Abstract
Obesity is a global problem and effective drug therapy treatment is still unavailable. Obesity develops due to an imbalance between energy intake and energy expenditure (EE). Understanding what happens to EE in obesity may be the key to developing new treatments for obesity. If EE in obesity can be elevated, it could be a potential therapeutic target. We recently discovered that in baseline conditions obese mice have increased EE, in terms of thermogenesis. However, this increase in EE is not great enough to offset the elevated calorie intake that leads to the development of obesity. In obesity, the adipose derived hormone leptin is significantly elevated. This elevated leptin concentration appears to cause an increase in thermogenesis through increased sympathetic nerve activity (SNA) to brown adipose tissue deposits. The brain region of the dorsomedial hypothalamus (DMH) appears to be a key region that leptin activates in obesity to cause this increased thermogenesis. One unsettling finding is that the sympathetic nervous system (SNS) in obesity is elevated via leptin and it seems unlikely that SNA would be selectivity increased to only brown adipose tissue. Previously, it has been observed that leptin can increase SNA to numerous organs including the kidney. Furthermore, in obesity, SNA is increased in numerous organs. This leads to the critical question: is the leptin-mediated elevation of SNA and thermogenesis also chronically activating the kidney and contributing to the development of hypertension in obesity?
Keywords: dorsomedial hypothalamus, leptin, obesity, sympathetic nerve activity, thermogenesis
Thermogenesis in Obesity
Energy homeostasis is the balance between food intake and energy expenditure (EE). Obesity develops as a consequence of an imbalance between energy intake and output. The prevalence of obesity continues to increase in the majority of countries examined.1 Not one new drug therapy with the capacity to treat obesity has been approved by the FDA in the past 10 years and surgical intervention still remains an expensive and unsustainable method to remove excess body fat for the majority of sufferers. This leaves a gap as to how to treat the millions of people that are currently obese. One of the key problems associated with obesity is that it increases the risk of metabolic disorders, including type II diabetes, cardiovascular diseases and dyslipidemia—diseases that greatly increase the risk of mortality.2,3
Reducing calorie intake in the short-term does decrease body weight. However, in the longer term, caloric restriction leads to limited success as the body enters a state of “starvation,” where weight loss plateaus and the limited intake of calories the body does receive is stored. Research is now increasingly focused on determining how the body expends energy through adaptive thermogenesis and the production of heat. Gaining an understanding of this pathway could allow for its manipulation in obesity by enabling more energy to be expended and by counteracting increased energy intake. The other benefit of understanding how the body expends energy is that the same mechanisms regulating thermogenesis in obesity possibly contribute to the development of metabolic syndrome, especially hypertension and even insulin resistance through changes in the activity of the SNS. Thermogenesis is increased in obese animals as well as when animals are exposed to cold.4,5 In both situations, thermogenesis is increased by increased SNS input to brown adipose tissue (BAT). Pioneer works, by either surgical or chemical denervation, have demonstrated the crucial importance of the SNS for the activation of BAT.6,7 Also, mice that lack the ability to produce catecholamines (the products of increased SNA) lack the ability to elevate thermogenesis in response to cold, highlighting the importance of this system in mediating messages from the brain to initiate thermogenesis.8 BAT cells are also crucially important to enable thermogenesis to take place as these cells contain uncoupling protein 1 (UCP1) and this protein has the capacity to convert the energy dense substrates into heat rather than being converted into ATP energy.9,10 For animals to effectively expend energy through thermogenesis, the central nervous system increases SNA causing increased catecholamines (noradrenaline) release, this leads to increased activation of β-3 adrenergic receptors (β-3R) on BAT cells. Activation of β-3R causes activation of G-coupled cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA). This results in increased lipolysis of triglyceride droplets, thereby increasing the levels of intracellular free fatty acids. The elevated levels of free fatty acids in turn activate UCP1 and initiate heat production.8,10 UCP1 enables the proton produced by the mitochondrial reaction to be released as heat rather than using the proton gradient to produce ATP. With therapeutic intervention, the hope in obesity research is that if more stored energy can be converted into heat a new therapy to help combat excess body fat may be developed.
