Abstract
Mesenchymal stromal cells (MSCs) derived from adipose tissue have immunomodulatory effects, suggesting that they may have therapeutic potential for crescentic GN. Here, we systemically administered adipose-derived stromal cells (ASCs) in a rat model of anti-glomerular basement membrane (anti-GBM) disease and found that this treatment protected against renal injury and decreased proteinuria, crescent formation, and infiltration by glomerular leukocytes, including neutrophils, CD8+ T cells, and CD68+ macrophages. Interestingly, ASCs cultured under low-serum conditions (LASCs), but not bone marrow-derived MSCs (BM-MSCs), increased the number of immunoregulatory CD163+ macrophages in diseased glomeruli. Macrophages cocultured with ASCs, but not with BM-MSCs, adopted an immunoregulatory phenotype. Notably, LASCs polarized macrophages into CD163+ immunoregulatory cells associated with IL-10 production more efficiently than ASCs cultured under high-serum conditions. Pharmaceutical ablation of PGE2 production, blocking the EP4 receptor, or neutralizing IL-6 in the coculture medium all significantly reversed this LASC-induced conversion of macrophages. Furthermore, pretreating LASCs with aspirin or cyclooxygenase-2 inhibitors impaired the ability of LASCs to ameliorate nephritogenic IgG-mediated renal injury. Taken together, these results suggest that LASCs exert renoprotective effects in anti-GBM GN by promoting the phenotypic conversion of macrophages to immunoregulatory cells, suggesting that LASC transfer may represent a therapeutic strategy for crescentic GN.
Mesenchymal stromal cells (MSCs; formally known as mesenchymal stem cells) derived from cord blood, bone marrow, connective, and adipose tissues have the capacity to differentiate into multiple mesenchymal lineages, including osteoblasts, chondrocytes, and adipocytes.1,2 Apart from the classic regenerative property of MSCs, mounting evidence from studies focusing on bone marrow–derived MSCs (BM-MSCs) suggests that MSCs can modulate inflammatory immune responses.3–8 This effect is currently believed to be mediated through MSC-derived growth factors, cytokines, and PGs, which negatively regulate inflammatory immune responses and the proliferation of leukocytes and resident cells that are systemically or locally activated.9–14
As a potential clinical therapeutic agent, ASCs may have a number of practical advantages over BM-MSCs relating to their abundance and availability.15,16 Several studies have demonstrated ASC-mediated immunomodulation of particular leukocyte subsets, including lymphocytes and dendritic cells.14,17,18 This immunomodulatory property of ASCs has already been exploited for therapeutic intervention in inflammatory diseases. A number of clinical trials are underway in which ASCs have been administered to patients with autoimmune disorders, including graft versus host disease, Crohn’s disease, multiple sclerosis, and SLE.19–21 However, the precise mechanism of ASC-mediated immunomodulation is unclear, and a direct comparison of the efficacy of ASCs versus that of BM-MSCs has not been undertaken.
For cell transfer therapy, a reduction in the concentration of serum in MSC cultures is beneficial for recipients because this reduces concerns about infection with microorganisms or pathogenic proteins originating from culture media. However, the concentration of serum in culture media influences MSC expansion ex vivo, and thus affects cell proliferation. Moreover, the serum concentration modulates regeneration and immunomodulation.14,15 For instance, the proliferation rate of rat BM-MSCs in low-serum media is significantly lower than in high-serum media.14,15 In contrast, human ASCs grown under low-serum conditions (LASCs) display comparable growth to human BM-MSCs cultured in high-serum media.14,15 Of further interest, human LASCs more effectively suppress phytohemagglutinin-stimulated T cell proliferation in vitro than ASCs grown under high-serum conditions (HASCs), despite a similar ability of the two cell types to differentiate into the mesenchymal cell lineage.14 Therefore, LASC-mediated immunomodulation may have great potential as a cell-based therapy.
Anti-GBM GN is characterized by poor prognostic GN with crescent formation (crescentic GN [CGN]), which rapidly results in renal failure after onset of the disease.22 This is characteristic of Goodpasture’s disease in humans, in which patients may also occasionally present with pulmonary hemorrhage, with dire consequences. Although effector responses by neutrophils, macrophages/monocytes, T lymphocytes, and immune regulation by regulatory T cells and alternatively activated macrophages have been extensively investigated as part of efforts to understand the distinct mechanisms that cause glomerular crescent formation,23–29 a potent and feasible therapeutic strategy to inhibit leukocyte effector functions and/or augment their regulatory functions has not emerged for CGN patients. Thus, in this study, we investigated the potential of ASCs to exert immunomodulatory functions in anti-GBM GN.
Results
Administration of ASCs Ameliorates Disease Activity in a Rat Anti-GBM GN Model
The anti-inflammatory properties of ASCs were compared with those of BM-MSCs in a rat anti-GBM GN model. We administered 2.0×106 BM-MSCs or ASCs cultured under high-serum conditions (20% FBS) (HASCs) to TF78-treated WKY/NCrj rats for 6 consecutive days. Elevations in BUN, serum creatinine (sCr), and proteinuria in BM-MSC–treated rats were comparable with those in untreated rats. In contrast, HASC treatment significantly attenuated renal dysfunction and proteinuria compared with the BM-MSC–treated and control groups (Figure 1, A and B). Renal histologic analysis on day 7 revealed milder glomerular crescent formation and tubular damage in HASC-treated animals than in BM-MSC–treated rats (Figure 1, C and D). Moreover, a marked increase in the CD163+ macrophage population, which represents alternatively activated M2 macrophages,30,31 was observed in HASC-treated rats versus the BM-MSC–treated group (Figure 1E). Because the number of infiltrated CD68+ cells in the glomerulus (which represent infiltrated macrophages or dendritic cells) was similar between the BM-MSC–treated and HASC-treated groups (Figure 1E), we hypothesized that ASCs may exert their function by phenotypic conversion of macrophages into immunoregulatory cells in inflamed glomeruli after anti-GBM GN induction.
