Abstract
Adipocyte progenitors are thought to play a fundamental role in white adipose tissue (WAT) plasticity, which enables dynamic modulation of WAT metabolic and cellular characteristics in response to various stimuli. In general, two main strategies have been used to identify adipocyte progenitor cells: fluorescence-activated cell sorting (FACS)-based prospective analysis and lineage tracing. Although FACS-isolation is highly useful in defining multipotential stem cell populations for in vitro analysis and transplantation, lineage tracing is essential to identify endogenous progenitors that do, in fact, differentiate into adipocytes in vivo. Our recent lineage tracing studies have shown that cells expressing the surface marker platelet-derived growth factor receptor α (PDGFRα) give rise to white and brown adipocytes in adult WAT, depending on inductive cues. PDGFRα+ cells are a subpopulation of those expressing CD34 and Sca1, and have unique morphology whereby long dendritic processes contact numerous cell types in the microenvironment. The significant contribution of PDGFRα+ cells to browning and hyperplastic expansion of WAT leads us to propose that PDGFRα+ cells are remodeling stem cells in adult WAT. Application of advanced imaging technology and genetic tools to this progenitor population will allow greater understanding of cellular plasticity in adipose tissue.
Keywords: adipose tissue plasticity, adipocyte progenitors, brown adipocytes, white adipocytes, lineage tracing
Introduction and Scope
The central function of adipose tissue is management of long-term energy reserves. Although excessive fat mass is associated with increased risk of diabetes and is certainly the most salient feature of obesity, fat accumulation per se is not the critical underlying factor for disease risk. Rather, growing evidence indicates that obesity-related disease occurs when the interrelated metabolic, endocrine and immune functions of adipose tissue are dysregulated.1,2 The fact that the cellular composition and metabolic character of adipose tissues are responsive to nutritional and pharmacologic stimuli3 raises the possibility that adipose tissue plasticity might be targeted for therapeutic benefit.4 The plasticity can take many forms, including expanding anabolic functions of white adipocytes5 or, conversely, increasing the catabolic functions by brown adipocyte recruitment and white adipose tissue “browning.”6,7
The mechanisms mediating adipose tissue remodeling involve complex and poorly understood interactions among numerous cell types within the adipose tissue microenvironment. It is well established that adipose tissues contain a large number of resident stromal cells that are capable of adipogenesis and are a likely source of progenitors for adaptive remodeling (for recent comprehensive review see refs. 8 and 9). Indeed, the pluripotency of adipose stem cells hold great promise for restorative medicine.10 Nonetheless, relatively little is known about the cell types within adipose tissue that directly contribute to tissue pharmacologic and nutritional tissue remodeling in vivo. We recently utilized in vivo lineage tracing techniques to identify a population of cells that are capable of becoming brown or white adipocytes, depending on inductive signals.11 In this brief report, we place this work in the contexts of therapeutic tissue remodeling and progenitor identification strategies, and speculate on the functional role of dendritic PDGFRα+ cells in tissue remodeling and repair.
