Abstract
Angiopoietin-like 4 (Angptl4) is a secreted protein modulating triacylglycerol homeostasis. Its transcription is induced by glucocorticoids, which act to elevate circulating Angptl4 levels during fasting. In investigating the role of Angptl4 in glucocorticoid action, we identified that in addition to its known ability to inhibit lipoprotein lipase, Angptl4 stimulates intracellular adipocyte lipolysis. Fatty acid release by murine adipocytes following fasting or treatment with glucocorticoids or catecholamines is highly Angptl4-dependent. In fact, Angptl4 can directly stimulate cAMP-dependent PKA signaling and lipolysis when added to adipocytes. Here, we detail this novel Angptl4-dependent lipolytic regulatory mechanism and discuss its physiological and therapeutic implications.
Keywords: Angptl4, adipocyte, cAMP, catecholamine, fasting, glucocorticoids, lipolysis
Mammals benefit from the ability to switch between fuel substrates to power the body’s energy needs in response to changing nutritional, environmental, and circadian states. Flexible substrate utilization allows for the provision of an even supply of energy to working tissues during the most adverse circumstances; for example the running of a marathon or the scaling of a high-altitude mountain peak. On the other hand, the failure to switch seamlessly between fuels can wreak havoc, as when runners hit “the wall” and collapse during endurance races.
This capacity is not reserved for extreme situations. Even during a simple overnight fast, coordinated fuel switching is taking place within the liver. Specifically, the liver must switch from using glycogen early in a fast to relying on fatty acids (FAs) mobilized from the triacylglycerol (TG) pool of white adipose tissue (WAT) as a fuel for everything from β-oxidation to ketone body synthesis when fasting is extended. An inability to steadily flux FAs from the WAT to the liver during prolonged fasting could therefore be life-threatening.
Despite its requirement for normal intermediary metabolism, conditions in which FA release by the WAT is excessive can also be deleterious. For example, in patients with obesity and insulin resistance, lipolysis by white adipocytes is no longer restricted to the fasted state, occurring even in the fed state when insulin normally suppresses it.1,2 The resulting increased flux of FA from the WAT to other tissues, including the liver, skeletal muscles, pancreas and other metabolic tissues, is associated with their dysfunction.1-3 This injurious process is termed lipotoxicity and has been implicated in the development of type 2 diabetes. Therefore, beyond providing a better understanding of normal physiology, efforts to determine new factors that stimulate and limit lipolysis may yield therapeutic targets relevant to diabetes.
Several factors are known to participate in regulating TG hydrolysis (intracellular lipolysis) in the WAT and the lipolytic release of stored fatty acids during fasting. These include sympathetic neurotransmitters, such as norepinephrine, and a slate of counter-regulatory hormones, including glucagon, thyroid hormone, growth hormone and glucocorticoids.4-7 Of these, glucocorticoids exert a profound effect on lipid partitioning between tissues and can produce extreme alterations in body fat distribution when chronically in excess, as in the Cushing syndrome.8,9 Glucocorticoids exert their complex effects by binding to intracellular glucocorticoid receptors (GRs) that controls the transcription of hundreds of genes across tissues. The normal diurnal variation in glucocorticoid levels, therefore, may be an important component dictating the daily fluctuation in gene transcription in several metabolic tissues. We have explored glucocorticoid-dependent transcription as a means to identify potential targets to limit WAT lipolysis and prevent lipotoxicity in obesity states.10
We initially identified angiopoietin-like 4 (Angptl4) as a glucocorticoid-regulated gene in several cell types, including A549 human lung epithelial cells, rat primary hepatocytes and human primary adipocytes.11,12 We further confirmed the induction of Angptl4 mRNA in mouse liver and WAT in response to the systemic administration of dexamethasone (DEX), a synthetic glucocorticoid.12 Angptl4 held promise as a glucocorticoid-dependent modulator of lipid flux for several reasons. First, hypertriglyceridemia is promoted both by glucocorticoid excess and in models where ANGPTL4 levels are increased.13-15 Second, Angptl4–/– mice display increased plasma TG clearance and decreased liver TG synthesis, two components of the phenotype seen when the ratio of active to inactive glucocorticoids is reduced by pharmacologically inhibiting 11β-hydroxysteroid dehydrogenase type I.16,17 Third, Angptl4 synthesis and secretion by the WAT and liver are profoundly induced by fasting [it is also called fasting-induced adipose factor (FIAF)],18,19 a condition also associated with elevated glucocorticoid levels.