Figure 1.
Systemic administration of BM-MSCs and HASCs has a protective effect against rat anti-GBM GN. (A) Analysis of renal function in BM-MSC–treated and HASC-treated rats after induction of anti-GBM GN. The levels of both BUN (left) and sCr (right) at day 7 after TF78 injection are significantly reduced in the HASC-treated group (n=7) compared with the PBS-treated (n=7) and BM-MSC–treated (n=13) groups. Dotted line indicates the value in healthy rats (n=3). (B) 16-hour proteinuria value on day 5 in the HASC-treated group (n=7) compared with the PBS-treated (n=7) and BM-MSC–treated (n=6) groups. (C and D) Representative micrographs of PAS-stained renal sections harvested on day 7. Histologic scores for glomerular crescent formation (C) and tubular injury (D) in the indicated groups (n=6–7 per group) at day 7 are determined semiquantitatively. Dotted areas indicate glomerular crescent formation. (E) Representative micrographs of glomeruli stained with specific monoclonal IgGs for rat CD68 (upper) and CD163 (lower) on day 7 after disease induction. Accumulation of CD68+ and CD163+ (arrowheads) macrophages per glomerulus at day 7 is also determined. All data are mean ± SD. *P<0.01 and **P<0.05 as determined by ANOVA. Scale bars indicate 50 μm, except for the micrograph illustrating tubular injury, in which the bar indicates 200 μm. PAS, periodic acid–Schiff.
Low-Serum Culture Conditions Amplify the Renoprotective Effects of ASCs
Our previous reports demonstrated the therapeutic superiority of LASC transfer versus HASC transfer in several animal models.14,15,32 Systemic or local administration of LASCs improved rat ischemic hind limb injury15 and folic acid–induced acute renal tubular injury,32 and impeded production of antibodies against exogenous porcine red blood cells.14 Moreover, LASCs produce higher amounts of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) in vitro than HASCs (Supplemental Figure 1C). Therefore, we examined whether LASCs exhibit enhanced renoprotective effects compared with HASCs in anti-GBM GN. Administration of LASCs to TF78-treated rats significantly improved survival over the 28-day observation period (Figure 2A). Functional analysis of the diseased kidneys also demonstrated a dramatic reduction in BUN, sCr, and proteinuria in LASC-treated versus HASC-treated rats (Figure 2B). Despite comparable TF78 deposition on the GBM in both HASC- and LASC-treated groups (Supplemental Figure 2), histologic analysis clearly demonstrated that glomerular crescent formation was significantly reduced on days 4, 7, and 14 in LASC-treated rats compared with the HASC-treated group (43% decrease in glomerular crescent formation in HASC-treated rats versus a 75% decrease in LASC-treated rats compared with the control group on day 4; Figure 2C). The decrease in the number of CD68+ glomerular macrophages was more significant in LASC-treated rats, and a significant increase in the number of glomerular CD163+ macrophages was observed in LASC-treated rats over the entire study period compared with the HASC-treated and control groups (Figure 2E).
Figure 2.
Comparison of HASC and LASC administration for ASC-mediated protection against anti-GBM GN. (A) Survival curves for LASC-treated (n=14) and untreated (n=13) rats after anti-GBM antibody injection. (B) Renal function is assessed by determining BUN and sCr concentrations in PBS-treated (n=7), HASC-treated (n=7), and LASC-treated (n=8) anti-GBM GN rats on days 7 and 14. Dotted lines indicate value in healthy rats (n=3). The concentration of protein in the urine of rats in each group measured on days 3, 5, and 14 (n=7 per group). (C and D) Representative micrographs of kidney sections stained with PAS on day 4 to assess crescent formation (C) and on day 7 to assess tubular injury (D). Histologic scores for glomerular crescent formation and tubular injury are determined on days 4, 7, and 14 in the indicated groups (n=6–8 per group). Dotted areas indicate glomerular crescent formation. (E and F) Representative micrographs of glomeruli at day 4 and the interstitium at day 7, stained with specific antibodies against CD68+ and CD163+ macrophages. Glomerular (E) and interstitial (F) accumulation of CD68+ and CD163+ cells at indicated time points (n=7–8 per group). All data are mean ± SD. *P<0.01 and **P<0.05 as determined by ANOVA. (G) Representative micrographs of glomeruli obtained by double immunostaining for CD163 (green) and CD206 (red) at day 4 after disease induction with LASC treatment. The inset shows CD163+CD206+ macrophages under high-power magnification. (H) Representative micrographs illustrating double immunostaining for IL-10 (green) and CD163 (red) or CD206 (red) and quantification of CD163+ or CD206+ cells expressing IL-10 at day 4 after disease induction with LASC treatment. Arrowheads indicate IL10+CD163+ cells in the glomerulus and the inset shows IL-10+CD163+ cells under high-power magnification (n=4 per group). All data are mean ± SD. **P<0.05 as determined by t test. Scale bars, 50 μm in C and E; 100 μm in F; 200 μm in D. PAS, periodic acid–Schiff.
In addition to amelioration of glomerular damage, renal tubular injury was also attenuated by LASC treatment at days 4 and 7 (Figure 2D). Notably, there was no significant difference in the accumulation of CD163+ macrophages in the renal interstitium between the experimental groups, despite the reduced number of CD68+ cells in the LASC-treated group (Figure 2F). Moreover, circulating monocytes isolated from LASC-treated rats with anti-GBM GN did not exhibit an increase in cytosolic or surface levels of CD163 compared with circulating monocytes isolated from diseased rats treated with PBS (Supplemental Figure 3). Together, these data indicate that ASCs promote an increase in the number of CD163+ cells specifically and locally at inflamed glomeruli.