Adipose Tissue and Obesity-Related Disorders
Obesity has been recognized as a predisposing factor for several common and severe diseases, including type 2 diabetes, cardiovascular diseases and certain types of cancer.12 While obesity is defined by increased lipid storage and adipose tissue mass, the consequences of obesity vary widely among individuals, even with a similar body mass index.13 Indeed, excess adipose tissue, as occurring in obesity, and the absence of adipose tissue, as occurring in lipodystrophy, both result in insulin resistance. This indicates that dysfunction of adipose tissue, rather than the level of total adiposity, is a key risk factor for developing metabolic disorders.13
Adipose tissue has evolved to store excess energy by hyperplastic and/or hypertrophic growth; however, under obesogenic conditions, such as chronic nutritional oversupply, adipose tissue is often unable to meet the demand for excess lipid storage.13 In many cases, limited expandability of adipose tissue cause ectopic accumulation of lipid and its byproducts in major metabolic organs, which leads to cellular dysfunction and disturbed systemic metabolism.14 Within this context, lipotoxicity resulting from dysfunctional adipose tissue is thought to be a key event underlying the development of obesity-induced insulin resistance and metabolic disease.15
In general, two adipocentric therapeutic strategies have been applied to reverse lipotoxicity: the first is to optimize the anabolic function of adipose tissue, thereby rerouting lipid from lipid-intolerant organs to fat tissue; the second is to increase the catabolic function of adipose tissue to reduce fat mass. For example, the thiazolidinedione (TZD) class of anti-diabetic drugs has been clinically used to restore adipose tissue insulin sensitivity, in part by enhancing fat deposition.16 In addition, TZDs normalize secretion of multiple adipokines to reduce inflammatory signaling and improve metabolic profiles.17 Another attractive approach is to augment energy expenditure by promoting oxidative metabolism in adipose tissue.18 For example, it is well known that β3-adrenergic receptor (ADRB3) agonists stimulate thermogenesis and catabolic metabolism in adipose tissue, and have anti-obesity and anti-diabetic effects in rodent models.19,20 While some of these effects involve typical brown fat tissue, adrenergic activation clearly increases the catabolic function of white adipose tissue via uncoupling protein 1 (UCP1)-dependent and independent mechanisms.21 In theory, it may be possible to improve the metabolic character of adipose tissue by activating progenitors to generate new adipocytes that ultimately have improved catabolic or anabolic characteristics.4 At the present time, the identities of the adipocyte progenitors and the mechanisms that control the progenitor fate and behaviors in vivo are poorly characterized. Nonetheless, recruitment of beneficial adipocytes by controlling progenitor dynamics would be one of the possible strategies to prevent or treat obesity and its associated diseases.
Cellular and Metabolic Plasticity of WAT
White adipose tissue (WAT) possesses exceptional plasticity, enabling dynamic modulation of its metabolic and cellular characteristics in response to various stimuli. For example, WAT can increase adipocyte mass and number dramatically under high fat feeding,3 and it can regenerate in the setting of injury or partial surgical removal.22-24 PPARγ agonists are known to expand WAT by recruiting progenitors and promoting adipogenesis of preadipocytes.25,26 In addition, WAT has the capacity to transition between white and brown-like.27 Multiple reports demonstrate that traditional white fat depots can adopt brown fat-like characteristics under certain physiological and pharmacological conditions, such as cold exposure and β-adrenergic stimulation.28,29 Since ADRB3 are mainly expressed in adipose tissues, selective ADRB3 agonists, such as CL316243 (CL), have been utilized to study induction of the catabolic phenotype in WAT upregulated catabolic gene transcription (mainly fatty acid oxidation), mitochondrial biogenesis and elevation of metabolic rate.19,30
