Abstract
A large number of studies have shown that mature adipocytes are able to dedifferentiate in vitro into progeny cells, which possess proliferative capacity and mutilineage potential. Our present study confirms that mature adipocytes derived from Angus cattle also dedifferentiate into proliferative-competent progeny cells. However, this report is unlike any published for all other breeds of cattle we have worked with or that we have seen in published reports, in which mature adipocytes retain and distribute lipids into daughter cells symmetrically or asymmetrically. In the present work, we noted that Angus-derived mature adipocytes extruded a majority of their cellular lipid droplets prior to cell division. In this manner, these cells are processing lipid in a manner observed in mature adipocytes isolated from swine tissue. These results suggest that regulation of the mechanism(s) underlying lipid processing might be different between and within animal breeds. Lipid processing in beef-derived adipocytes during dedifferentiation may serve as a unique animal model for studying lipid metabolism during reverse adipogenesis.
Keywords: mature adipocytes, lipids, dedifferentiation, proliferation, cattle
A key component of meat animal production is the consumer acceptance of the final product.1 While a number of factors influence final carcass quality, marbling/intramuscular fat (IMF) plays an important role in imparting flavor and juiciness to enhance the eating quality of meat.1,2 Devising a variety of regimens to improve IMF in meat animals, while decreasing undesirable fat accumulation (such as subcutaneous and visceral fat tissue), has been the focus of numerous studies.1-11 As the main contributor of adipose tissue, the cellularity of adipocytes and the underlying mechanisms regulating adipogenesis and lipid mobilization deserve considerable attention.1-3,5,8-10,12-16
Adipogenesis is a complex process involving cells of the adipose lineage, whereby cells proliferate, become committed, switch to differentiation mode and begin to express differentiated phenotypes.2,14,17-20 Mature adipocytes, possessing large-unilocular lipid vesicles, have traditionally been considered incapable of returning to a proliferative state and could only metabolize lipid.20 However, recent studies have shown that lipid-filled adipocytes may dedifferentiate into proliferative-competent progeny cells and these daughter cells may redifferentiate into adipocytes or transdifferentiate into other types of cells in vitro.2,9,10,12-14,17,18,21-42 As such, evaluation of mature adipocyte (cellular) physiology, dedifferentiation and plasticity may reveal a new understanding of adipogenesis.
To initiate studies with mature adipocytes, we previously documented that pure cultures of beef-derived (lipid-filled) adipocytes were capable of resuming proliferation even with considerable cytosolic lipid in either an asymmetric or symmetric manner.18,24,25 Alternatively, pig-derived mature (lipid-filled) adipocytes always dumped their lipid directly into the media (external) environment prior to proliferation.43,44 However, in our present work, during an ordinary cell expansion protocol, beef-derived mature (lipid-filled) adipocytes dedifferentiated into proliferative-competent cells by first dumping their cytosolic lipid in a manner similar to pig-derived adipocytes in vitro. As the present observations were through the use of a different breed of cattle (Angus), it is possible that different breeds or even individuals (Angus breed individuals) may possess diverse lipid metabolism and handling, which may contribute to our observations. As such, Angus-derived mature adipocytes possessing different dedifferentiation systems could be a novel mode for studying lipid routing during dedifferentiation of mature adipocytes.
Cells were observed daily and pictures were taken for the adipocytes derived from pig (Fig. 1) and Angus cattle (Fig. 2).
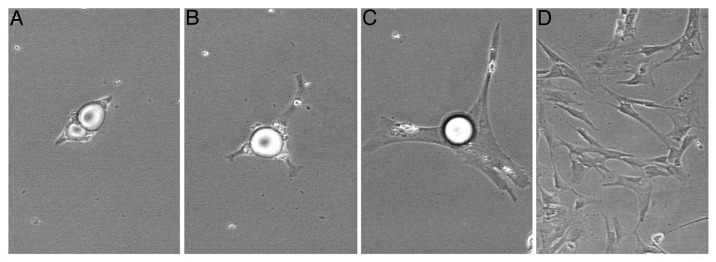
Figure 1. Lipid handing manner during dedifferentiation of pig-derived mature adipocyte. Pig-derived mature adipocyte extruded lipid prior to proliferation. Isolation method was described by Chen et al.43,44 [(A–C), 200× magnification; (D), 100× magnification; in time order.]