Immunohistochemical analysis of diseased glomeruli revealed that the expression of CD206, another specific marker for alternatively activated M2 macrophages,31,33 did not specifically overlap with that of CD163 (Figure 2G). This is suggestive of heterogeneous differentiation of macrophages into M2 cells in diseased glomeruli.30 We then investigated whether CD163+ or CD206+ cells produce IL-10, a representative anti-inflammatory cytokine induced by LASC treatment, and confirmed that expression of IL-10 is higher in CD163+ cells than in CD206+ cells in diseased glomeruli (Figure 2H).
It has been proposed that glomerular inflammation in anti-GBM GN is a multistep process that includes glomerular recruitment of leukocytes such as neutrophils, monocytes/macrophages, and lymphocytes, as well as production of proteinases, cytokines/chemokines, and oxygen radicals by inflammatory cells.23–26,34,35 Histologic assessment of neutrophils and T cells in diseased glomeruli after TF78 injection revealed a decrease in the accumulation of neutrophils and CD8+ T cells, but not CD4+ T cells, in both HASC- and LASC-treated rats; however, these differences were limited to day 1 (Supplemental Figure 4). Together, these results indicate that during the initial phase, LASCs prevent recruitment of broad leukocyte subsets, including CD8+ T cells, neutrophils, and CD68+ macrophages, and then play an anti-inflammatory role in subsequent phases by promoting an increase in the number of CD163+ macrophages in glomeruli.
Profiles of IL-1β, IL-12 p70, and IL-10 Cytokines in the Renal Cortex of Diseased Kidneys of Rats Treated with ASCs
One subtype of alternatively activated M2 macrophages is known to play an immunoregulatory role by generating IL-10, an immunosuppressive cytokine. To clarify whether ASC-mediated attenuation of rat anti-GBM GN results from a phenotypic switch of renal macrophages to CD163+ cells, we examined cytokine profiles in the renal cortex of diseased animals. Renal concentrations of IL-1β and IL-12 p70, representative proinflammatory cytokines, were significantly decreased in LASC-treated animals at day 4 after disease induction, but no differences in the concentrations of these cytokines were observed on day 7 compared with the HASC- and PBS-treated groups. In contrast, higher IL-10 levels were observed in the LASC-treated group than in both the HASC-treated and control groups on both days 4 and 7 (Figure 3A). Notably, solid correlations existed between the IL-10 concentration and the number of glomerular CD163+ infiltrating cells and sCr concentration in all experimental groups (Figure 3, B and C). These results strongly suggest that administration of ASCs, in particular LASCs, protects against TF78-mediated renal damage by converting macrophages from an inflammatory to an immunoregulatory phenotype.
Figure 3.
Renal cytokine profiles in ASC-treated anti-GBM GN rats. (A) Concentrations of the proinflammatory cytokines IL-1β and IL-12p70 and the anti-inflammatory cytokine IL-10 in the kidneys of PBS-treated, HASC-treated, and LASC-treated anti-GBM GN rats. Cytokine concentrations in nanograms per milligram of total protein in homogenates from renal cortex are determined. All data are mean ± SD. *P<0.01 as determined by ANOVA (n=7–8 per group). (B and C) Linear regression analysis reveals a tight correlation between renal IL-10 concentration and glomerular accumulation of CD163+ macrophages at days 4 and 7 (B) (r2=0.77) and sCr (C) (r2=0.66) at day 7. Each dot represents an individual kidney sample from a PBS-treated, HASC-treated, and LASC-treated rat.
Recruitment of HASCs and LASCs into Diseased Kidneys Is Comparable
It is important to demonstrate the accumulation of circulating exogenous ASCs in inflamed organs where their therapeutic potential is observed. Therefore, we examined the delivery of carboxyfluorescein succinimidyl ester (CFSE)–treated ASCs into diseased rat kidneys. Intravenous administration of ASCs via the tail vein led to their entrapment in the liver and lung at day 5, because these are the first organs encountered and possess large vascular beds. After day 5, the cells were distributed in multiple organs (Figure 4A and Supplemental Figure 5). CFSE+ cells were observed primarily in the glomeruli but were also present in glomerular crescents and peritubular capillaries on day 5 (Figure 4A); however, they were not evident from day 14 onward (Figure 4B). Importantly, no difference between HASC- and LASC-treated rats with respect to the number of glomerular CFSE+ cells was observed at day 5 (Figure 4C), suggesting that differential accumulation of HASCs and LASCs in the kidney cannot explain the differential renoprotective effects of these two cell populations.
Figure 4.
Recruitment of transferred ASCs in the kidneys of anti-GBM GN rats. (A) CFSE-stained LASCs are administered to anti-GBM GN rats on days 0–4 via the tail vein, and the kidneys are harvested on day 5. Exogenous LASCs (arrowheads) in frozen sections are identified using a polyclonal anti-CFSE antibody. In kidneys, CFSE+ cells are observed in the glomeruli, glomerular crescents, and interstitium. (B) Glomerular accumulation of CFSE+ cells at days 5, 14, and 28 is shown. (C) A representative immunofluorescence image of kidney tissue from an LASC-treated anti-GBM GN rat on day 5 (left). Dotted area indicates a glomerulus. Glomerular accumulation of CFSE+ cells (arrowheads) from HASC- or LASC-treated rats is analyzed (right). No significant difference is observed between HASC-treated and LASC-treated rats with respect to the number of glomerular CFSE+ ASCs. Data are mean ± SD (n=4 per group). Scale bar, 50 μm.