One of the most prominent events of catabolic remodeling is the appearance of UCP1 expressing multilocular brown adipocytes as clusters diffused within WAT.31 It remained unclear, however, which cell types give rise to these inducible brown adipocytes (iBA). Current views on cellular plasticity suggest that alterations in cellular identity might occur through genetic reprogramming of adult somatic cells: by transdifferentiation from one mature cell type to another, also called metaplasia; or by reversion to a stem cell-like or a less-differentiated state with subsequent alternative differentiation, called dysplasia.32 Evidence from detailed morphological analysis on iBA at ultrastructural levels suggests that conversion from mature white adipocyte (WA) to BA during cold acclimatization and β-adrenergic stimulation is achieved by metaplasia.33,34 Although mature WA can undergo transdifferentiation to generate iBA, this mechanism cannot explain the population of iBA derived from proliferating cells.30
Search for Adipocyte Progenitors: Prospective Analyses and Lineage Tracing
It is widely believed that stem cells residing in adipose tissue provide a source of progenitors for adipose tissue expansion and regeneration.9 In general, two main strategies have been used to identify adipocyte progenitors: “prospective analysis” and lineage tracing. Prospective analysis involves isolating progenitors from dissociated tissue by fluorescence-activated cell sorting (FACS), and evaluating differentiation potential either in vitro or in vivo following transplantation. This approach has been highly successful in identifying multipotent stem cells, including those with high adipogenic potential35,36 (for recent review see refs. 9 and 10). For example, Friedman’s group identified a cell population (Lin-CD29+CD34+Sca-1+CD24+) with an increased in vitro adipogenic potential over heterogeneous stromal vascular fraction (SVF), and these cells reconstitute functional WAT when transplanted into a lipodystrophic mouse model.36
Although FACS-based approaches have proven useful in defining adipogenic potential from SVF of WAT and has great potential for restorative medicine, the approach does not directly address the identity of progenitors that contribute to adipose tissue during normal development or in response to endogenous stimuli. For this purpose, genetic lineage tracing is an essential tool since it allows labeling and tracking of putative progenitors in intact tissue.37 Recently, several elegant lineage tracing studies enabled breakthrough findings on developmental origin of brown adipose tissue (BAT) and discovered common progenitors for muscle and BAT.38 These studies have established that the early myogenic factor, MYF5, is expressed in progenitors that give rise to both skeletal muscle and BAT in the interscapular and perirenal depots. Moreover, the iBA emerging in WAT in response to ADRB3 stimulation never express MYF5, suggesting that BA that reside in “classic” interscapular BAT and iBA in WAT originate from distinct lineages. In a study conducted by Graff’s group, the direct precursors of WA in developing mouse WAT were identified as a population that express PPARγ and several pericyte markers.39 A recent study also demonstrated that TZD treatment accelerated differentiation of PPARγ+ pericyte-like adipocyte progenitors.26 However, it is not known whether these committed precursors of WA serve as adult adipose stem cells in response to other adipogenic conditions or ADRB3 stimulation.
Identification of Bipotential Adipocyte Progenitors in Adult WAT
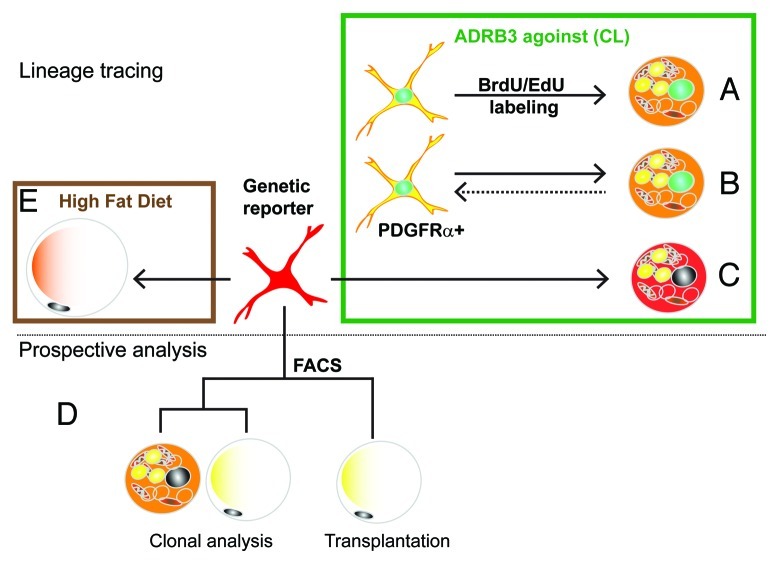
We recently reported that iBA are derived from proliferation of PDGFRα-expressing resident progenitors in abdominal WAT.11 The key observations of this work are summarized in Figure 1. In previous work, we found that catabolic WAT remodeling by ADRB3 activation, proceeded in two phases: a transient inflammation (due to excessive fatty acid mobilization), followed by upregulation of catabolic gene expression, including UCP1.30 We noted in those studies that inflammation and catabolic gene expression were inversely related.30,40 Given this reciprocal relation, we reduced the level of ADRB3-mediated inflammation (by lowering the dose) and found that induction of the catabolic phenotype was facilitated. Importantly, we found that > 85% of the multilocular UCP1+ adipocytes that appeared in abdominal (perigonadal) fat were derived from proliferating cells (Fig. 1A, green box). This discovery of conditions that promoted the proliferation and differentiation of progenitors into BA was key to developing a strategy to identify iBA progenitors in vivo.