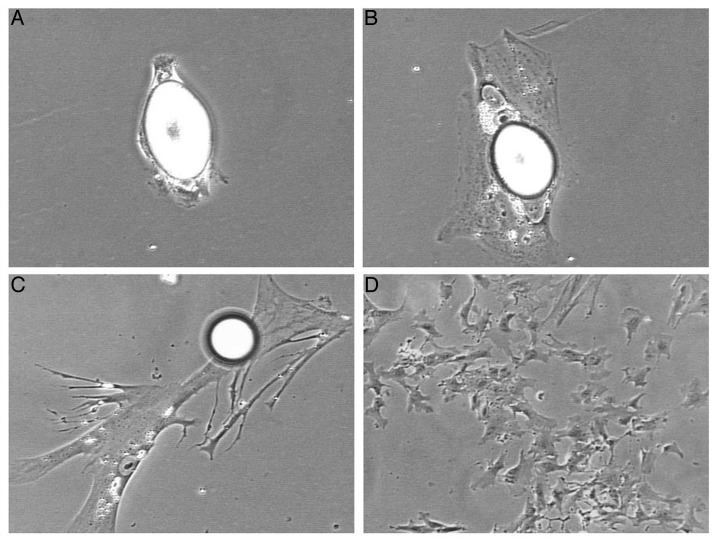
Figure 2. Lipid processing during dedifferentiation of purified cultures of mature adipocytes derived from Angus cattle. At the end of ceiling culture, mature adipocytes appeared like a spindle-shaped oval (A). One day after reinverting the flask the lipid-filled adipocyte began to enlarge and adhered tightly to the flask surface (B). Two days after reinverting the flask the cell membrane spreaded significantly and a large lipid droplet was extruding into the medium (C). Eight days after reinverting the flask, lipid has been expelled, and the cells proliferated into a number of daughter cells in vitro (D). [(A–C), 200× magnification; (D), 100× magnification.]
Results showed that fat cells derived from pig adhered firmly to the surface of the flask after the purified ceiling culture. Subsequently, the attached fat cells stretched membrane and big lipid droplets were extruded from cytoplasm, followed by cell division (Fig. 1). This phenomenon was in accord with what we found before, of which lipid-filled adipocytes derived from pig could dedifferentiate into proliferative-competent progeny cells, extruding lipids prior to cell division.
Similarly, pure mature adipocytes derived from Angus cattle adhered on the flask after the ceiling culture regimen. Adipocytes filled with lipid droplets showed oval-shaped conformation with little (attachment) spindles on apical and dorsal poles of the cell. After a few hours mature adipocytes began to spread out (Fig. 2A and B). Then, however, unlike what we have previously observed in 100% of our beef-derived adipocyte cultures (lipid-laden cells retained lipids and segmented/processed them into daughter cells symmetrically or asymmetrically),2,12,17,18,23-26,38,41 numerous Angus-derived mature adipocytes deposited cytosolic lipid directly into medium environment prior to proliferation (process took approximately 2 d after flask re-inversion) (Fig. 2C), showing the similar lipid handling with fat cells derived from pig. Subsequently, lipid extruded cells divided into daughter cells at next day and presented strong proliferative capacity (Fig. 2D). These results suggest that different beef cattle-derived mature adipocytes possess different dedifferentiation mechanisms.