LASCs Directly Promote Functional Polarization of Macrophages into Immunoregulatory M2 Cells
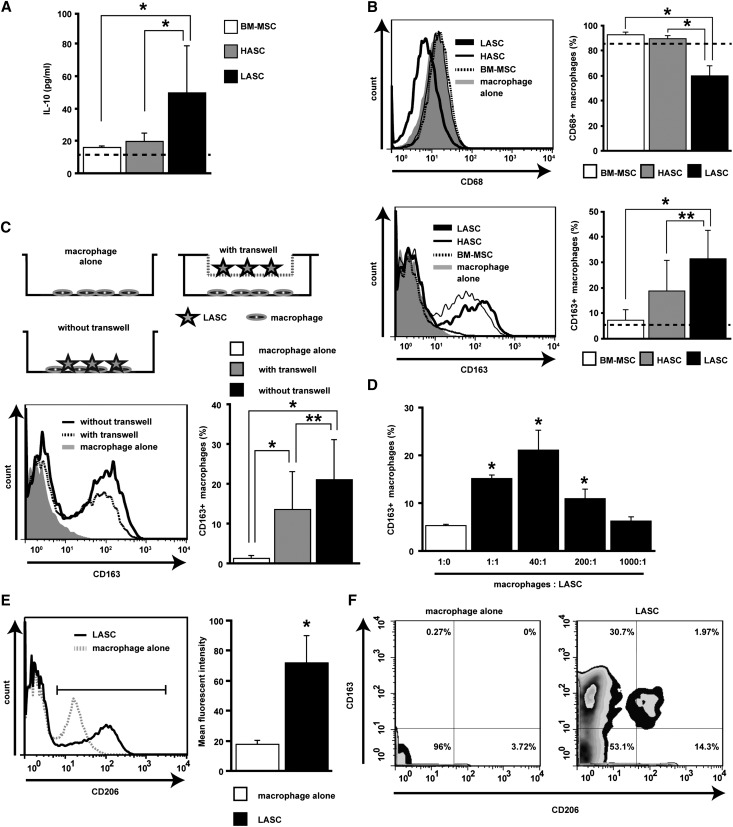
We conducted in vitro studies to explore the possibility that LASCs can directly polarize macrophages into an immunoregulatory phenotype. In a coculture system involving MSCs and rat peritoneal macrophages, LASCs strongly induced IL-10 production and enhanced the number of CD163+ macrophages compared with BM-MSCs and HASCs (Figure 5, A and B). LASC-mediated induction of CD163 expression on macrophages was also evident by real-time imaging (Supplemental Figure 6). However, the number of CD68+ macrophages significantly decreased when cocultured with LASCs (Figure 5B). LASCs also induced expression of CD206, another marker of alternatively activated M2 macrophages,31,33 but the proportion of CD206high+CD163+ cells was small (around 6%) in the macrophage population cocultured with LASCs (Figure 5, E and F).
Figure 5.
ASC-mediated functional polarization of macrophages into immunoregulatory cells. (A) IL-10 concentration in culture supernatants of peritoneal macrophages cultured for 48 hours with BM-MSCs, HASCs, or LASCs at a 20:1 ratio. Dotted line indicates value in macrophages cultured alone (n=9–13 per group). (B) Expression of CD68 (upper) and CD163 (lower) on peritoneal macrophages cultured with MSCs at a 2:1 ratio is evaluated by flow cytometry. The percentage of CD68+ and CD163+ cells is determined. The population of CD68+ macrophages is significantly reduced and that of CD163+ macrophages is significantly increased when macrophages are cultured with LASCs compared with macrophages cultured with BM-MSCs or HASCs (n=7 per group). Dotted line represents value for macrophages cultured alone (n=7). (C) Macrophage polarization into M2 cells after direct and indirect contact with LASCs. A trans-well plate system prevents direct contact between macrophages and LASCs. Macrophages are cultured on the bottom of the plate and LASCs are cultured in the upper well at a 2:1 ratio. LASC-mediated CD163 expression on macrophages is evaluated using trans-well plates, and the percentage of CD163+ cells is determined. Macrophages with or without LASCs were subjected to positive and baseline control, respectively (n=7 per group). (D) Analysis of LASC-mediated polarization of macrophages into CD163+ cells under different cell ratios. The percentage of CD163+ cells is determined. (E) CD206 induction on macrophages cocultured with LASCs (left). Mean fluorescence intensity of CD206+ cells (right) is evaluated in the indicated gate. (F) Quadrants and numbers indicate percentage of cells from each gate of CD163+ and/or CD206+ macrophages alone (left) or with LASCs (right) (n=4 per group). All data are mean ± SD. *P<0.01 and **P<0.05 as determined by ANOVA.
Next, we cultured peritoneal macrophages with LASCs in a trans-well plate system that prevents close contact between the LASCs and macrophages in order to determine whether cell-to-cell contact is needed for polarization of macrophages to CD163+ cells. Induction of CD163 expression on macrophages was observed in this culture system, indicating that LASC-derived soluble factors play a role in macrophage M2 polarization. However, a further increase in the number of CD163+ macrophages was observed in the absence of the membrane insert, suggesting that cell contact enhances this process (Figure 5C). Although LASCs injected in vivo successfully accumulated in the glomeruli, the number of cells was relatively small. To evaluate the efficiency with which LASCs induce a phenotypic change in macrophages, we incubated peritoneal macrophages with LASCs at ratios ranging from 1:1 to 1000:1. Remarkably, an individual LASC could induce a phenotypic change on 200 macrophages (Figure 5D). Together, these data provide compelling evidence that a very small number of LASCs may be sufficient to promote polarization of macrophages into M2 cells in the inflamed glomerulus.