Figure 1. Experimental strategies for the identification of bipotent adipocyte progenitors. Initial experiments used tracing techniques (top panel) to investigate the origin of brown adipoyctes that are induced by the ADRB3 agonist stimulation (green box). (A) In vivo tagging with thymidine analogs demonstrated that iBA come from proliferating cells. (B) Immunotyping of cells tagged with thymidine analogs indicated that iBA are derived from progenitors that express PDGFRα+. (C) Inducible genetic tagging with the PDGFRα-CreER reporter model proved that PDGFRα+ cells become iBA during CL stimulation. Prospective analysis (lower panel) of FACS-isolated cells indicate that PDGFRα+ have the potential to become either brown and white adipocytes. (D) FACS-isolated cells formed colonies that contain both brown and white adipocytes, and following transplantation became white adipocytes. (E) Lineage tracing using the PDGFRα-CreER reporter model demonstrated that high fat feeding greatly increases the recruitment of white adipocytes from PDGFRα+ progenitors.
We first determined the time course of ADRB3-induced progenitor proliferation, then determined that actively proliferating cells expressed the cell surface markers PDGFRα, Sca1 and CD34 (Fig. 1B). When cells were flash-tagged with thymidine analogs and traced over the next 24 h, we found that most labeled cells lost expression of PDGFRα as they differentiated into BA. Interestingly, newly differentiated BA also could divide, and double labeling experiments suggest progenitor/newly differentiating cells could undergo multiple rounds of cell division resulting in clusters of new BA in WAT. Importantly, a few PDGFRα+ cells that underwent cell division retained morphological and immunochemical characteristics of progenitors; thus, the progenitor population remained stable over time.
Although fate tracing of proliferating cells strongly indicated that PDGFRα+ cells are iBA progenitors, definitive data can only be obtained using genetic lineage tracing, in which cells are labeled prior to treatment, and their fate followed over time. For this purpose we took advantage of a mouse model developed by the Richardson lab41 in which treatment of mice with tamoxifen induces expression of fluorescent reporter protein in PDGFRα+ cells via Cre-mediated recombination. Using this model we conclusively demonstrated that PDGFRα+ cells give rise to iBA during ADRB3 stimulation (Fig. 1C).
We also performed conventional prospective analyses of PDGFRα+ progenitors that were isolated by FACS (Fig. 1D, lower panel). Single cells isolated by FACS formed colonies that contained a mixture of both WA (UCP1−) and BA (UCP1+) to varying degrees (Fig. 1D). Interestingly, the adipogenic potential of PDGFRα+ cells, assessed as the percentage of adipogenic colonies, was similar for cells derived from various fat depots, suggesting that differences in responsiveness to ADRB3 stimulation reflects extrinsic influences of the microenvironment. In vitro experiments with FACS-isolated cells demonstrated dual white/brown potential. To extend these findings in vivo, we transplanted FACS-purified PDFGRα+ cells into mice and found that these cells become exclusively WA in the absence of ADRB3 stimulation. Interestingly, subsequent stimulation of ADRB3 receptors failed to instate expression of UCP1 in these cells, suggesting that the white vs. brown cell fate may be set by inductive signals generated early during proliferation/differentiation.
The dual potential of purified PDGFRα+ cells suggested that PDGFRα+ cells might contribute to homeostatic turnover and adipose tissue expansion during over-nutrition. Using genetic lineage tracing, we found that PDGFRα+ cells contributed to the low level (1.5%/2 mo) adipogenesis in adult abdominal WAT (Fig. 1E). Importantly, 2 mo of high fat feeding dramatically increased the recruitment and differentiation of PDGFRα+ cells into WA. High fat feeding is associated with adipocyte death, as indicated by numerous “crown-like structures,” implying a dynamic process of cell death and restoration.42,43 However, it is difficult to estimate the magnitude of this remodeling by using static histological approaches. For example, in our experiments, high fat feeding did not significantly alter total fat cell number in gonadal fat pads. However, inducible tagging of progenitors demonstrated that high fat feeding recruits a substantial population of new adipocytes (up to 25% of total fat cells in 2 mo) from PDGFRα+ progenitors despite little of no change in total (net) adipocyte number. These observations indicate that high fat feeding increases adipocyte turnover, in which adipocyte death is largely balanced by replacement from PDGFRα+ progenitors.