In further investigating the transcriptional regulation of rat Angptl4 gene by glucocorticoids, we used chromatin immunoprecipitation along with a bioinformatics approach to identify a putative binding site for GR in the genomic region of Angptl4.12 DEX treatment markedly stimulated the activity of a luciferase reporter coupled to this GR binding region positioned in front of the TATA box in H4IIE rat hepatoma cells.12 This region was ultimately identified as a functional glucocorticoid-responsive element (GRE) by mutagenesis and gel shift experiments.12 Our work pinpointed that GR-control of Angptl4 transcription involves modulating DNase I accessibility and the levels of histone acetylation within the genomic region containing the GRE.12 We linked these effects to in vivo physiology by studying mice lacking Angptl4. These mice had reductions in DEX-induced hypertriglyceridemia and hepatic steatosis, indicating that Angptl4 is required for these effects.12 Despite our transcriptional characterization, we wondered how Angptl4 and glucocorticoids conspire to regulate lipid fluxes in vivo.
In exploring this question, our most recent work has focused on the role of Angptl4 during fasting. A net flux of FFAs out of the WAT can result when the rate at which adipocytes hydrolyze intracellular triacylglycerols (TGs) and release FFAs is greater than the rate at which they take up and esterify dietary fats. The uptake of dietary fats stored within circulating lipoproteins by adipocytes requires the action of lipoprotein lipase (Lpl) enzymes (extracellular lipolysis), whereas the mechanisms governing TG hydrolysis (intracellular lipolysis) by adipocytes are more complex. Although the involvement of glucocorticoid action in fasting-induced WAT lipolysis has been described,15,20 determining the extent to which glucocorticoids regulate intracellular adipocyte lipolysis and the mechanisms by which this occurs has been elusive. In considering how Angptl4 functions, it is intriguing to note that, in addition to inhibiting Lpl, Angptl4 also promotes the expression of WAT genes involved in TG hydrolysis and the lipolytic release of intracellular FFAs by adipocytes.21,22 Therefore, we thought that Angptl4 might modulate both extracellular and intracellular lipolysis.
Our studies reveal that, beyond inhibiting extracellular Lpl, Angptl4 also stimulates intracellular TG hydrolysis and FFA release by murine adipocytes during fasting in response to classic physiological cues. Angptl4–/– mice failed to appropriately release glycerol, a marker of intracellular lipolysis, in response to a physiological fast.23 Furthermore, we showed that glucocorticoid action is a direct determinant of the TG-hydrolytic potential of the WAT during fasting23 and that, interestingly, this action also requires Angptl4.23 In our experiments, TG hydrolysis by adipocytes in response to short-term fasting (6 h) and glucocorticoid treatment in vivo and catecholamine treatment in vitro was preceded by increases in cytosolic levels of cAMP.23 Angptl4 was necessary for each of these stimuli to elevate cAMP levels and to stimulate the PKA-dependent phosphorylation of key components of the lipolytic machinery.23 These findings combine to suggest that Angptl4 may regulate intracellular adipocyte lipolysis by modulating a common step in the cAMP-dependent signaling cascade. In exploring this possibility further, we found that purified human ANGPTL4, when added on its own to cultured murine adipocytes, remarkably increased intracellular cAMP levels and rescued the lipolytic impairment seen in Angptl4-deficient cells.23
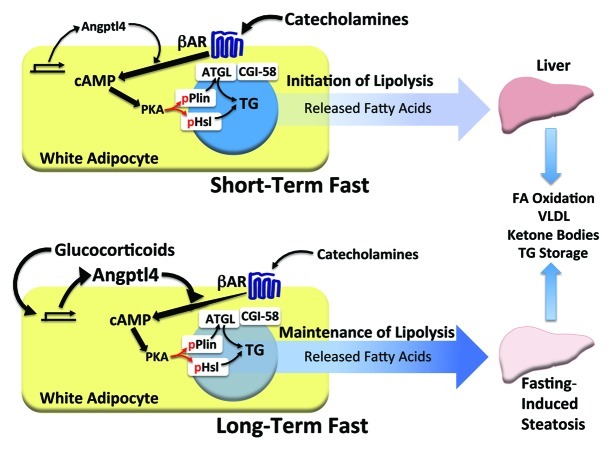