PGE2 Produced by LASCs Strongly Induces the Phenotypic Conversion of Macrophages into Immunoregulatory Cells
A variety of cytokines, growth factors, and PGs that pleiotropically affect tissue regeneration, cell proliferation, and immune modulation are produced by MSCs. In particular, PGE2 derived from BM-MSCs was recently described as playing a critical role in stimulating IL-10 production by macrophages.12 Therefore, we hypothesized that ASC-derived PGE2 may be a key modulator of macrophage conversion to an immunoregulatory phenotype in our anti-GBM GN experimental model. Despite comparable amounts of 15d-PGJ2, a metabolite of PGD2, in the supernatants of MSCs cultured with and without macrophages, LASCs cultured in the absence of macrophages constitutively secreted abundant amounts of PGE2 compared with BM-MSCs and HASCs (Figure 6A). Secretion of PGE2 by LASCs was even more prominent in cocultures with macrophages (Figure 6B). Moreover, pharmaceutical ablation of LASC-derived PGE2 synthesis by a cyclooxygenase-2 (COX-2) inhibitor or aspirin and blocking of the EP4 receptor (but not the PGD receptor) clearly impaired macrophage conversion to CD163+ cells in the coculture system (Figure 6, C–E). In vivo pharmaceutical ablation of LASC-derived PGE2 synthesis by a COX-2 inhibitor or aspirin also decreased the therapeutic potency of LASCs and the induction of glomerular macrophage polarization to CD163+ cells (Figure 7). Treatment with synthetic PGE2 alone resulted in a significant increase in the conversion of cultured macrophages into CD163+ cells (Figure 6F), but was less efficient than LASC treatment, suggesting that interaction between LASCs and macrophages as well as persistent stimulation or other humoral factors are also required.
Figure 6.
LASC-derived PGE2 is an essential soluble factor that promotes polarization of macrophages toward immunoregulatory cells in vitro. (A and B) The concentrations of 15d-PGJ2, a metabolite of PGD, and PGE2 in culture supernatants of MSCs cultured alone (A) or with peritoneal macrophages at a 1:2 ratio (B) are measured. Dotted line indicates the value in medium (A) and in macrophage culture supernatant (B). LASCs constitutively secrete a higher amount of PGE2 than BM-MSCs or HASCs do, and this is enhanced in coculture with macrophages (n=4 in each group). (C and D) The Cox-2 inhibitor CAY10404 (C) and aspirin (D) impaired PGE2 excretion by LASCs cultured with macrophages in a dose-dependent manner. Reduction of LASC-mediated macrophage polarization by CAY10404 (C) and aspirin (D) is also evaluated by flow cytometry as macrophage CD163 expression. Representative histograms and percentage of CD163+ macrophages incubated with CAY10404 or aspirin at indicated concentrations are shown. Dotted line indicates value in cultures containing macrophages alone. (E) The decrease in the CD163+ population is evaluated as the percentage of CD163+ cells cultured with each EP or PGD receptor antagonist compared with CD163+ cells cultured with the respective vehicle control (black column). (F) Macrophage polarization into CD163+ cells after a single stimulation with PGE2. CD163 expression on macrophages incubated for 48 hours with synthetic PGE2 at the indicated concentrations is determined by flow cytometry (n=4–5 per group). All data are mean ± SD. **P<0.05 as determined by ANOVA.
Figure 7.
Pharmaceutical ablation of PGE2 synthesis abrogated the therapeutic potency of LASCs. (A–C) LASCs pretreated with 50 μM CAY10404 or 1000 μM aspirin are transferred into anti-GBM GN rats. The 16-hour proteinuria level at day 5 (A), histologic score of glomerular crescent formation at day 7 (B), and glomerular accumulation (C) of CD68+ (left) and CD163+ (right) macrophages at day 7 are evaluated as described in the legend for Figure 2. Dotted lines indicate value in anti-GBM GN rats not subjected to LASC transfer (n=10). All data are mean ± SD. **P<0.05 compared with untreated LASC group as determined by ANOVA (n=7–10 per group).
LASC-Derived IL-6 Promotes Conversion of Macrophages to the Immunoregulatory Phenotype
As discussed above, LASC-derived PGE2 alone was not sufficient to cause conversion of macrophages to an immunoregulatory phenotype. Whereas reports indicate that IL-10, M-CSF, IL-4, and IL-13 promote differentiation of macrophages into M2 cells,31,36 these cytokines were largely absent in LASC supernatants in our study (data not shown). However, secretion of considerable amounts of IL-6 by LASCs was observed (Figure 8A). Interestingly, polarization of macrophages to CD163+ cells was significantly reversed by antibody neutralization of IL-6 in the culture supernatant, and was induced by IL-6 stimulation in vitro (Figure 8, B and C). These data suggest that besides PGE2, LASC-derived IL-6 may also mediate macrophage polarization.
Figure 8.
LASC-derived IL-6 is another soluble factor that promotes polarization of macrophages into CD163+ cells in vitro. (A) IL-6 concentration in the culture supernatants of BM-MSCs, HASCs, and LASCs incubated for 24 hours. (B) Neutralization of IL-6 using an anti-IL-6 antibody results in a dose-dependent decrease in the population of LASC-induced CD163+ macrophages compared with cultures treated with normal goat IgG (black column). (C) Recombinant IL-6 alone promotes the phenotypic switch of macrophages into CD163+ cells (n=5 per group). All data are mean ± SD. **P<0.05 as determined by ANOVA.
Discussion
Crescent formation is a hallmark of active renal disease and determines the outcome in patients with human GN, including anti-GBM GN, ANCA-associated GN, lupus nephritis, and IgA nephropathy. Although BM-MSC administration has been shown to ameliorate acute tubular injury induced by cisplatin or ischemia reperfusion,37,38 and subcapsular injection of ASCs in rat kidneys has been shown to reverse folic acid–induced acute tubular damage,32 the capacity of MSCs to ameliorate glomerular damage has not been demonstrated until this report. Here, we clearly demonstrate the therapeutic superiority of ASCs to BM-MSCs in ameliorating renal damage in a rat crescentic GN model that recapitulates aspects of human anti-GBM GN. In addition, we demonstrate that LASCs in particular attenuate neutrophil, CD8+ T cell, and CD68+ macrophage recruitment during the initial phases of the disease. We also show that LASCs promote the phenotypic switching of glomerular macrophages to immunoregulatory cells during later phases of anti-GBM GN, and that this switching is dependent on LASC-derived PGE2 and IL-6 (Figure 9). Together, these activities of LASCs reduce formation of the glomerular crescents that lead to progressive renal dysfunction and proteinuria.