The present study showed that mature adipocytes derived from cattle and pig can dedifferentiate into proliferative-competent progeny cells in vitro. This result is consistent with numerous studies, which have suggested that lipid-filled adipocytes derived from cattle and other species may not be terminally differentiated.18,21,22,24-28,34,35,43,44 In previous studies, two different processes were described for lipid handling/processing during dedifferentiation of mature adipocytes. For lipid-filled adipocytes derived from cattle, the lipid droplet is sectioned/partitioned and transferred into daughter cells symmetrically (retain the same amount of lipids) or asymmetrically (retain different amount of lipids).18,24-26 Another (more definitive) manner of lipid processing was determined for pig-derived mature adipocytes, which suggested that pig-derived mature adipocytes extruded lipids into the medium directly prior to cell division.43,44 However, in the present study with Angus cattle, mature adipocytes also began to extrude lipids quite rapidly (the first day after inverting the culture flask), followed by most cells on the second day, showing the similar manner with pig-derived fat cells. This has never been observed in other cattle cell systems that we have conducted on numerous occasions. These results suggest that not only different species may possess diverse lipid metabolism manner but also the different breeds or even individuals. Another explanation for the difference in cell handling of lipid might be a simple result of slight divergence in methods used for cell isolation. If so, then more attention in isolation procedures may result in ability to acquire representatives of specific populations of cells or of for a wide-divergence of mature adipocytes possessing more general properties. As such, the regulation underlying the different modes of lipid handling manners remains unclear.
Recently, several studies were reported regarding the gene expression profile during the opposite process of traditional adipogenesis (what we term as dedifferentiation). Ono et al.36 indicated that the genes involving movement, growth, proliferation and morphogenesis were upregulated during pig-derived mature adipocyte dedifferentiation and that lipid metabolism-related genes were significantly downregulated (these genes involved in the oxidation, quantity, modification and metabolic process of fatty acid metabolism). However, some regulation pathways of adipocytes derived from beef cattle were different from other species-derived adipocytes.1,10,12,20,45-47 Different breeds of beef-derived gene expression profiles involved in dedifferentiation need to be examined.
In our ceiling culture work to isolate and purify homogeneous cultures of progeny cells from dedifferentiated (mature) adipocytes, not every lipid-filled cell possesses the ability to dedifferentiate and form proliferative-competent progeny cells.29 In the present study, the purified mature adipocytes derived from Angus cattle were evaluated by daily microscopic examination. A total of 10 mature adipocytes were obtained after cell isolation and early differential plating (step 1 of culture purification); nine cells extruded lipids after 1 or 2 d, while one cell retained lipid, did not proliferate but did survive during the subsequent 20 d after cell isolation, when we ended the cell culture. However, from the nine lipid-extruded cells, eight cells died and disappeared several days after lipid-extrusion or evidence of progeny cell division, and homogeneous progeny cells were obtained from one mature adipocyte possessing dedifferentiation and proliferation capacity. The lack of complete epigenetic events for cell self-renewal might be the reason for the failure of dedifferentiation and proliferation. Generation of increased numbers of cultures of homogeneous progeny cells is a consideration, as studies on the progeny of one single cell may not reflect the real physiology of a whole body. Such a cell system could, however, provide considerable information about the ability of progeny cells to undergo adipogenesis to form lipid-filled adipocytes in vitro, and whether the regulation of such is consistent with data derived from cell lines or stromal vascular cells.19,20 Moreover, the cell physiology of pure cell type has provided us the basis for better understanding the complicated network for all kinds of cells. For example, although the rate for dedifferentiation of mature adipocytes might be 1% (based on whole animal estimates) there are millions of mature adipocytes that possess the potential to dedifferentiate, proliferate and form new cells when exposed to an appropriate environment. We simply need to devise more efficient ways to isolate and purify (to homogeneity) sufficient cells for a variety of experiments.31 In addition, even if limited to only a few cells, the dedifferentiation phenomenon of mature adipocytes in vitro will provide us a novel model for studying lipid metabolism/handling/processing.
A number of studies, including this one, suggest that domestic animals (like cattle and pig) may be used as models for humans and other animals because the interactions between adipose and (other) tissues may be more easily evaluated.10,46,48 Considering that the dedifferentiation process of cattle-derived (mature) adipocytes may involve individual-specific lipid mobilization, we may need to resolve this issue prior to expounding on the usefulness of such a system for human-related dysfunctions.49 Even so, as stored lipid serves as an energy-providing substrate, it has an important role on regulating human health and animal production. Consequently, the underlying mechanisms involved in lipid processing/handling during dedifferentiation of pure cultures of Angus-derived adipocytes may be useful in deciphering regulating carcass composition and meat quality (for meat animal production) and in mediating adverse effects related to human health.
