Abstract
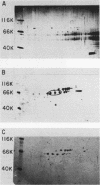
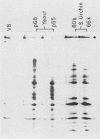
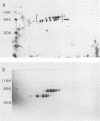
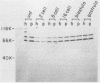
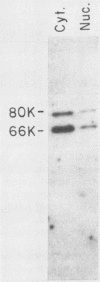
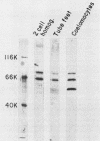
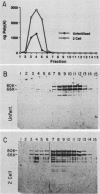
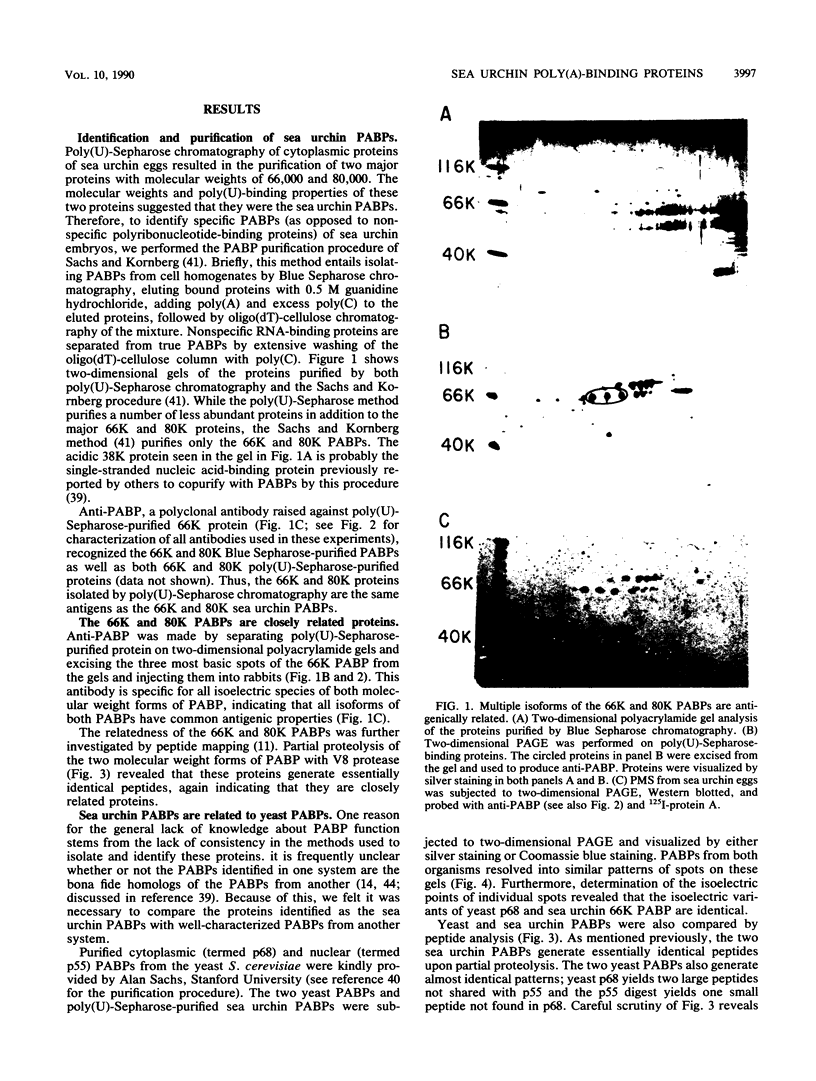
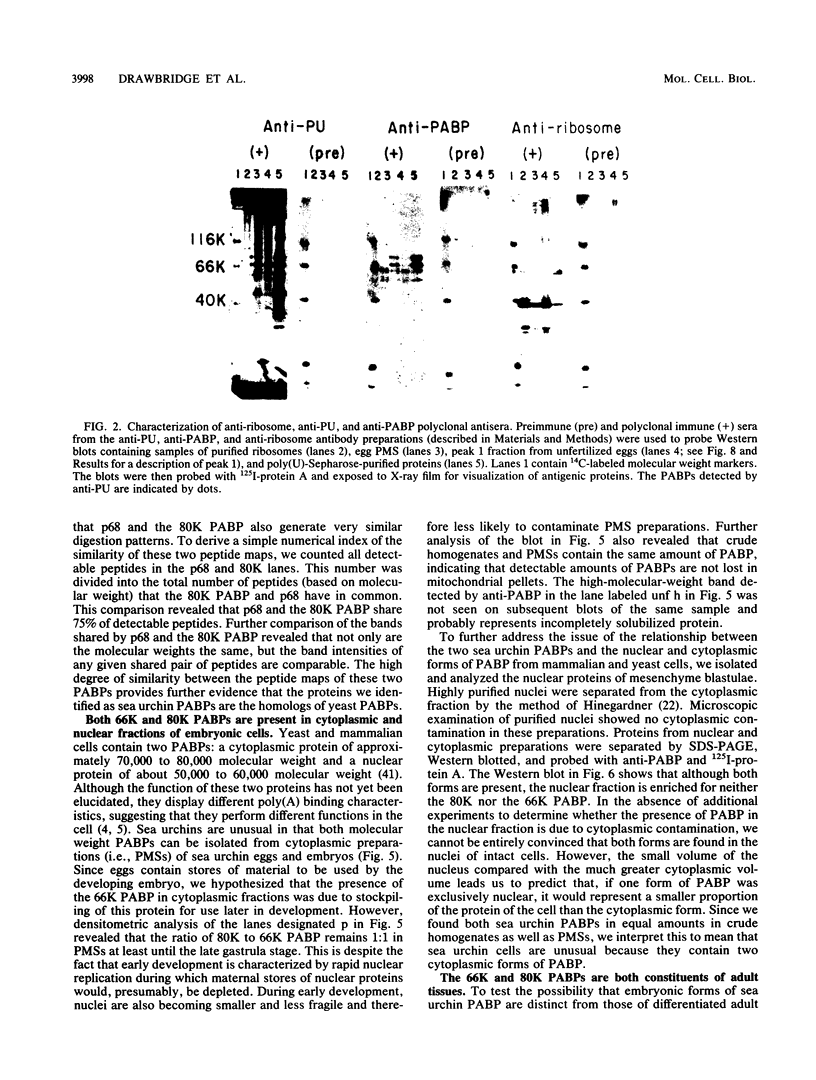
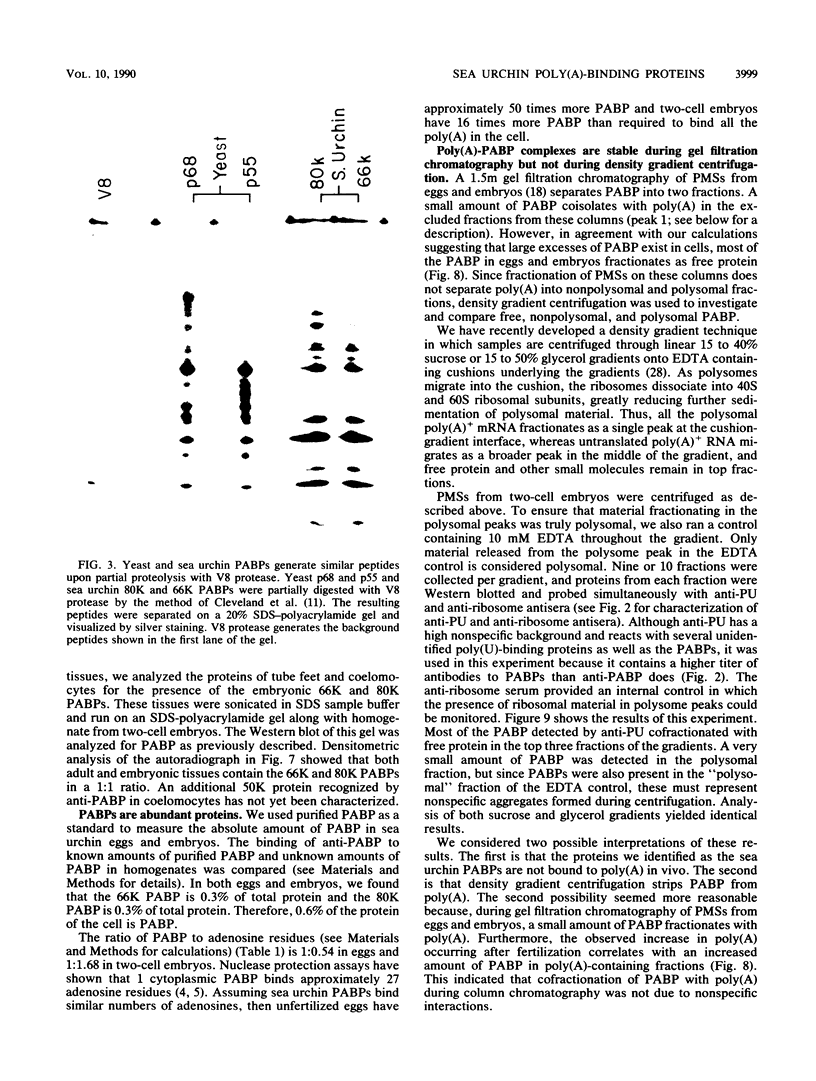
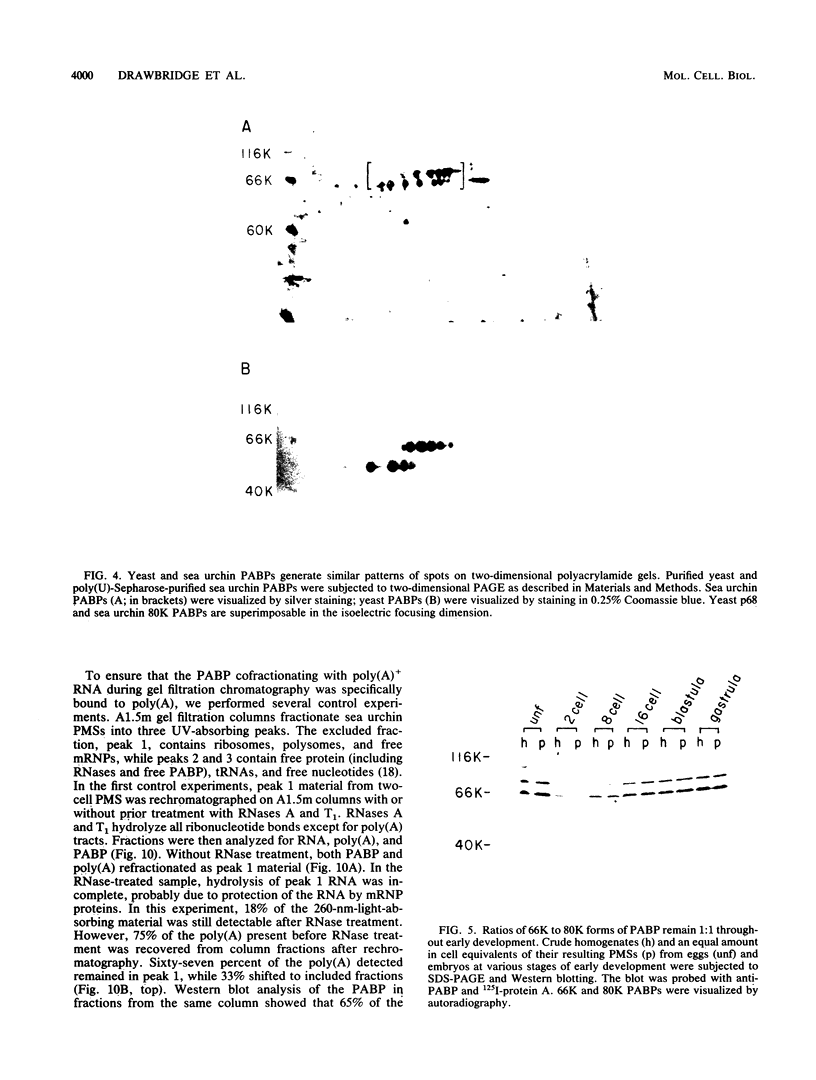
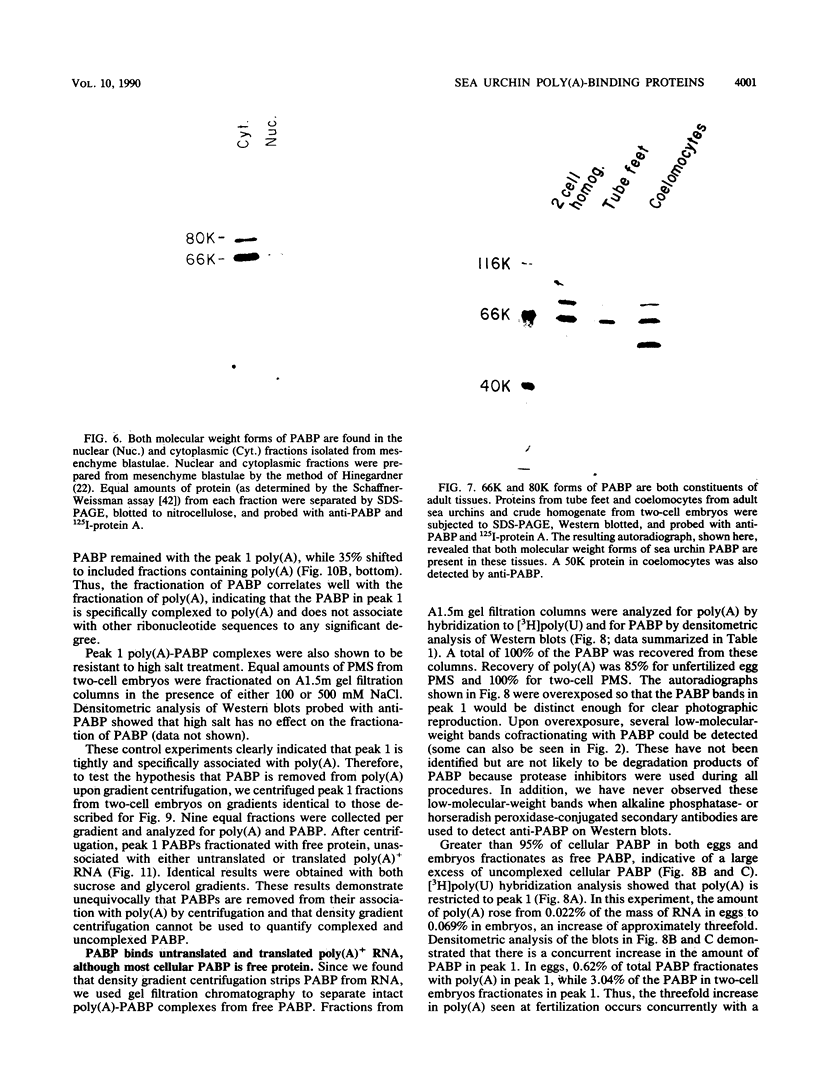
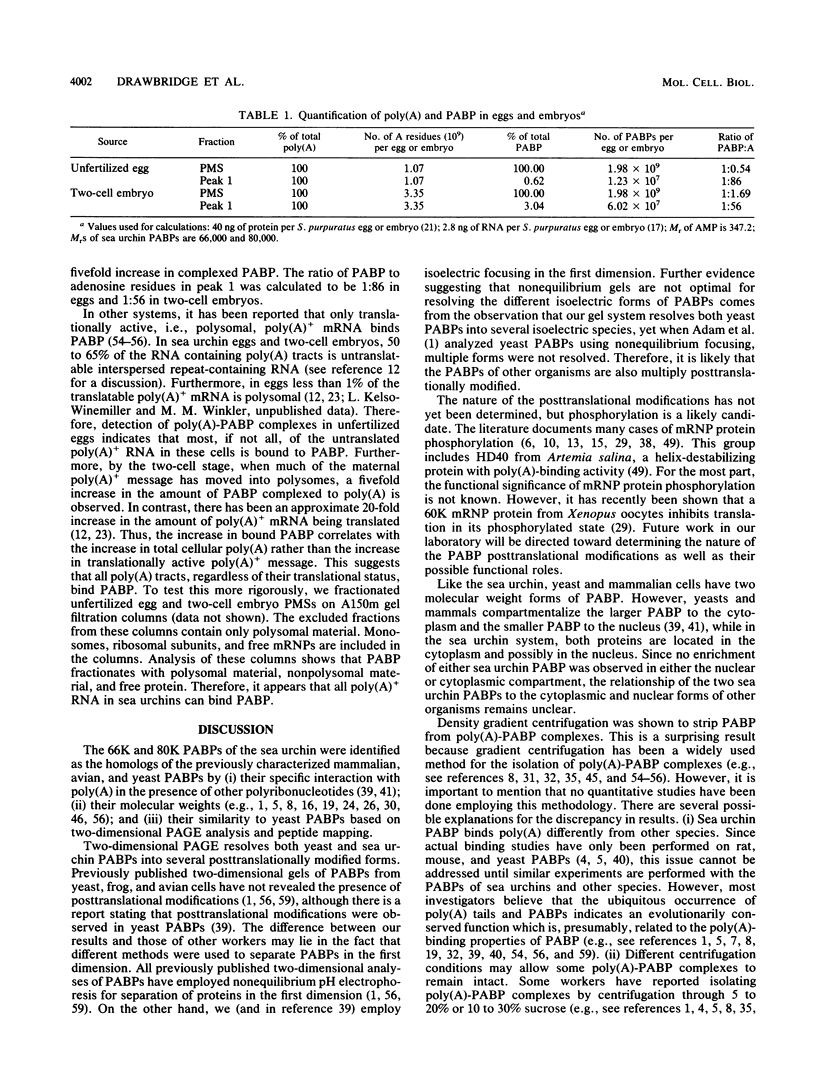