Our in vivo and in vitro findings allow for the construction of a more advanced model depicting the temporal contribution of several components of fasting-induced lipolysis and the role of Angptl4 in this process (Fig. 1). Early on during a fast, catecholamines and other counter-regulatory defenses act on the WAT early on to increase cAMP levels, leading to activation of PKA and phosphorylation of hormone-sensitive lipase (Hsl) and perilipin-1 (Plin1), two proteins that localize to lipid droplets and participate in lipolysis upon undergoing specific PKA-dependent phosphorylation.6 During this phase (modeled by fasting mice for 6 h), Angptl4 serves two roles: it inhibits Lpl to limit extracellular lipolysis and fat uptake by adipocytes and also potentiates the actions of catecholamines by enhancing their effect on cAMP-dependent TG hydrolysis. When fasting is extended (modeled by fasting mice for 24 h), glucocorticoids exert a greater effect on WAT TG hydrolysis by controlling the transcription of many genes, one of which is Angptl4. For both catecholamines and glucocorticoids, the ability to stimulate the release of stored TG by the WAT is linked to their capacity to increase adipocyte cAMP levels. For this, they require Angptl4. We have therefore identified Angptl4 as a common downstream mediator that integrates the acute lipolytic actions of glucocorticoids and catecholamines during physiological fasting in adipocytes.
Figure 1. The physiological role of Angptl4 in adipocyte lipolysis. Model depicting the concert of factors proposed to mediate the pace of adipocyte lipolysis during short-term (6 h) and extended (24 h) fasting. Early on during a fast (top), TG hydrolysis by adipocytes is largely under control of catecholamines, which act through β-adrenergic receptors (βAR) and stimulatory G-proteins to increase cAMP levels and activate PKA-dependent phosphorylation of key elements of the lipolytic machinery, including perilipin-1 (Plin) and hormone-sensitive lipase (Hsl). Phosphorylated Plin (pPlin), in turn, facilitates the interaction between adipocyte triglyceride lipase (ATGL) and CGI-58, which is associated with lipase activation. Activated ATGL and Hsl work together to release fatty acids from TG stores, which flux to the liver and are used for oxidation, VLDL synthesis, and ketone body production. Basal levels of Angptl4 facilitate catecholamine action during this phase of fasting. When fasting is prolonged (bottom), the influence of catecholamines wanes, giving way to the more dominant role of glucocorticoids. Glucocorticoids strongly induce Angptl4, the levels of which rise proportionally in the WAT. Angptl4 mediates glucocorticoid-dependent PKA signaling, acting to maintain ATGL and Hsl-dependent TG hydrolysis and the steady flux of fatty acids from the WAT to the liver. Angptl4 action in this setting is required for fasting-induced hepatic steatosis, a hallmark of prolonged fasting in mouse models.
The net flux of lipids between tissues in intermediary metabolism is complex, and involves a balance between rates of both lipogenesis and lipolysis within the WAT. As such, it is interesting to note that glucocorticoids can stimulate both lipolysis and lipogenesis.10 Although the overall effect of glucocorticoid action during a physiological fast may be to stimulate lipid flux from the WAT to the liver, this effect can be remarkably different in other states. For example the Cushing syndrome of chronic glucocorticoid excess is associated not with a uniform increase or decrease in the size of fat depots in the body, but rather with a redistribution of fat from the periphery to visceral and hepatic depots.8,9 This shift is due in part to depot-specific differences in glucocorticoid-responsive lipolysis and lipogenesis. Specifically, visceral depots increased lipogenesis and TG storage, allowing them to expand, whereas a more dominant effect of lipolysis in peripheral depots is associated with their atrophy. Determining what factors determine depot-specific differences in these two counteracting processes is of biomedical importance. With this in mind, it will be important to know whether differences in Angptl4-dependent lipid metabolism between visceral and peripheral adipocytes may contribute, at least in part, to the depot-specific effects of glucocorticoids.