Collectively, our study indicated that Angus cattle unlike other cattle breeds examined before possess mechanisms similar to pigs, during the dedifferentiation of mature adipocytes. This suggests that lipid metabolism may be different among species or even individuals. Our results suggest that breed-specific dedifferentiation systems may be at play and that we have devised a solid experimental base for better understanding this unique model of lipid metabolism and adipogenesis.
Materials and Methods
Animal/tissue samples
Sternomandibularis muscle samples of Angus cattle (n = 1) and fat tissues of swine (n = 3) were collected at Washington State University (WSU) Abattoir. The WSU Animal Care and Use Committee screened the use of animals in this research, and the animal use met the standards imposed by both the United States Department of Agriculture (USDA) and the United States Public Health Service (PHS).
Cell isolation and cell culture
Beef- and pig-derived adipocytes isolation methods were previously described by Fernyhough et al.26 and Chen et al.43,44 Briefly, isolated cells derived from IMF of Angus cattle and fat tissues of swine were cultured in inverted 12.5 ml flasks (ceiling culture, upside down) filling with DMEM/F12 + 10% horse serum (HS) after collagenase digestion and wash, respectively. Early differential plating was done on the 2 d of cell isolation.26 Transferred mature adipocytes were cultured for 4 d until floating lipid-filled adipocytes firmly attached the surface of flask. Subsequently, the flask was re-inverted to normal position, and medium was replaced by 5 ml DMEM/F12 + 10% fetal bovine serum (FBS) and medium was changed every 2 d. Cells were monitored daily to prevent any contaminations according to adipocyte purification methods and cautions.26 All the cells were cultured in 37°C incubator with 95% air and 5% CO2. Cell numbers were recorded and each mature adipocyte was marked and observed under microscope after flask re-inversion. All photomicrographs were taken with a Sony RGB digital camera (3/4-inch chip) married to a Nikon Diaphot phase contrast microscope and Image Pro Plus® image analysis software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/21447
References
- 1.Dodson MV, Jiang Z, Chen J, Hausman GJ, Guan L, Novakofski J, et al. Allied industry approaches to alter intramuscular fat content and composition in beef animals. J Food Sci. 2010;75:R1–8. doi: 10.1111/j.1750-3841.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- 2.Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, et al. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci. 2009;87:1218–46. doi: 10.2527/jas.2008-1427. [DOI] [PubMed] [Google Scholar]
- 3.Du M, Dodson MV. Advanced techniques to enhance marbling in meat. In: Joo S, ed. Control of Meat Quality. 2011:105-15. [Google Scholar]
- 4.Mir PS, He ML, Travis G, Entz T, McAllister T, Marchand S, et al. Periodic 48 h feed withdrawal improves glucose tolerance in growing pigs by enhancing adipogenesis and lipogenesis. Nutr Metab (Lond) 2012;9:10–22. doi: 10.1186/1743-7075-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Zhu MJ, Dodson MV, Du M. Developmental programming of fetal skeletal muscle and adipose tissue development. J Genomics. 2012;1:29–38. doi: 10.7150/jgen.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin W, Dodson MV, Moore SS, Basarab JA, Guan LL. Characterization of microRNA expression in bovine adipose tissues: a potential regulatory mechanism of subcutaneous adipose tissue development. BMC Mol Biol. 2010;11:29–37. doi: 10.1186/1471-2199-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao YM, Basu U, Dodson MV, Basarb JA, Guan L. Proteome differences associated with fat accumulation in bovine subcutaneous adipose tissues. Proteome Sci. 2010;8:14. doi: 10.1186/1477-5956-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Xue C, Wang X, Liu H, Xu Y, Zhao R, et al. Differential display of expressed genes reveals a novel function of SFRS18 in regulation of intramuscular fat deposition. Int J Biol Sci. 2009;5:28–33. doi: 10.7150/ijbs.5.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi M, Guan LL, Zhang B, Dodson MV, Okine E, Moore SS. Adipogenesis of bovine perimuscular preadipocytes. Biochem Biophys Res Commun. 2008;366:54–9. doi: 10.1016/j.bbrc.2007.11.110. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi M, Guan LL, Zhang B, Dodson MV, Okine E, Moore SS. Gene expression patterns of bovine perimuscular preadipocytes during adipogenesis. Biochem Biophys Res Commun. 2008;366:346–51. doi: 10.1016/j.bbrc.2007.11.111. [DOI] [PubMed] [Google Scholar]