Poly(A)-binding proteins (PABPs) are the best characterized messenger RNA-binding proteins of eucaryotic cells and have been identified in diverse organisms such as mammals and yeasts. The in vitro poly(A)-binding properties of these proteins have been studied intensively; however, little is known about their function in cells. In this report, we show that sea urchin eggs have two molecular weight forms of PABP (molecular weights of 66,000 and 80,000). Each of these has at least five posttranslationally modified forms. Both sea urchin PABPs are found in approximately 1:1 ratios in both cytoplasmic and nuclear fractions of embryonic cells. Quantification in eggs and embryos revealed that sea urchin PABPs are surprisingly abundant, composing about 0.6% of total cellular protein. This is 50 times more than required to bind all the poly(A) in the egg based on the binding stoichiometry of 1 PABP per 27 adenosine residues. We found that density gradient centrifugation strips PABP from poly(A) and therefore underestimates the amount of PABP complexed to poly(A)+ RNA in cell homogenates. However, large-pore gel filtration chromatography could be used to separate intact poly(A)-PABP complexes from free PABP. Using the gel filtration method, we found that the threefold increase in poly(A) content of the egg after fertilization is paralleled by an approximate fivefold increase in the amount of bound PABP. Furthermore, both translated and nontranslated poly(A)+ RNAs appear to be complexed to PABP. As expected from the observation that PABPs are so abundant, greater than 95% of the PABP of the cell is uncomplexed protein.
