness V., Silver J., Hollis G. F. Regulated expression of the c-myb and c-myc oncogenes during erythroid differentiation. J Cell Biochem. 1986;32(1):11–21. doi: 10.1002/jcb.240320103. [DOI] [PubMed] [Google Scholar]
- Kume T. U., Takada S., Obinata M. Probability that the commitment of murine erythroleukemia cell differentiation is determined by the c-myc level. J Mol Biol. 1988 Aug 20;202(4):779–786. doi: 10.1016/0022-2836(88)90558-x. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Hatton K. S., Skoultchi A. I., Schildkraut C. L. c-myc mRNA levels in the cell cycle change in mouse erythroleukemia cells following inducer treatment. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5323–5327. doi: 10.1073/pnas.82.16.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Ramsay R., Sheffery M., Rifkind R. A. Changes in gene expression during hexamethylene bisacetamide induced erythroleukemia differentiation. Prog Clin Biol Res. 1987;251:253–268. [PubMed] [Google Scholar]
- Marks P. A., Sheffery M., Rifkind R. A. Induction of transformed cells to terminal differentiation and the modulation of gene expression. Cancer Res. 1987 Feb 1;47(3):659–666. [PubMed] [Google Scholar]
- Mechti N., Piechaczyk M., Blanchard J. M., Marty L., Bonnieu A., Jeanteur P., Lebleu B. Transcriptional and post-transcriptional regulation of c-myc expression during the differentiation of murine erythroleukemia Friend cells. Nucleic Acids Res. 1986 Dec 22;14(24):9653–9666. doi: 10.1093/nar/14.24.9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murate T., Kaneda T., Rifkind R. A., Marks P. A. Inducer-mediated commitment of murine erythroleukemia cells to terminal cell division: the expression of commitment. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3394–3398. doi: 10.1073/pnas.81.11.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson M. A., Bush E. W., Vanderburg C. Transcriptional-translational regulation of muscle-specific protein synthesis and its relationship to chondrogenic stimuli. J Biol Chem. 1986 Jan 25;261(3):1477–1486. [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F. Growth-dependent synthesis of c-myc-encoded proteins: early stimulation by serum factors in synchronized mouse 3T3 cells. Mol Cell Biol. 1985 Nov;5(11):2903–2912. doi: 10.1128/mcb.5.11.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J. Deregulated expression of c-myc by murine erythroleukaemia cells prevents differentiation. 1986 Aug 28-Sep 3Nature. 322(6082):848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J., Rodgers C. c-myc antisense transcripts accelerate differentiation and inhibit G1 progression in murine erythroleukemia cells. Mol Cell Biol. 1988 Sep;8(9):3683–3695. doi: 10.1128/mcb.8.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. G., Ikeda K., Rifkind R. A., Marks P. A. Changes in gene expression associated with induced differentiation of erythroleukemia: protooncogenes, globin genes, and cell division. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6849–6853. doi: 10.1073/pnas.83.18.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V. M., Ramsay R. G., Rifkind R. A., Marks P. A. Modulation of the c-myb, c-myc and p53 mRNA and protein levels during induced murine erythroleukemia cell differentiation. Oncogene. 1989 Feb;4(2):165–173. [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J. Expression of the c-myb and c-myc genes is regulated independently in differentiating mouse erythroleukemia cells by common processes of premature transcription arrest and increased mRNA turnover. Mol Cell Biol. 1988 Sep;8(9):3938–3942. doi: 10.1128/mcb.8.9.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt R. A., Shatzman A. R., Rosenberg M. Expression and characterization of the human c-myc DNA-binding protein. Mol Cell Biol. 1985 Mar;5(3):448–456. doi: 10.1128/mcb.5.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove T. G., Watt R., Keng P., Macara I. G. Stabilization of myc proto-oncogene proteins during Friend murine erythroleukemia cell differentiation. J Biol Chem. 1988 Jun 25;263(18):8918–8924. [PubMed] [Google Scholar]