Figure 9.
Model for LASC-mediated amelioration of anti-GBM GN. LASCs impair recruitment of neutrophils and CD8+ T cells into the glomerulus during the initial phase of anti-GBM GN and promote PGE2-EP4 receptor-dependent phenotypic conversion of infiltrating macrophages to immunoregulatory macrophages during subsequent phases of the disease. Other soluble factors, including IL-6, are also required for this process. The accumulation of a significant number of immunoregulatory macrophages in the glomeruli protect against development of proteinuria and glomerular crescent formation, which is critical for a positive prognosis in anti-GBM GN.
We showed that intravenous administration of LASCs, HASCs, or BM-MSCs significantly reduces total macrophage infiltration in diseased glomeruli. Phenotypic conversion of macrophages to CD163+ cells in diseased glomeruli was demonstrated in the LASC-treated group, but was less prominent after HASC treatment and was minimal in BM-MSC–treated animals. It is well known that CD163+ macrophages represent anti-inflammatory M2 macrophages,30,31,39–41 and we and others have clearly demonstrated colocalization of CD163 and cytosolic IL-10 in glomerular macrophages in anti-GBM GN.28,29 Furthermore, blockage of the angiotensin II receptor or treatment with statins has been shown to attenuate anti-GBM GN, together with augmentation of CD163+ glomerular macrophages.28,29 In our in vitro study, LASC treatment effectively increased the number of CD163+ and CD206+ cells and the level of IL-10 secretion, but only a small population of rat peritoneal macrophages expressed both CD206+ and CD163+ in our coculture system. In addition to our in vitro evidence, we found a greater increase in IL-10 secretion in CD163+ cells than in CD206+ cells in diseased glomeruli after LASC transfer. Therefore, we speculate that LASC-mediated conversion of macrophages to IL-10–producing CD163+ cells ameliorates glomerular injury in rat anti-GBM GN. In mice and humans, CD163+ and CD206+ macrophages are classified as anti-inflammatory M2c cells producing IL-10 and profibrotic M2a-like cells, respectively.30 Interestingly, M2a- or M2c-macrophage transfer has been shown to dramatically attenuate renal injury after mouse adriamycin nephropathy,42–44 suggesting both M2a and M2c macrophages have renoprotective effects. Considering the above evidence, a precise characterization of the M2 cell subtype induced by LASC treatment and an evaluation of the efficacy of adoptive transfer of CD163+ macrophages is needed for a more complete understanding of the therapeutic significance of LASC-mediated conversion of macrophages into CD163+ cells in anti-GBM GN.
However, it remains unclear whether LASC-induced CD163+ macrophages are directly involved in protecting against anti-GBM GN-induced renal damage. Previous studies involving sepsis models have shown that MSC administration ameliorates tissue injury and neutrophil infiltration into the kidney that are associated with a reduction in the respiratory burst of neutrophils exposed to formyl-Met-leu-Phe-OH (fMLP) in vitro.11,12 With respect to lymphocytes, MSCs have been shown to suppress CD8+ T cell proliferation and cytotoxic activity in cells stimulated with allogeneic peripheral blood lymphocytes, DCs, or phytohemagglutinin in vitro.3,45 In this study, it was clear that LASC treatment reduced glomerular infiltration of CD8+ T cells, neutrophils, and CD68+ macrophages. Therefore, it is possible that LASC-mediated inactivation of neutrophils, CD8+ T cells, and CD68+ macrophages, rather than polarization of CD163+ macrophages is associated with amelioration of anti-GBM GN.
Anti-GBM IgG activates leukocytes and glomerular endothelial cells to elevate local cytokine/chemokine production, and increases expression of adhesion molecules such as intercellular adhesion molecule-1 on leukocytes and endothelial cells in the glomerulus. Subsequent thrombus formation and collapse of capillaries can impair glomerular microcirculation24,46; consequently, circulating cells, including leukocytes, platelets, and presumably administrated ASCs as well, tightly adhere to glomerular capillaries and accumulate in diseased glomeruli. We found administered ASCs both in glomerular capillaries and crescents in which CD163+ cells were primarily observed. This proximity to macrophages might be associated with the phenotypic conversion of macrophages in inflamed glomeruli. Administered ASCs were also occasionally observed in the peritubular capillaries of diseased kidneys, but we found no evidence of direct contact between LASCs and CD163+ macrophages in the interstitial capillaries. Whereas interstitial accumulation of CD68+ macrophages and tubular injury were significantly reduced by LASC transfer, there was no difference between the LASC- and HASC-treated groups with respect to the number of interstitial CD163+ macrophages. Therefore, the renoprotective effect of LASCs against tubulointerstitial damage in anti-GBM GN is not dependent on macrophage phenotypic conversion, but rather on LASC-derived humoral factors such as HGF32 or on LASC-mediated amelioration of glomerular injury.