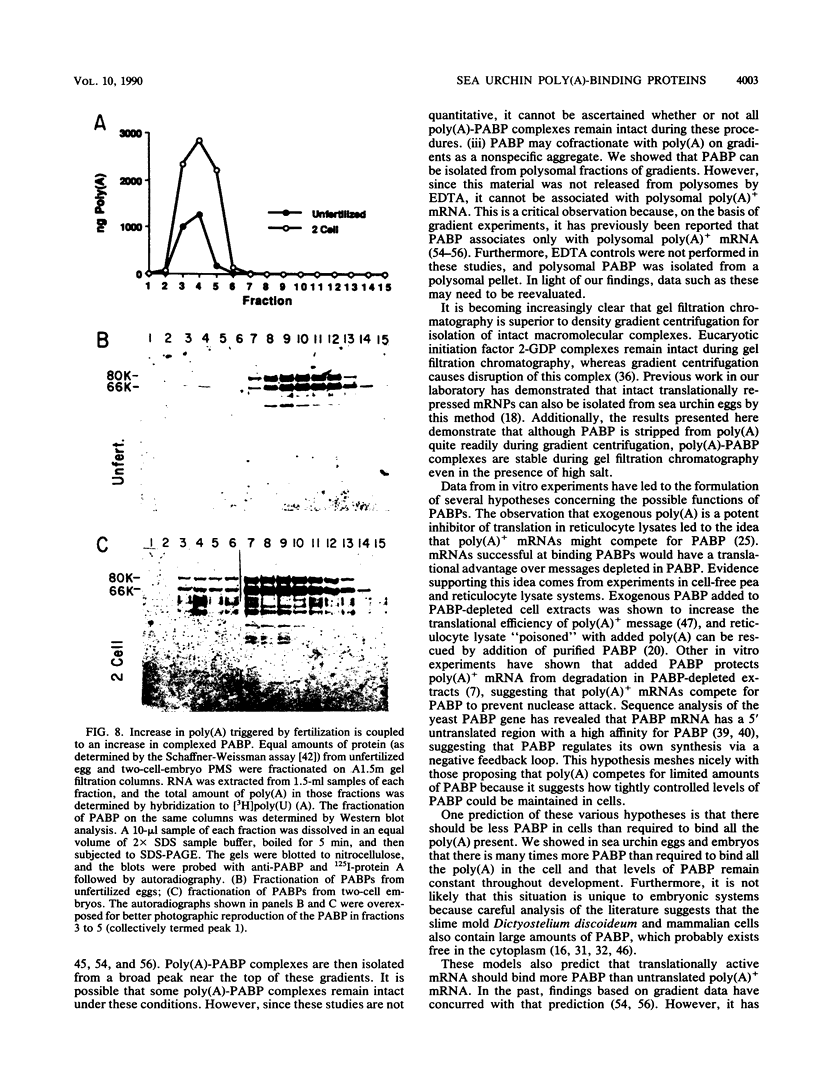
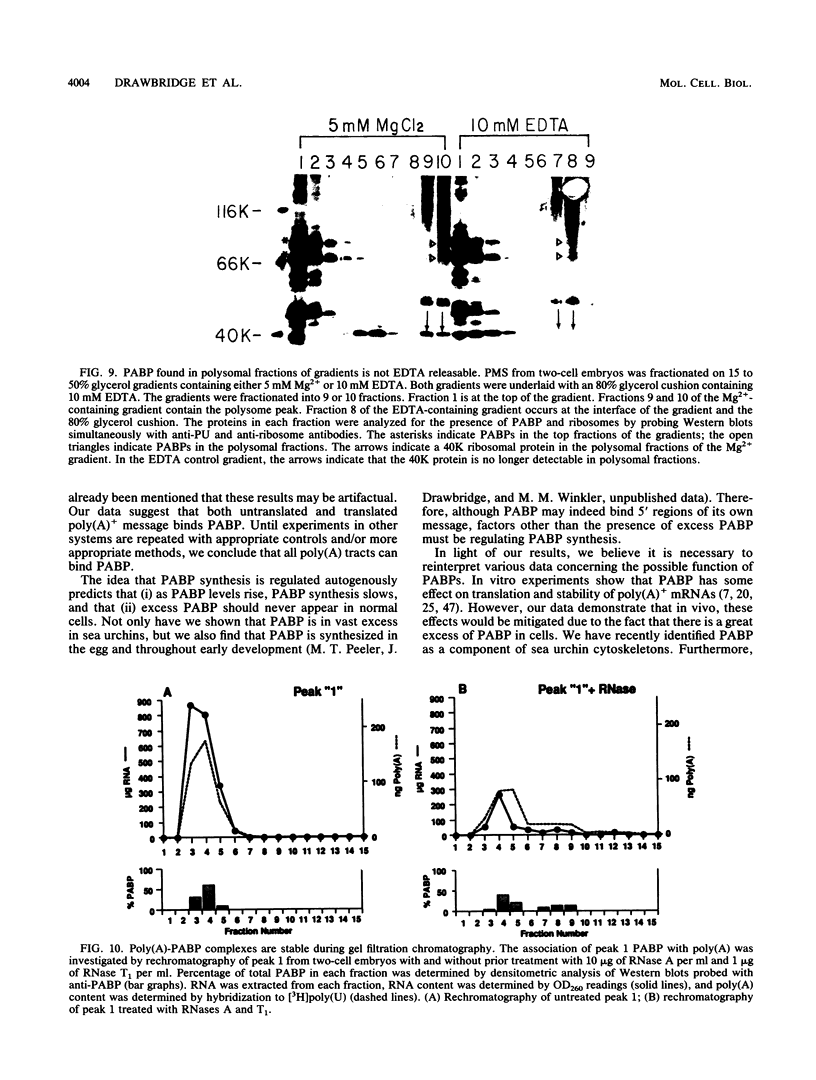
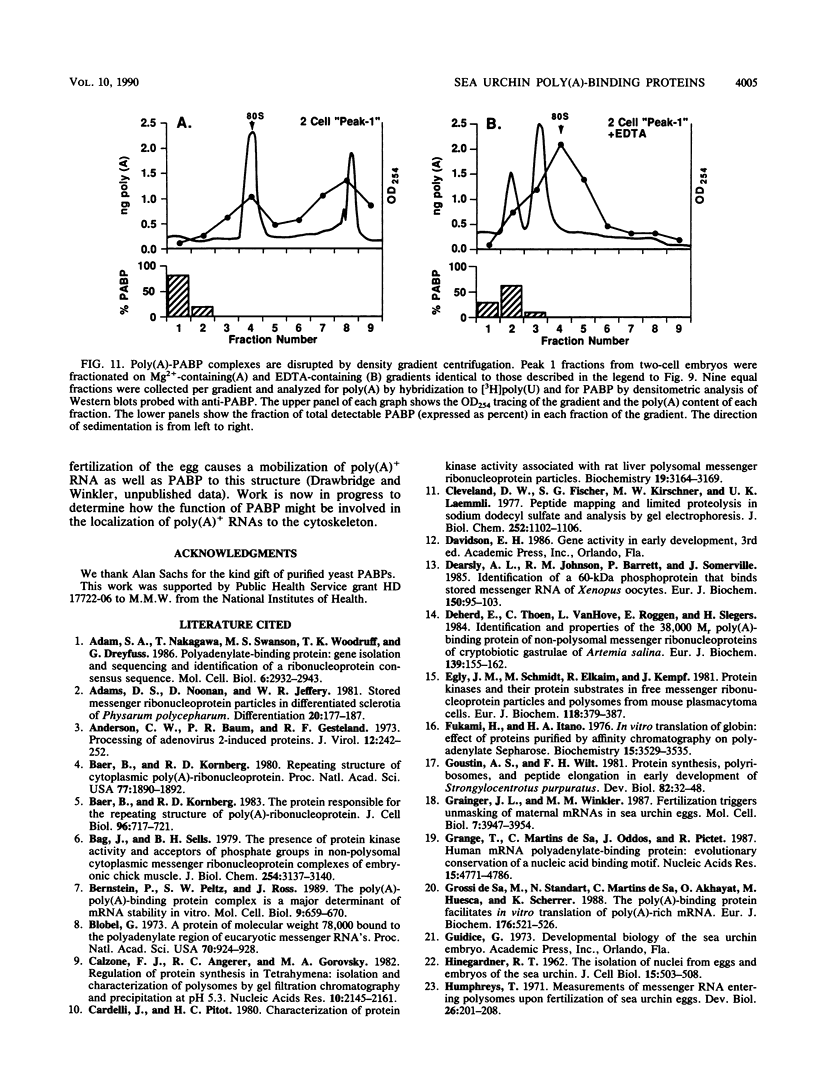
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Nakagawa T., Swanson M. S., Woodruff T. K., Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986 Aug;6(8):2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. S., Noonan D., Jeffery W. R. Stored messenger ribonucleoprotein particles in differentiated sclerotia of Physarum polycephalum. Differentiation. 1981;20(3):177–187. doi: 10.1111/j.1432-0436.1981.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B. W., Kornberg R. D. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1890–1892. doi: 10.1073/pnas.77.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B. W., Kornberg R. D. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983 Mar;96(3):717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag J., Sells B. H. The presence of protein kinase activity and acceptors of phosphate groups in nonpolysomal cytoplasmic messenger ribonucleoprotein complexes of embryonic chicken muscle. J Biol Chem. 1979 May 10;254(9):3137–3140. [PubMed] [Google Scholar]
- Bernstein P., Peltz S. W., Ross J. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol. 1989 Feb;9(2):659–670. doi: 10.1128/mcb.9.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F. J., Angerer R. C., Gorovsky M. A. Regulation of protein synthesis in Tetrahymena: isolation and characterization of polysomes by gel filtration and precipitation at pH 5.3. Nucleic Acids Res. 1982 Mar 25;10(6):2145–2161. doi: 10.1093/nar/10.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J., Pitot H. C. Characterization of protein kinase activity associated with rat liver polysomal messenger ribonucleoprotein particles. Biochemistry. 1980 Jul 8;19(14):3164–3169. doi: 10.1021/bi00555a008. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- De Herdt E., Thoen C., Van Hove L., Roggen E., Piot E., Slegers H. Identification and properties of the 38 000-Mr poly(A)-binding protein of non-polysomal messenger ribonucleoproteins of cryptobiotic gastrulae of Artemia salina. Eur J Biochem. 1984 Feb 15;139(1):155–162. doi: 10.1111/j.1432-1033.1984.tb07989.x. [DOI] [PubMed] [Google Scholar]
- Dearsly A. L., Johnson R. M., Barrett P., Sommerville J. Identification of a 60-kDa phosphoprotein that binds stored messenger RNA of Xenopus oocytes. Eur J Biochem. 1985 Jul 1;150(1):95–103. doi: 10.1111/j.1432-1033.1985.tb08993.x. [DOI] [PubMed] [Google Scholar]