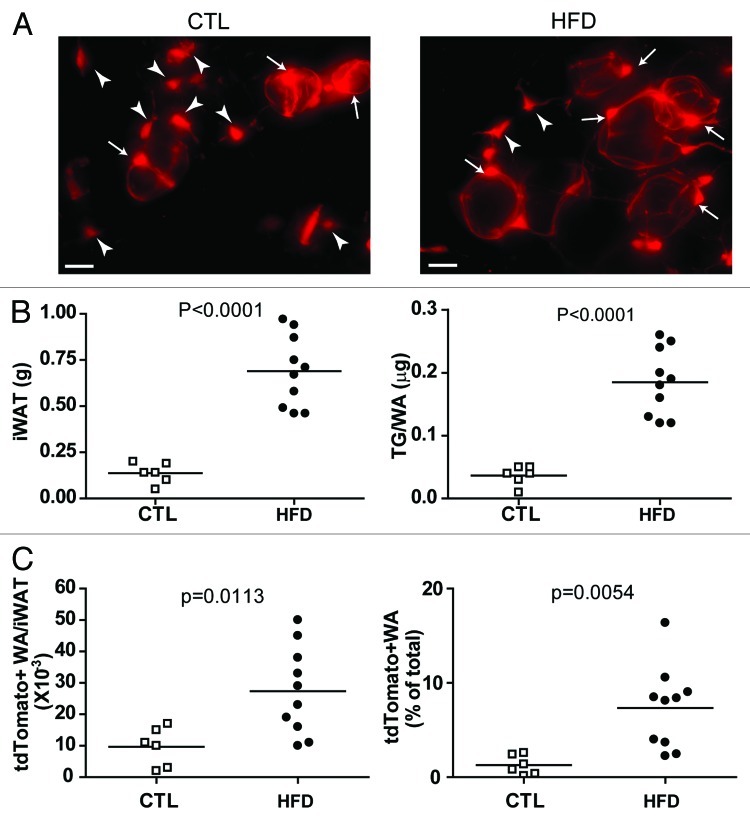
Our initial studies concentrated on adipogenesis in abdominal fat pads. We have now evaluated the contribution of PDGFRα+ cells to adipogenesis in subcutaneous (inguinal) WAT in vivo. As shown in Figure 2, the tdTomato genetic reporter was efficiently induced in subcutaneous WAT, and both stellate progenitors and tagged adipocytes were observed 8 weeks later (Fig. 2A, left). In chow-fed mice, approximately 2% of total adipocytes were derived from PDGFRα+ progenitors over the 2 mo tracing period (Fig. 2C). High fat feeding produced pronounced adipocyte hypertrophy (Fig. 2B) and significantly accelerated the recruitment of PDGFRα+ progenitors into adipocytes (Fig. 2C). These results indicate that recruitment and differentiation of PDGFRα+ progenitors by high fat feeding occurs similarly in both abdominal and subcutaneous WAT. Clearly, the ability to inducibly tag progenitors will allow investigation of progenitor/adipocyte dynamics in response to physiological and pathological challenges across adipose tissue depots.
Figure 2. PDGFRα+ progenitors in iWAT contribute to white adipogenesis during adipose tissue expansion induced by high-fat diet feeding. (A) Representative images of tdTomato+ cells in iWAT whole mount from control and HFD mice. Arrowheads mark tdTomato+ stellate progenitors, and arrows mark tdTomato+ adipocytes. (B) Effect of HFD on iWAT expansion. HFD increased the weight of iWAT pads and adipocyte triglyceride content, compared with chow-fed mice. (C) Effect of HFD on adipogenesis from tdTomato+ cells. HFD increased the number and density of tdTomato+ adipocytes in iWAT. Circles indicate individual animals and the horizontal lines represent group means.
Unique and Unexpected Properties of PDGFRα+ Cells
PDGFRα+ cells have been reported to exist in several tissues at various developmental stages. During early development, PDGFRα+ cells are involved in broad spectrum of development, including neural crest formation, central nervous system development, and organogenesis.44 PDGFRα expression in adult cells seems more confined in specific progenitor populations. For instance, PDGFRα+ cells in the brain are known as oligodendrocyte progenitors.41,45 Muscle-derived PDGFRα+ cells have been identified as a progenitor population that is responsible for ectopic adipocyte formation and fibrosis in damaged muscle.46-48
The PDGFRα+ progenitors identified in adult adipose tissue can be distinguished from previously described progenitors of WA by several criteria.11 As noted above, Tang et al.39 identified direct precursors of WA in rapidly growing perinatal WAT as pericyte-like cells that express PPARγ and several pericyte markers. The PDGFRα+ cells were negative for PPARγ, SMA and PDGFRβ. While often localized near blood vessels and capillaries, they reside outside of the mural compartment and have extended processes that contact other cell types.