Since 2007, it is acknowledged that humans, like rodents, express BAT deposits/cells in adulthood.11-14 Via positron emission tomography (PET) scanning, brown adipose tissue depots were identified in humans and appear to be particularly concentrated in the neck, supraclavicular area, para-aortic, para-vertebral and suprarenal areas.11-13 The protein UCP1 is increased at baseline conditions in obese animals as well as when animals, including humans, are exposed to cold conditions, suggesting protein activity and increased thermogenesis in these conditions.4,9,15 Interestingly, UCP1 expression and thermogenesis are only elevated in obese mice that are hyperleptinemic, but not in adipose tissue mass matched obese mice that are genetically leptin-deficient (ob/ob mice) or leptin receptor-deficient (db/db) mice, suggesting a role of leptin in increasing thermogenesis in obesity.8,16,17
Selective Leptin Resistance could be the Link between Adiposity and Elevated Sympathetic Outflow
Leptin, a cytokine discovered in 1994, is secreted from adipocytes in proportion to the mass of adipose tissue an animal accumulates.18-20 Since its discovery, and the recognition that leptin is the inhibitory signal from fat informing the brain of the body’s stocks of stored energy, leptin has been extensively studied regarding its ability to produce anorexigenic actions. Leptin decreases food intake and this is particularly dependent on the depolarization and hyperpolarization of neurons in the arcuate nucleus of the hypothalamus (ARH).21,22 Leptin resistance develops in obesity, a condition in which the ARH neurons expressing leptin receptors do not become further activated from baseline in response to exogenous leptin, and therefore the elevated leptin levels do not decrease food intake or increase energy expenditure.23,24 How leptin resistance develops in obesity and how it could be corrected is now under substantial research. Recently, we directly measured thermogenesis from tissue expressing BAT cells in obese leptin-resistant mice (diet induced obese mice, DIO) and discovered that despite the leptin resistance, these mice still experience increased thermogenesis in response to leptin.4,23 DIO mice have an elevated baseline BAT temperature compared with lean mice, and ob/ob mice have an even lower BAT temperature at baseline compared with lean mice, confirming previous research that leptin appears to have a role at baseline in controlling sympathetic outflow to BAT and regulating the body’s levels of thermogenesis.
We discovered that leptin elevated thermogenesis by actions in the brain because intracerebroventricular injection, like peripheral injections of leptin, elevated thermogenesis. The DMH has been recognized for a number of years to influence sympathetic activity to organs, including BAT. The role of leptin in this region, however, has not been thoroughly studied.25,26 The DMH region appears to be at least one region in which leptin mediates its ability to increase thermogenesis, even in obesity and despite leptin resistance developing in ARH.4 A large concentration of leptin receptors is present in the DMH, although the exact chemical characteristics of these neurons expressing leptin receptors are still not clearly understood.27,28 Leptin acts via the DMH, increasing SNA, and we have demonstrated that blocking β-3 receptors abolishes the increased thermogenetic response in DIO mice.4 The DMH contains no direct projections to the premotor sympathetic neurons and therefore the DMH neurons that respond to leptin must be innervating premotor neurons in other areas of the brain. These areas potentially include the paraventricular nucleus (PVN), an area previously demonstrated to receive direct innervations from leptin receptor-expressing neurons in the DMH.29,30 Other sympathetic premotor areas, including the rostral ventrolateral medulla and the raphe pallidus, receive projections from the DMH that influence the sympathetic outflow to a number of organs. Whether these are leptin receptor-expressing neurons is currently unknown.31-33
Our data suggests that the elevated leptin levels in obesity activate the leptin receptors in the DMH, increasing sympathetic outflow to BAT, increasing the activity of UCP1 and increasing thermogenesis. Physiologically this could be a potential way that the body tries to balance energy homeostasis in obesity, but obviously this fails and energy intake still exceeds energy expenditure.
Recently, a paper by Chao et al. identified a key role for neuropeptide Y (NPY) neurons in the DMH for mediating thermogenetic actions.34 The expression of NPY neurons in the DMH is elevated in models of obesity, so these neurons may indeed play a role in mediating the increased thermogenic response in obesity.35,36 However, very low concentrations of leptin receptors have been reported in NPY neurons of the DMH in young animals, whether this level changes in obesity deserves to be examined.37 For the leptin-mediated increase in thermogenesis to be targeted and manipulated in obesity, an understanding of the leptin receptor-expressing neurons in the DMH is needed in order to exacerbate thermogenesis and rebalance energy homeostasis. An understanding of the neuronal circuitry leading from the DMH to the increased sympathetic nerve outflow to BAT cells would also have to be established. This is critical as the leptin-mediated increase in SNA and thermogenesis in BAT, is unlikely to be selectively elevating SNA solely to BAT in obesity.