- Egly J. M., Schmitt M., Elkaim R., Kempf J. Protein kinases and their protein substrates in free messenger ribonucleoprotein particles and polysomes from mouse plasmacytoma cells. Eur J Biochem. 1981 Aug;118(2):379–387. doi: 10.1111/j.1432-1033.1981.tb06413.x. [DOI] [PubMed] [Google Scholar]
- Fukami H., Itano H. A. In vitro translation of globin: effect of proteins purified by affinity chromatography on polyadenylate-Sepharose. Biochemistry. 1976 Aug 10;15(16):3529–3535. doi: 10.1021/bi00661a021. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Wilt F. H. Protein synthesis, polyribosomes, and peptide elongation in early development of Strongylocentrotus purpuratus. Dev Biol. 1981 Feb;82(1):32–40. doi: 10.1016/0012-1606(81)90426-7. [DOI] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M. Fertilization triggers unmasking of maternal mRNAs in sea urchin eggs. Mol Cell Biol. 1987 Nov;7(11):3947–3954. doi: 10.1128/mcb.7.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange T., de Sa C. M., Oddos J., Pictet R. Human mRNA polyadenylate binding protein: evolutionary conservation of a nucleic acid binding motif. Nucleic Acids Res. 1987 Jun 25;15(12):4771–4787. doi: 10.1093/nar/15.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi de Sa M. F., Standart N., Martins de Sa C., Akhayat O., Huesca M., Scherrer K. The poly(A)-binding protein facilitates in vitro translation of poly(A)-rich mRNA. Eur J Biochem. 1988 Oct 1;176(3):521–526. doi: 10.1111/j.1432-1033.1988.tb14309.x. [DOI] [PubMed] [Google Scholar]
- HINEGARDNER R. T. The isolation of nuclei from eggs and embryos of the sea urchin. J Cell Biol. 1962 Dec;15:503–508. doi: 10.1083/jcb.15.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys T. Measurements of messenger RNA entering polysomes upon fertilization of sea urchin eggs. Dev Biol. 1971 Oct;26(2):201–208. doi: 10.1016/0012-1606(71)90122-9. [DOI] [PubMed] [Google Scholar]
- Irwin D., Kumar A., Malt R. A. Messenger ribonucleoprotein complexes isolated with oligo(dT)-cellulose chromatography from kidney polysomes. Cell. 1975 Feb;4(2):157–165. doi: 10.1016/0092-8674(75)90122-1. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery W. R. Characterization of polypeptides associated with messenger RNA and its polyadenylate segment in Ehrlich ascites messenger ribonucleoprotein. J Biol Chem. 1977 May 25;252(10):3525–3532. [PubMed] [Google Scholar]
- Kelso-Winemiller L., Drawbridge J., Winkler M. M. A new ultracentrifugation technique for analysis and isolation of polysomes. Nucleic Acids Res. 1989 Jun 26;17(12):4896–4896. doi: 10.1093/nar/17.12.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kick D., Barrett P., Cummings A., Sommerville J. Phosphorylation of a 60 kDa polypeptide from Xenopus oocytes blocks messenger RNA translation. Nucleic Acids Res. 1987 May 26;15(10):4099–4109. doi: 10.1093/nar/15.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Poly (A)-rich ribonucleoprotein complexes from HeLa cell messenger RNA. J Biol Chem. 1976 Oct 10;251(19):5888–5894. [PubMed] [Google Scholar]