- 11.Duckett SK, Byrne KM, Hossner KL, Dodson MV. Farm animal models applicable for cellular and molecular skeletal muscle growth research. Basic Appl Myol. 1998;8:169–73. [Google Scholar]
- 12.Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, et al. Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci. 2010;6:691–9. doi: 10.7150/ijbs.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei S, Duarte MS, Zan L, Du M, Jiang Z, Guan L, et al. Cellular and molecular implications of mature adipocyte dedifferentiation. J Genomics. 2012;1:5–12. doi: 10.7150/jgen.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, et al. Skeletal muscle stem cells from animals I. Basic cell biology. Int J Biol Sci. 2010;6:465–74. doi: 10.7150/ijbs.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodson MV, Hausman GJ. Metabolic syndromes: Resolving a malady that involves numerous tissues, cells, regulators and regulatory pathways. J Metabol Syndro. 2012;1:e101. [Google Scholar]
- 16.Dodson MV, Mir PS, Hausman GJ, Guan LL, Du M, Jiang Z, et al. Obesity, metabolic syndrome, and adipocytes. J Lipids. 2011;2011:721686. doi: 10.1155/2011/721686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodson MV, Jiang Z, Du M, Hausman GJ. Adipogenesis: it is not just lipid that comprises adipose tissue. J Genomics. 2012;1:1–4. doi: 10.7150/jgen.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernyhough ME, Bucci LR, Hausman GJ, Antonio J, Vierck JL, Dodson MV. Gaining a solid grip on adipogenesis. Tissue Cell. 2005;37:335–8. doi: 10.1016/j.tice.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Hausman GJ, Dodson MV. Stromal vascular cells and adipogenesis: cells within adipose depots regulate adipogenesis. J Genomics. 2012;1:56–66. doi: 10.7150/jgen.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood) 2010;235:1185–93. doi: 10.1258/ebm.2010.010063. [DOI] [PubMed] [Google Scholar]
- 21.Vierck JL, McNamara JP, Dodson MV. Proliferation and differentiation of progeny of ovine unilocular fat cells (adipofibroblasts) In Vitro Cell Dev Biol Anim. 1996;32:564–72. doi: 10.1007/BF02722983. [DOI] [PubMed] [Google Scholar]
- 22.Vierck JL, McNamara JP, Dodson MV. Two alternative procedures to isolate adipofibroblasts from sheep skeletal muscle. Methods Cell Sci. 1996;18:309–14. doi: 10.1007/BF00127908. [DOI] [Google Scholar]
- 23.Fernyhough ME, Vierck JL, Dodson MV. Assessing a non-traditional view of adipogenesis: adipocyte dedifferentiation--mountains or molehills? Cells Tissues Organs. 2006;182:226–8. doi: 10.1159/000093970. [DOI] [PubMed] [Google Scholar]
- 24.Fernyhough ME, Helterline DL, Vierck JL, Hausman GJ, Hill RA, Dodson MV. Dedifferentiation of mature adipocytes to form adipofibroblasts: more than just a possibility. Adipocytes. 2005;1:17–24. [Google Scholar]
- 25.Dodson MV, Fernyhough ME, Vierck JL, Hausman GJ. Adipocytes may not be a terminally differentiated cell type: Implications for animal production. Anim Sci. 2005;80:239–40. [Google Scholar]
- 26.Fernyhough ME, Vierck JL, Hausman GJ, Mir PS, Okine EK, Dodson MV. Primary adipocyte culture: adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology. 2004;46:163–72. doi: 10.1007/s10616-005-2602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tholpady SS, Aojanepong C, Llull R, Jeong JH, Mason AC, Futrell JW, et al. The cellular plasticity of human adipocytes. Ann Plast Surg. 2005;54:651–6. doi: 10.1097/01.sap.0000158065.12174.40. [DOI] [PubMed] [Google Scholar]