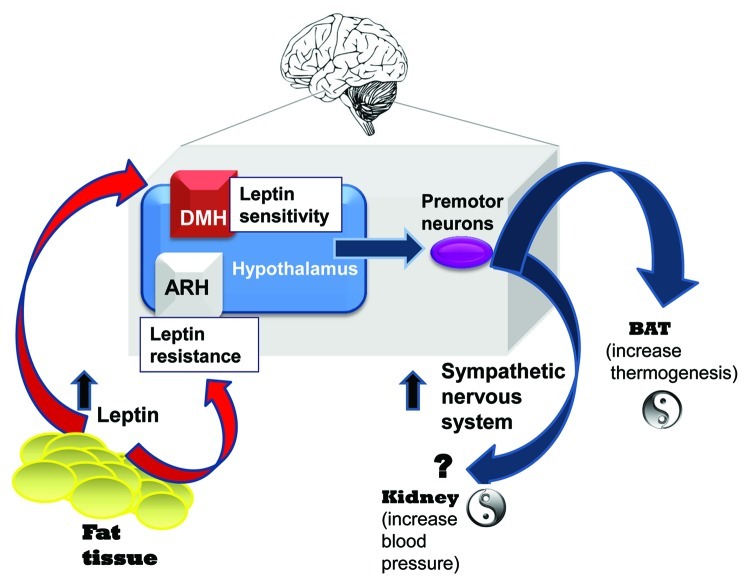
In obesity (Fig. 1), SNA is increased.38-40 Controversy remains as to whether this elevation leads to innervation of all organs throughout the body or whether SNA elevation in obesity is organ specific. Leptin has previously been demonstrated to have the ability to increase SNA in anaesthetised rats in a dose-dependent manner not only to BAT, but also to the adrenal, hind limb and kidney regions.41 Overall, it is generally recognized that SNA is increased in obesity and, as we recently demonstrated, in BAT. A concerning finding, as it is now readily hypothesized, is that increasing SNA chronically to the kidney contributes to the development of hypertension. Hypertension is one of the metabolic diseases that often develops in obesity and it significantly contributes to the development of cardiovascular diseases, the number one cause of death globally. Therefore, future research in this area is warranted. Studies have established that leptin can acutely elevate SNA.42,43 Nevertheless, no conclusive results demonstrating that leptin chronically elevates SNA, leading to hypertension, have yet been shown.
Figure 1. Potential “yin-yang” effect of hyperleptinemia on thermogenesis and blood pressure. As humans become obese, leptin levels increase due to adipose tissue mass accumulation. However, this hyperleptinemia fails to cause weight loss because resistance develops, restricting leptin’s ability to produce anorectic actions. Several studies demonstrate that neurons in the ARH become resistant to leptin. Our published data provides strong evidence that leptin retains the ability to activate leptin receptor-expressing neurons in the DMH and increase SNA in obese mice. These results raise the possibility that the increased sympathetic outflow in obesity occurs to tissues other than just BAT, such as the kidney involved in the control of blood pressure. Selective leptin resistance could be a crucial mechanism linking adiposity and elevated sympathetic outflow.
Overactivity of SNS is a common feature of obesity in humans. The increased SNA in obesity also appears to cause organ damage, which exacerbates the risk of cardiovascular disease and metabolic syndrome.44 Research has already established that acute microinjection of leptin into the DMH of anesthetized rats elevates heart rate and blood pressure.45 Determining if hyperleptinemia is the cause of chronically elevated SNA in obesity, via activation of leptin receptors in higher brain regions, will potentially generate new therapeutics and improves the care of patients with metabolic syndrome. Moreover, it may even provide a new alternative treatment for obesity itself.
Our study shows that leptin retains the ability through actions in the DMH to increase thermogenesis via the SNS, and in obesity, provides an exciting prospect for new therapies. Indeed, thermogenesis could be therapeutically manipulated to counteract increased caloric intake and restore energy homeostasis. Nevertheless, it should be viewed with caution as the same system and pathways mediating a leptin evoked increase in thermogenesis in obesity may also be adversely elevating the risk of hypertension and the ultimate development of cardiovascular diseases.