- Manrow R. E., Jacobson A. Identification and characterization of developmentally regulated mRNP proteins of Dictyostelium discoideum. Dev Biol. 1986 Jul;116(1):213–227. doi: 10.1016/0012-1606(86)90058-8. [DOI] [PubMed] [Google Scholar]
- Manrow R. E., Jacobson A. Increased rates of decay and reduced levels of accumulation of the major poly(A)-associated proteins of Dictyostelium during heat shock and development. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1858–1862. doi: 10.1073/pnas.84.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkes P. E. Messenger ribonucleoprotein complexes isolated by oligodeoxythymidylate-cellulose chromatography from Neurospora crassa polysomes. J Bacteriol. 1977 Jul;131(1):240–246. doi: 10.1128/jb.131.1.240-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peters C., Jeffery W. R. Postfertilization poly(A) . protein complex formation on sea urchin maternal messenger RNA. Differentiation. 1978;12(2):91–97. doi: 10.1111/j.1432-0436.1979.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Maitra U. Identification of ribosome-bound eukaryotic initiation factor 2.GDP binary complex as an intermediate in polypeptide chain initiation reaction. J Biol Chem. 1986 Jun 15;261(17):7723–7728. [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Reversible inhibition of translation by Xenopus oocyte-specific proteins. Nature. 1984 May 24;309(5966):378–380. doi: 10.1038/309378a0. [DOI] [PubMed] [Google Scholar]
- Rittschof D., Traugh J. A. Identification of casein kinase II and phosphorylated proteins associated with messenger ribonucleoproteins particles from reticulocytes. Eur J Biochem. 1982 Apr 1;123(2):333–336. doi: 10.1111/j.1432-1033.1982.tb19772.x. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Bond M. W., Kornberg R. D. A single gene from yeast for both nuclear and cytoplasmic polyadenylate-binding proteins: domain structure and expression. Cell. 1986 Jun 20;45(6):827–835. doi: 10.1016/0092-8674(86)90557-x. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W., Kornberg R. D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987 Sep;7(9):3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B., Kornberg R. D. Nuclear polyadenylate-binding protein. Mol Cell Biol. 1985 Aug;5(8):1993–1996. doi: 10.1128/mcb.5.