- 28.Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–74. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 29.Dodson MV, Hausman GJ, Guan LL, Jiang Z, Du M. Potential impact of mature adipocyte dedifferentiation in terms of cell numbers. Inter J Stem Cells. 2011;4:76–8. doi: 10.15283/ijsc.2011.4.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, et al. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355–65. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 31.Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565–75. doi: 10.1016/j.yjmcc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780–5. doi: 10.1016/j.bbrc.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–22. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 34.Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008;332:435–46. doi: 10.1007/s00441-008-0593-9. [DOI] [PubMed] [Google Scholar]
- 35.Nobusue H, Kano K. Establishment and characteristics of porcine preadipocyte cell lines derived from mature adipocytes. J Cell Biochem. 2010;109:542–52. doi: 10.1002/jcb.22431. [DOI] [PubMed] [Google Scholar]
- 36.Ono H, Oki Y, Bono H, Kano K. Gene expression profiling in multipotent DFAT cells derived from mature adipocytes. Biochem Biophys Res Commun. 2011;407:562–7. doi: 10.1016/j.bbrc.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 37.Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct. 2008;33:211–22. doi: 10.1247/csf.08038. [DOI] [PubMed] [Google Scholar]
- 38.Dodson MV, Fernyhough ME. Mature adipocytes: are there still novel things that we can learn from them? Tissue Cell. 2008;40:307–8. doi: 10.1016/j.tice.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Dodson MV, Jiang Z. Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells. Int J Biol Sci. 2010;6:80–8. doi: 10.7150/ijbs.6.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodson MV, Wei S, Duarte M, Du M, Jiang Z, Hausman GJ, et al. Cell Supermarket: Adipose Tissue as a Source of Stem Cells. J Genomics. 2012;1:39–44. doi: 10.7150/jgen.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs. 2008;188:359–72. doi: 10.1159/000134007. [DOI] [PubMed] [Google Scholar]
- 42.Fernyhough ME, Hausman GJ, Guan LL, Okine E, Moore SS, Dodson MV. Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun. 2008;368:455–7. doi: 10.1016/j.bbrc.2008.01.113. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Guridi M, Fernyhough ME, Jiang Z, Guan L, Hausman G, et al. Initial differences in lipid processing leading to pig- and beef-derived mature adipocyte dedifferentiation. Basic Appl Myol. 2009;19:243–6. [Google Scholar]
- 44.Chen J, Guridi M, Fernyhough ME, Jiang Z, Guan L, Hausman GJ, et al. Clonal mature adipocyte production of proliferative-competent daughter cells requires lipid export prior to cell division. Inter J Stem Cells. 2009;2:76–9. doi: 10.15283/ijsc.2009.2.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romao JM, Jin W, Dodson MV, Hausman GJ, Moore SS. Guan le L. MicroRNA regulation in mammalian adipogenesis. Exp Biol Med. 2011;236:997–1004. doi: 10.1258/ebm.2011.011101. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi M, Guan LL, Basarab JA, Dodson MV, Moore SS. Comparative analysis of gene expression profiles in subcutaneous fat tissues of beef cattle. Comp Biochem Physiol Part D Genomics Proteomics. 2008;3:251–6. doi: 10.1016/j.cbd.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Fernyhough ME, Okine E, Hausman G, Vierck JL, Dodson MV. PPARgamma and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007;33:367–78. doi: 10.1016/j.domaniend.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Dodson MV, Vierck JL, Hausman GJ, Guan LL, Fernyhough ME, Poulos SP, et al. Examination of adipose depot-specific PPAR moieties. Biochem Biophys Res Commun. 2010;394:241–2. doi: 10.1016/j.bbrc.2010.02.170. [DOI] [PubMed] [Google Scholar]
- 49.Kokta TA, Dodson MV, Gertler A, Hill RA. Intercellular signaling between adipose tissue and muscle tissue. Domest Anim Endocrinol. 2004;27:303–31. doi: 10.1016/j.domaniend.2004.05.004. [DOI] [PubMed] [Google Scholar]