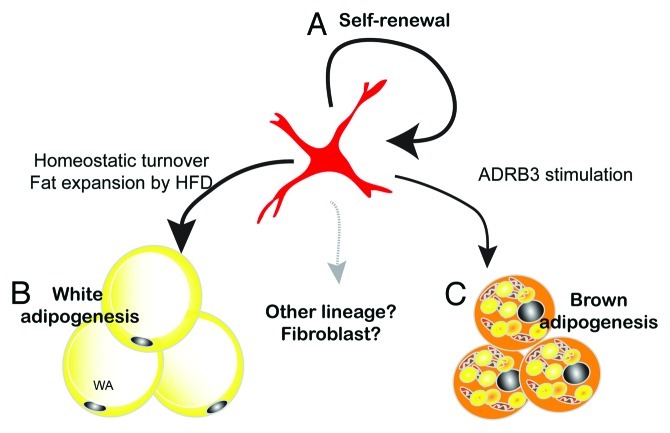
Three-dimensional imaging demonstrated unique dendritic morphology of PDGFRα+ progenitors, characterized by thin cytoplasmic processes that cover long distances and contact numerous cell types. These observations suggest that PDGFRα+ cells actively monitor the microenvironment and might be recruited during metabolic stress or damage. These morphological characteristics, in addition to their ability to contribute to tissue maintenance and plasticity, lead us to propose that PDGFRα+ cells are remodeling stem cells in adult adipose tissue (Fig. 3). PDGFRα+ cells are capable of clonal growth in vitro in which > 70% of colonies contain a mixture of brown and white adipocytes. Furthermore, a fraction of dividing PDGFRα+ cells retains stem cell characteristics in vivo and thus the tissue population of progenitors remains constant following recruitment by ADRB3 agonist. These observations suggest that PDGFRα+ cells are capable of self-renewal. One important unresolved question is whether PDGFRα+ cells are capable of differentiating into other cellular phenotypes, such as muscle and bone. PDGFRα+ cells derived from muscle can differentiate into adipocytes or fibroblasts, but do not have myogenic potential.46-48 It seems possible, if not likely, that PDGFRα+ cells contribute to adipose tissue fibrosis under conditions of chronic inflammation. If so, the fate of these cells may determine adaptive vs. maladaptive remodeling during adipose tissue expansion.
Figure 3. PDGFRα+ cells are remodeling stem cells in adult WAT. Adult mouse WAT contains white/brown bipotent progenitors. (A) The ability of FACS-isolated PDGFRα+ cells to form clones in vitro, and the stable tissue content of these cells over the course of ADRB3 recruitment, indicates that PDGFRα+ cells have self-renewing properties. (B) PDGFRα+ cells contribute to basal homeostatic turnover of WA and to hyperplasic remodeling induced by high fat feeding. (C) PDGFRα+ progenitors are responsible for BA generation induced by ADRB3 stimulation. Whether PDGFRα+ cells contribute to other cell types within adipose tissue is currently under investigation.
Significance of Tools (Pdgfra-CreER and Model)
In vivo identification of adipocyte progenitors is essential to understand how adipose tissue remodels in response to altered metabolic status. The genetic reporter system utilized in our studies, in combination with imaging techniques and isolation methods, will greatly aid our comprehensive understanding of adipocyte progenitor biology and adipose tissue plasticity. For example, advanced imaging technology, including multiphoton microscopy and time-lapse imaging, will permit tracking of fluorescently tagged progenitors in the context of a live organism and organotypic culture.49 Such in vivo labeling of progenitors may provide a basis for defining progenitor niches that liberate adipogenic signals, and the biological responses of progenitors to particular signaling molecules can be investigated more directly in the highly controlled environment of in vitro tissue culture. Importantly, identification of a progenitor marker enables manipulation progenitors by genetic tools. Conditional knockout models using Pdgfra-CreERT2 will be valuable to identify molecular mechanisms of proliferation and differentiation of progenitors. Moreover, the ability to alter candidate signaling molecules in progenitor cells will offer a great opportunity to address long-standing questions regarding cellular plasticity in the context of intact adipose tissue.