Acknowledgments
We thank Dr Daphne Vogiagis for her kind help in editing the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/20690
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The continuing epidemic of obesity in the United States. JAMA. 2000;284:1650–1. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–97. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–60. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton JM, Bartness TJ, Wade GN. Effects of norepinephrine and denervation on brown adipose tissue in Syrian hamsters. Am J Physiol. 1989;257:R396–404. doi: 10.1152/ajpregu.1989.257.2.R396. [DOI] [PubMed] [Google Scholar]
- 7.Himms-Hagen J, Cui J, Lynn Sigurdson S. Sympathetic and sensory nerves in control of growth of brown adipose tissue: Effects of denervation and of capsaicin. Neurochem Int. 1990;17:271–9. doi: 10.1016/0197-0186(90)90149-N. [DOI] [PubMed] [Google Scholar]
- 8.Commins SP, Marsh DJ, Thomas SA, Watson PM, Padgett MA, Palmiter R, et al. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140:4772–8. doi: 10.1210/en.140.10.4772. [DOI] [PubMed] [Google Scholar]
- 9.Bouillaud F, Ricquier D, Mory G, Thibault J. Increased level of mRNA for the uncoupling protein in brown adipose tissue of rats during thermogenesis induced by cold exposure or norepinephrine infusion. J Biol Chem. 1984;259:11583–6. [PubMed] [Google Scholar]
- 10.Fan W, Voss-Andreae A, Cao WH, Morrison SF. Regulation of thermogenesis by the central melanocortin system. Peptides. 2005;26:1800–13. doi: 10.1016/j.peptides.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 12.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 14.Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46:339–45. doi: 10.1007/BF00422121. [DOI] [PubMed] [Google Scholar]
- 15.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci. 2010;1212:E20–36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 16.Trayhurn P, James P. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse Pflügers Archiv European. J Physiol. 1978;373:189–93. doi: 10.1007/BF00584859. [DOI] [PubMed] [Google Scholar]
- 17.Davis TR, Mayer J. Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am J Physiol. 1954;177:222–6. doi: 10.1152/ajplegacy.1954.177.2.222. [DOI] [PubMed] [Google Scholar]
- 18.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 19.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 21.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–5. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 22.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 23.Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–94. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–9. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–84. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Münzberg H. Differential leptin access into the brain--a hierarchical organization of hypothalamic leptin target sites? Physiol Behav. 2008;94:664–9. doi: 10.1016/j.physbeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci U S A. 1998;95:741–6. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautron L, Lazarus M, Scott MM, Saper CB, Elmquist JK. Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach. J Comp Neurol. 2010;518:2090–108. doi: 10.1002/cne.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol. 2004;287:R824–32. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- 32.Horiuchi J, McDowall LM, Dampney RA. Differential control of cardiac and sympathetic vasomotor activity from the dorsomedial hypothalamus. Clin Exp Pharmacol Physiol. 2006;33:1265–8. doi: 10.1111/j.1440-1681.2006.04522.x. [DOI] [PubMed] [Google Scholar]
- 33.Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280:H2891–901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- 34.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–83. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11:630–7. doi: 10.1210/me.11.5.630. [DOI] [PubMed] [Google Scholar]
- 36.Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998;9:3415–9. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- 37.Draper S, Kirigiti M, Glavas M, Grayson B, Jiang B, Smith MS, et al. Microarray analysis of gene expression in Neuropeptide Y expressing neurons of the Dorsomedial nucleus of the Hypothalamus. Appetite. 2009;52:828. doi: 10.1016/j.appet.2009.04.058. [abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–9. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 39.Landsberg L, Young JB. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978;298:1295–301. doi: 10.1056/NEJM197806082982306. [DOI] [PubMed] [Google Scholar]
- 40.Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–40. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 41.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galletti F, D’Elia L, Barba G, Siani A, Cappuccio FP, Farinaro E, et al. High-circulating leptin levels are associated with greater risk of hypertension in men independently of body mass and insulin resistance: results of an eight-year follow-up study. J Clin Endocrinol Metab. 2008;93:3922–6. doi: 10.1210/jc.2008-1280. [DOI] [PubMed] [Google Scholar]
- 43.Eikelis N, Schlaich M, Aggarwal A, Kaye D, Esler M. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–9. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 44.Lambert E, Sari CI, Dawood T, Nguyen J, McGrane M, Eikelis N, et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension. 2010;56:351–8. doi: 10.1161/HYPERTENSIONAHA.110.155663. [DOI] [PubMed] [Google Scholar]
- 45.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–93. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]