From results obtained using a mouse sepsis model, Németh et al. reported that BM-MSC–derived PGE2 is essential for IL-10 production by macrophages that downregulate the systemic inflammatory response.12 They demonstrated production of PGE2 by BM-MSCs after LPS stimulation only when the BM-MSCs were cocultured with macrophages after the LPS exposure. Our real-time observations of peritoneal macrophages cocultured with MSCs demonstrated that macrophages are attracted to and contact LASCs, and subsequently express CD163 on the cell surface. Of note, LASC-mediated PGE2 generation and subsequent conversion of M2 macrophages did not require LPS stimulation, indicating that LASCs possess enhanced therapeutic potential compared with BM-MSCs. Moreover, a moderate level of CD163 expression on macrophages was observed in the trans-well culture system in which macrophages and LASCs were not in direct contact, suggesting that humoral factors also contribute to macrophage polarization. This cell contact-independent effect of MSCs upon macrophages was largely absent with BM-MSCs in the sepsis model.12
Compared with HASCs, LASCs generated significant amounts of PGE2. Production of PGE2 by BM-MSCs was minimal. Interestingly, PGE2 levels were further enhanced when LASCs were cocultured with macrophages, implying that physical interaction between LASCs and macrophages affects LASC-derived PGE2 synthesis. Ablation of LASC-derived PGE2 production significantly diminished CD163 expression on macrophages in vitro, and impeded efficacy of LASCs against rat anti-GBM GN in vivo. On the other hand, phenotypic conversion of macrophages to CD163+ cells after a single treatment with PGE2 was not as striking as that resulting from LASC treatment. Therefore, additional signals may be required for induction of M2 polarization of macrophages by LASCs. In addition to its proinflammatory effects, MSC-derived IL-6 has been shown to exhibit anti-inflammatory functions, such as inhibition of DC differentiation and production of reactive oxygen species by neutrophils.11,47 More recently, it was shown that tumor-derived IL-6 and PGE2 promote M2 polarization.48 We found that LASC-derived IL-6 and PGE2 induce CD163+ macrophages in our in vitro coculture system. Although IL-6 could not be detected in sera of diseased animals, regardless of whether they were subjected to LASC transfer (unpublished data), LASC-derived IL-6 in inflamed sites of the kidneys may be effective for M2 cell polarization. Furthermore, limiting MSC-mediated immunomodulation to inflamed sites would be more attractive and beneficial for the reduction of adverse effects than would systemic administration of a therapeutic agent.
In conclusion, although previous studies involving disease models and clinical trials have demonstrated that BM-MSC administration can be beneficial,7,49–51 we could not demonstrate the therapeutic efficiency of BM-MSC treatment in anti-GBM GN, because the immunomodulatory ability of MSCs may be context dependent. Further investigations into the functional differences between ASCs and BM-MSCs will be valuable at better defining the suitability of one cell type versus the other for clinical applications. Immunosuppressive therapy with corticosteroids and cyclophosphamide remains the prevailing approach for treating CGN, but the potential for adverse effects such as infection and cytotoxicity restrict the use of these agents. Our study suggests that LASC administration may be a desirable and feasible therapeutic alternative to improve the prognosis of anti-GBM GN patients.
Concise Methods
Animals
WKY/NCrj female rats were purchased from Charles River Inc. (Yokohama, Japan); CAG-EGFP-transgenic Lewis rats were kindly provided by Mito Otsuki (Kyoto University, Kyoto, Japan). All experimental animals were housed at a constant temperature and humidity, with a 12-hour light/dark cycle, and had unrestricted access to a standard diet and tap water in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For 16-hour collection of urine, animals were housed in metabolic cages on days 3, 5, and 14 after disease induction. The experimental protocols were in accordance with the Animal Experimentation Guidelines of Nagoya University Graduate School of Medicine.
Cell Preparation
Ex Vivo Expansion of MSCs from Rat Bone Marrow and Adipose Tissue
Isolation and expansion of BM-MSCs and ASCs from WKY/NCrj rats were performed as previously described.14,15,52 Adipose-derived stromal cells cultured in conventional high-serum (20% v/v) and low-serum (4% v/v) media were designated HASCs and LASCs, respectively. In addition, rat BM-MSC cultures were established in culture media containing 20% FBS. All three MSC types expressed CD44 (homing-associated cell adhesion molecule), CD54 (intercellular adhesion molecule-1), and CD90 (Thy-1), as previously demonstrated for rat MSCs,14 whereas they lacked surface expression of CD34 for hematopoietic stem cells and CD45 (leukocyte common antigen), a common marker for leukocytes (Supplemental Figure 1A). Rat BM-MSCs barely proliferated under low-serum (4%) culturing conditions (data not shown), whereas adipose-derived MSCs cultured under these conditions proliferated at rates comparable to BM-MSCs cultured under high-serum (20%) conditions (Supplemental Figure 1B). Notably, LASCs produced substantial amounts of VEGF and HGF (Supplemental Figure 1C).
Differentiation of MSCs into a Mesenchymal Lineage
Cultures of BM-MSCs, HASCs, and LASCs were examined using differentiation kits for adipocytes, chondrocytes, and osteoblasts (Invitrogen-Gibco, Carlsbad, CA). Adipocytes, chondrocytes, and osteoblasts were identified by oil red O, Alcian Blue, and alkaline phosphatase staining, respectively.1,15 All MSCs exhibited multipotency because they were competent for adipogenesis, osteogenesis, and chondrogenesis, as previously shown for human MSCs (Supplemental Figure 1D).15
Isolation of Peritoneal Macrophages
Peritoneal lavages as a source of rat macrophages were obtained by intraperitoneal injection of 50 ml of sterile saline. The abdomen was gently massaged before retrieval of the lavage. Mononuclear cells within the pellet resulting from centrifugation (400×g, 30 minutes) of the lavage were isolated using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO), and were then transferred to culture dishes. After overnight incubation at 37°C in a 5% CO2 atmosphere, floating cells were depleted from culture dishes and CD11b/c+ adhesive cells were isolated as macrophages.
Cell Culture Using a Trans-Well Plate System
A trans-well plate system (0.4-μm pore size; Costar, Boston, MA) was used to prevent close contact between LASCs and macrophages. The upper chamber contained 1×106 LASCs on an inserted membrane, and the bottom chamber contained 2×106 macrophages. Trans-well plates were incubated for 48 hours at 37°C in humidified air containing 5% CO2.