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schmid H. P., Köhler K., Setyono B. Possible involvement of messenger RNA-associated proteins in protein synthesis. J Cell Biol. 1982 Jun;93(3):893–898. doi: 10.1083/jcb.93.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H., Darnell J. E. The association of protein with the polyadenylic acid of HeLa cell messenger RNA: evidence for a "transport" role of a 75,000 molecular weight polypeptide. J Mol Biol. 1976 Jul 15;104(4):833–851. doi: 10.1016/0022-2836(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Schönfelder M., Horsch A., Schmid H. P. Heat shock increases the synthesis of the poly(A)-binding protein in HeLa cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6884–6888. doi: 10.1073/pnas.82.20.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Slater I., Gillespie D., Slater D. W. Cytoplasmic adenylylation and processing of maternal RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):406–411. doi: 10.1073/pnas.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slegers H., De Herdt E., Kondo M. Non-polysomal poly(A)-containing messenger ribonucleoproteins of cryptobiotic gastrulae of Artemia salina. Eur J Biochem. 1981 Jun;117(1):111–120. doi: 10.1111/j.1432-1033.1981.tb06309.x. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Belitsina N. V., Lerman M. I. Use of formaldehyde fixation for studies of ribonucleoprotein particles by caesium chloride density-gradient centrifugation. J Mol Biol. 1965 Dec;14(2):611–615. doi: 10.1016/s0022-2836(65)80213-3. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Akhayat O., Goldenberg S., Scherrer K. Differential repression of specific mRNA in erythroblast cytoplasm: a possible role for free mRNP proteins. EMBO J. 1983;2(11):1869–1876. doi: 10.1002/j.1460-2075.1983.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. M., Nelson E. M., Lashbrook C., Hershey J. W. Multiple levels of regulation of protein synthesis at fertilization in sea urchin eggs. Dev Biol. 1985 Feb;107(2):290–300. doi: 10.1016/0012-1606(85)90312-4. [DOI] [PubMed] [Google Scholar]
- Zelus B. D., Giebelhaus D. H., Eib D. W., Kenner K. A., Moon R. T. Expression of the poly(A)-binding protein during development of Xenopus laevis. Mol Cell Biol. 1989 Jun;9(6):2756–2760. doi: 10.1128/mcb.9.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., van Eekelen C. A., Jansen R. T., Princen J. M. Specific poly-A-binding protein of 76,000 molecular weight in polyribosomes is not present on poly A of free cytoplasmic mRNP. Nature. 1977 Nov 10;270(5633):189–191. doi: 10.1038/270189a0. [DOI] [PubMed] [Google Scholar]