Glucocorticoids do not regulate intermediary metabolism in isolation. Indeed, in addition to the several hormones controlling WAT lipolysis, others are anabolic during the fed state. Primary among these is insulin, which acts to stimulate fat storage, both in the WAT and liver. Insulin is known to increase the expression of Lpl and certain lipogenic genes, an effect mirroring that of glucocorticoids24,25 in some fat depots and in the liver during the fasted state. However, insulin also represses the expression of Angptl4,26,27 an effect reciprocal to that of glucocorticoids, which induce its expression. As such, Angptl4 expression levels are an integrated function of various hormonal inputs, and this integration may important in determining how Angptl4 levels rise and fall during the diurnal cycle of feeding and fasting. The influence of other hormones and signaling systems on the Angptl4-dependent component of glucocorticoid-regulated lipid metabolism is only beginning to be understood.
Our specific work has also brought several other key questions to light.
First, does Angptl4 modulate adipocyte lipolysis in a tissue-specific or systemic manner? The liver and the WAT are the two main tissues responsible for fasting-induced secretion of Angptl4. Given that the resulting rise in Angptl4 levels can be clearly measured in human and mouse blood, it is reasonable to assume that Angptl4 may exert endocrine effects. In this model, the liver could use Angptl4 as a hormone to modulate WAT lipolysis from a distance. On the other hand, adipocyte-derived Angptl4 could act in a local paracrine or autocrine manner within the WAT, modulating lipolytic activity in the secreting or adjacent cells. These two possibilities remain to be examined. Interestingly, Angptl4 is secreted both as a full-length and truncated protein. Whether one version of Angptl4 functions more as an endocrine hormone and the other as a tissue-specific paracrine modulator is a question worthy of future study. Furthermore, many other tissues, including the brown adipose tissue, skeletal muscle, lungs and mammary glands, also express Angptl4. It is unclear whether fasting increases the expression of Angptl4 in these tissues and how this Angptl4 contributes to lipolysis.
Second, what roles do other angiopoietin-like family members play in systemic lipid metabolism? The relationship between Angptl4 and fat metabolism is seen in humans as well as in mice. For example, a large population-based study showed that sequence variations in human ANGPTL4 are associated with reduced plasma TG levels,28 and another showed that ANGPTL4 levels in the WAT correlate with body weight in monozygotic twins.29 However, these studies have also revealed that genetic alterations in several other members of the angiopoietin-like family of proteins may also play key roles in systemic lipid metabolism. For example, nonsynonymous sequence variations in human ANGPTL3, ANGPTL4 and ANGPTL5 analyzed from a large study population were each associated with circulating TG levels in the lowest quartile, and many of these variant alleles produced proteins with a loss of function when expressed in cells.30 Therefore, ANGPTL3 and 5 may mirror ANGPTL4 and play similarly important roles in maintaining normal TG levels in mammals. Loss of function of ANGPTL3 can have lipid-lowering effects.31 Additionally, it is worth noting that ANGPTL5 expression is relatively high in human WAT.30 While ANGPTL3 is mainly expressed in the liver, it may still be able to affect adipocyte function through an endocrine mechanism. As such, the search for therapeutic targets to lower lipids levels should be extended to include members of the angiopoietin-like family other than just ANGPTL4.