Materials and Methods
All animal protocols were approved by the Institutional Animal Care and Use Committee at Wayne State University. Lineage tracing experiments during control condition and HFD feeding were performed in a PDGFRα-reporter system, as previously described.11 Briefly, Pdgfra-CreERT2/R26-LSL-tdTomato double transgenic mice were treated with tamoxifen (300 mg/kg) for 5 d, and HFD (60% fat diet, Research Diet, D12492) was introduced 1 week after the first dose of tamoxifen and continued for 8 weeks. Tissues were fixed with 10% formalin overnight at 4°C. Fluorescence microscopy was performed using an Olympus IX-81 microscope equipped with a spinning disc confocal unit, and confocal z stacks were processed using IPlabs software (Scanalytics, BD Biosciences). Total adipocyte numbers in iWAT were calculated as previously described.50 Total tdTomato+ adipocytes were estimated by extrapolation from the number of cells counted in 10 mg samples. Statistical significance was determined by unpaired t-test.
Acknowledgments
This work was supported by NIH grants DK 62292 and DK 76229.
Glossary
Abbreviations:
- ADRB3
β3-adrenergic receptor
- BA
brown adipocyte
- BAT
brown adipose tissue
- CL
- FACS
fluorescence-activated cell sorting
- iBA
inducible brown adipocytes
- SVF
stromal vascular fraction
- TZD
thiazolidinedione
- UCP1
uncoupling protein 1
- WA
white adipocyte
- WAT
white adipose tissue
Disclosure of Potential Conflicts of �Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/20804
References
- 1.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 3.Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 4.Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell. 2009;5:472–81. doi: 10.1016/j.stem.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50:1863–71. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- 6.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–61. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 7.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788–97. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–46. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cawthorn WP, Scheller EL, Macdougald OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab. 2012;23:270–7. doi: 10.1016/j.tem.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y-H, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–91. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 13.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10:306–15. doi: 10.1007/s11892-010-0122-6. [DOI] [PubMed] [Google Scholar]
- 15.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 17.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–9. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Tseng Y-H, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–82. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arch JRS. β(3)-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol. 2002;440:99–107. doi: 10.1016/S0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 20.Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: β-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18:2123–31. doi: 10.1210/me.2004-0193. [DOI] [PubMed] [Google Scholar]
- 21.Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–6. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 22.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Xaymardan M, Gibbins JR, Zoellner H. Adipogenic healing in adult mice by implantation of hollow devices in muscle. Anat Rec. 2002;267:28–36. doi: 10.1002/ar.10072. [DOI] [PubMed] [Google Scholar]
- 24.Koh YJ, Kang S, Lee HJ, Choi T-S, Lee HS, Cho C-H, et al. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–95. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, et al. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–53. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang W, Zeve D, Seo J, Jo AY, Graff JM. Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 2011;14:116–22. doi: 10.1016/j.cmet.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langin D. Recruitment of brown fat and conversion of white into brown adipocytes: strategies to fight the metabolic complications of obesity? Biochim Biophys Acta. 2010;1801:372–6. doi: 10.1016/j.bbalip.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–42. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 29.Young P, Arch JRS, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–4. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 30.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of β3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–16. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 31.Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol. 1997;54:121–31. doi: 10.1016/S0006-2952(97)00162-7. [DOI] [PubMed] [Google Scholar]
- 32.Cherry AB, Daley GQ. Reprogramming cellular identity for regenerative medicine. Cell. 2012;148:1110–22. doi: 10.1016/j.cell.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–86. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 34.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–53. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 35.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–8. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–9. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 37.Kretzschmar K, Watt FM. Lineage tracing. Cell. 2012;148:33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-α. Am J Physiol Endocrinol Metab. 2005;289:E617–26. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- 41.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 43.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–84. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 45.Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–51. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- 46.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uezumi A, Fukada S-i, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–52. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 48.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–64. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 49.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol Rev. 2010;90:1103–63. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 50.Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968;9:110–9. [PubMed] [Google Scholar]