Third, is the Angptl4 protein domain needed to inhibit extracellular lipolysis distinct from that needed to stimulate intracellular lipolysis? Among the known sequence variations in human angiopoietin-like family members, those that impair the ability of the protein to inhibit Lpl activity in vitro are located within regions coding for the N-terminal half of the protein.32 By contrast, none of the tested variants that altered the c-terminal portion of ANGPTL3 and 4 were shown to modify the Lpl-inhibitory capacity of the protein.30 Furthermore, purified N-terminus of ANGPTL4 is able to inhibit Lpl,33 and a single mutation at amino acid 40 of human ANGPTL4 (from glutamic acid to lysine, E40K) abolished its Lpl inhibitory activity (E40K).34 The domain or amino acids required for adipocyte lipolysis are unclear. Experimental dissociation of the Lpl inhibitory- and adipocyte lipolytic activities of ANGPTL4 would have intriguing metabolic and pharmacologic implications. Circulating ANGPTL4 is present in both full-length and truncated forms,18 and these two could have distinct effects on TG homeostasis. Moreover, ANGPTL4 mutants that promote adipocyte lipolysis without inhibiting Lpl activity could provide a potential therapeutic approach to reduce adiposity without raising plasma TG levels. On the other hand, mutants that specifically lack TG-hydrolytic activity could provide insight into how lipolysis may be limited in insulin resistant individuals, protecting against chronic lipotoxicity in non-adipose tissues.
Fourth, what is Angptl4 receptor and how does Angptl4 act to stimulate adipocyte TG hydrolysis? One of the most intriguing conclusions drawn from our studies to date is the concept that secreted Angptl4 can act on the extracellular aspect of adipocytes to modulate intracellular cAMP signaling and lipolysis. This conclusion directly invokes the presence of an Angptl4-responsive cell surface receptor on adipocytes. What is this receptor? As Angptl4 shares homology with the angiopoietins, which bind to the TIE family of receptor tyrosine kinases, one possibility for Angptl4 receptor is a member of the TIE family. However, the angiopoietin-like family members are notably different from the angiopoietins in that they do not have a TIE-binding domain. This makes it unlikely that these two otherwise similar families of secreted ligands share the same set of receptors.35 Rather, Angptl4 was shown to bind to fibronectin, vitronectin, integrin β1 and β5 in keratinocytes;36,37 however, it is unknown whether this receptor binding specificity plays a role in adipocyte biology.
We found that the inability of catecholamine treatment to stimulate TG hydrolysis or increase cAMP levels in Angptl4-deficient murine adipocytes could be rescued by adding either forskolin, 8 bromo-cAMP, or a phosphodiesterase inhibitor,23 indicating that the adrenergic signaling cascade leading to lipolysis requires the presence of Angptl4 at a point downstream of the β-adrenergic receptor but upstream of activated adenylate cyclase. The expression of GαS, Gβ, Gγ and distinct isoforms of β-adrenergic receptors and adenylyl cyclase were similar in WAT of wild-type and Angptl4−/− mice.23 However, it is possible that Angptl4 initiates signaling events that modulate the activity of these proteins. Angptl4 has been shown to regulate distinct signaling pathways in different cell types. In keratinocytes, ANGPTL4 has been shown to activate integrin-mediated signaling that includes the stimulation of focal adhesion kinase and other downstream signaling molecules, such as protein kinase C and the small GTPase Rac.36-38 Notably, Angptl4 in the hypothalamus has been shown to inhibit AMP-activated protein kinase (AMPK).39 AMPK action has been linked to adipocyte lipolysis.40,41 Angptl4 has also been shown to inhibit the MAP/ERK kinase pathway in endothelial cells.42 None of these signaling pathways has been examined for their role in the action of Angptl4 in adipocytes, and its potential effects on cAMP-dependent signaling requires investigation.
Determining the adipocyte receptor and downstream signaling processes for Angptl4 is definitely worthy of research, as it may greatly facilitate the testing of potential Angptl4-blocking approaches as another strategy to reduce the aberrant lipolysis and attendant lipotoxicity in metabolic tissues that is seen in insulin resistant individuals.
Acknowledgments
The work in the Wang laboratory is supported by NIHR01(DK83591). S.K.K. is supported by an NIH Career Development Grant (5K08DK080174).
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/20787
References
- 1.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–14. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Mariash CN. Thyroid hormone and the adipocyte. J Clin Endocrinol Metab. 2003;88:5603–4. doi: 10.1210/jc.2003-031800. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–72. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. 2009;23:1161–70. doi: 10.1210/me.2008-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pivonello R, De Leo M, Vitale P, Cozzolino A, Simeoli C, De Martino MC, et al. Pathophysiology of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):77–81. doi: 10.1159/000314319. [DOI] [PubMed] [Google Scholar]
- 9.Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology. 2010;92(Suppl 1):86–90. doi: 10.1159/000314213. [DOI] [PubMed] [Google Scholar]
- 10.Yu CY, Mayba O, Lee JV, Tran J, Harris C, Speed TP, et al. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS One. 2010;5:e15188. doi: 10.1371/journal.pone.0015188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq C, Yamamoto KR. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci U S A. 2004;101:15603–8. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker BR. Cortisol--cause and cure for metabolic syndrome? Diabet Med. 2006;23:1281–8. doi: 10.1111/j.1464-5491.2006.01998.x. [DOI] [PubMed] [Google Scholar]
- 14.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157:545–59. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197:189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- 16.Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A. 2007;104:11766–71. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–20. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 19.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–93. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JE, Peckett AJ, D’souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol. 2011;300:C198–209. doi: 10.1152/ajpcell.00045.2010. [DOI] [PubMed] [Google Scholar]
- 21.Sanderson LM, Degenhardt T, Koppen A, Kalkhoven E, Desvergne B, Müller M, et al. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol Cell Biol. 2009;29:6257–67. doi: 10.1128/MCB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattijssen F, Kersten S. Regulation of triglyceride metabolism by Angiopoietin-like proteins. Biochim Biophys Acta. 2012;1821:782–9. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Gray NE, Lam LN, Yang K, Zhou AY, Koliwad S, Wang JC. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. J Biol Chem. 2012;287:8444–56. doi: 10.1074/jbc.M111.294124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest. 1993;92:2191–8. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gathercole LL, Morgan SA, Bujalska IJ, Hauton D, Stewart PM, Tomlinson JW. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS One. 2011;6:e26223. doi: 10.1371/journal.pone.0026223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizutani N, Ozaki N, Seino Y, Fukami A, Sakamoto E, Fukuyama T, et al. Reduction of insulin signaling upregulates angiopoietin-like protein 4 through elevated free fatty acids in diabetic mice. Exp Clin Endocrinol Diabetes. 2012;120:139–44. doi: 10.1055/s-0031-1291258. [DOI] [PubMed] [Google Scholar]
- 27.Yamada T, Ozaki N, Kato Y, Miura Y, Oiso Y. Insulin downregulates angiopoietin-like protein 4 mRNA in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;347:1138–44. doi: 10.1016/j.bbrc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39:513–6. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robciuc MR, Naukkarinen J, Ortega-Alonso A, Tyynismaa H, Raivio T, Rissanen A, et al. Serum angiopoietin-like 4 protein levels and expression in adipose tissue are inversely correlated with obesity in monozygotic twins. J Lipid Res. 2011;52:1575–82. doi: 10.1194/jlr.P015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119:70–9. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–50. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 32.Yau MH, Wang Y, Lam KS, Zhang J, Wu D, Xu A. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J Biol Chem. 2009;284:11942–52. doi: 10.1074/jbc.M809802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge H, Yang G, Yu X, Pourbahrami T, Li C. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. J Lipid Res. 2004;45:2071–9. doi: 10.1194/jlr.M400138-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Yin W, Romeo S, Chang S, Grishin NV, Hobbs HH, Cohen JC. Genetic variation in ANGPTL4 provides insights into protein processing and function. J Biol Chem. 2009;284:13213–22. doi: 10.1074/jbc.M900553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346:603–10. doi: 10.1042/0264-6021:3460603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol. 2010;177:2791–803. doi: 10.2353/ajpath.2010.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–3009. doi: 10.1074/jbc.M110.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal M, Tan MJ, Huang RL, Goh YY, Wang XL, Tang MB, et al. Angiopoietin-like 4 regulates epidermal differentiation. PLoS One. 2011;6:e25377. doi: 10.1371/journal.pone.0025377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HK, Youn BS, Shin MS, Namkoong C, Park KH, Baik JH, et al. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59:2772–80. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaidhu MP, Fediuc S, Anthony NM, So M, Mirpourian M, Perry RL, et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50:704–15. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–48. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–40. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]