Flow Cytometry Analyses
Identification of BM-MSCs, HASCs, and LASCs
All antibodies were obtained from BD Biosciences Pharmingen (San Diego, CA) unless otherwise indicated. FITC mouse anti-rat CD34 (Santa Cruz Biotechnology, Santa Cruz, CA), PE mouse anti-rat CD45, FITC mouse anti-rat CD44H, PE mouse anti-rat CD54 (AbD Serotec, Oxford, UK), and FITC mouse anti-rat CD90 were used for identification of MSCs. Respective isotype controls, including PE mouse IgG1 for CD34 (Santa Cruz Biotechnology), PE mouse IgG1κ for CD45, FITC mouse IgG2aκ for CD44H, PE mouse IgG1 for CD54 (AbD Serotec), and FITC mouse IgG2aκ, were used as negative controls. Cells were acquired using a FACS Canto II flow cytometer (BD Biosciences).
Assessment of Functional Polarization of Macrophages
After 48-hour coculturing of 3×106 macrophages and 1.5×106 BM-MSCs, HASCs, or LASCs, adherent cells were subjected to flow cytometry analysis. Infiltrating macrophages and immunoregulatory macrophages were defined using mouse FITC anti-rat CD68 IgG1 (ED1; AbD Serotec) or mouse PE anti-rat CD163 IgG1 (ED2; AbD Serotec), and rabbit anti-rat CD206 IgG (mannose receptor; Abcam, Cambridge, MA), respectively, in cells gated by APC mouse anti-rat CD11b/c IgG2aκ (BioLegend, San Diego, CA). Intracellular staining using BUF09 (AbD Serotec) was essential for CD68 detection. The fluorescent PE signal for CD163 was enhanced using a FASER kit-PE (Miltenyi Biotec, Bergisch Gladbach, Germany). In all experiments, the Fcγ receptor was blocked using purified mouse anti-rat CD32 IgG1κ. Populations of CD68+ and CD163+ macrophages (M2) were evaluated as a percentage of CD11b/c+ cells.
Induction and Treatment of Rat Anti-GBM GN
Using a previously described method, we established a mouse monoclonal IgG clone designated TF78, which specifically binds to α4(IV)NC1 of the rat GBM to stably induce CGN.53 Intraperitoneal administration of TF78 induced the disease in WKY/NCrj rats in a dose-dependent manner (Supplemental Figure 7A), and treatment with 100 μg of TF78 led to progressive elevations in BUN, sCr, and proteinuria (Supplemental Figure 7B). At various time points, blood and urine samples were sent to Mitsubishi BCL Co. Ltd (Tokyo, Japan) and then BUN, sCr, and urine protein were measured by Mitsubishi BCL Co. Ltd. Histologic analyses of rat kidneys treated with TF78 demonstrated severe crescent formation in glomeruli together with linear IgG deposition in the GBM (Supplemental Figure 7, C and D). Unless otherwise indicated, 100 μg of TF78 was administered to each animal.
Assessment of Mouse IgG Deposition on the Rat GBM
Deposition of TF78 on the rat GBM was confirmed using frozen kidney sections and polyclonal rabbit FITC-labeled anti-mouse IgG (H+L) (Invitrogen). Deposition of IgG was semiquantitatively assessed by determining the end point positive titer for detection of staining using serial dilutions of anti-mouse IgG antibody ranging from 1:125 to 1:400027,54 (Supplemental Figure 2).
Transfer of BM-MSCs, HASCs, or LASCs for Treating Rat Anti-GBM GN
We administered 2×106 third to fifth passage BM-MSCs, HASCs, or LASCs in 2 ml of sterile PBS to each rat in the respective treatment group via the tail vein on days 0–5 after TF78 injection. Diseased rats in the control group received 2 ml of sterile PBS lacking cells.
Histologic Assessment of Glomerular Crescent Formation and Leukocyte Accumulation
Histologic evaluation of glomerular crescent formation was determined semiquantitatively on paraffin-embedded tissue sections using the periodic acid–Schiff staining method. The percentage of area occupied by crescents in each glomerulus was estimated and assigned one of the following scores: 0, no crescent; 1, 0%–25%; 2, 25%–50%; 3, 50%–75%; and 4, 75%–100% crescent occupation. The mean score was then calculated as the crescent score.55 Tubulointerstitial injury was defined as tubular dilation or atrophy, denudation of the tubular basement membrane, or tubular necrosis. Tubular injury scores were evaluated on a scale of 0–4 as follows: 0, no tubulointerstitial injury; 1, <25% injury; 2, 25%–50% injury; 3, 51%–75% injury; and 4, >75% injury. Buffered (1:100) formalin-fixed tissues were immunostained using mouse anti-rat CD68 monoclonal IgG1 (clone ED-1; BMA Biomedicals, Augst, Switzerland) as a marker for infiltrating macrophages or dendritic cells, mouse anti-rat CD163 monoclonal IgG1 (clone ED-2; BMA Biomedicals) as a marker for alternatively activated M2 macrophages, and rabbit anti-rat CD8 monoclonal IgG (Epitomics, Burlingame, CA) as a marker for cytotoxic T cells. Infiltration of glomerular neutrophils was assessed using the naphthol-AS-D chloroacetate (Sigma-Aldrich) esterase reaction, as described elsewhere.23 For each animal, crescent formation and the number of stained cells were evaluated in >40 glomeruli per renal cross-section. To evaluate the colocalization of CD163+ and CD206+ or IL-10 in glomerular cells, immunohistochemical staining using mouse biotin anti-rat CD163 IgG1 (ED2; AbD Serotec) and rabbit anti-rat CD206 IgG (mannose receptor; Abcam) or goat anti-rat IL-10 polyclonal IgG (R&D Systems, Minneapolis, MN) was performed after blocking endogenous biotin using an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). For the double stain of CD163 and CD206, the anti-CD163 antibody was detected by staining with Alexa488 streptavidin (Molecular Probes, Eugene, OR) followed by biotinylated anti-streptavidin (Vector Laboratories) to amplify the signal, and anti-CD206 antibody was detected by staining with Alexa555 goat anti-rabbit IgG (Molecular Probes). For the double stain of cytosolic IL-10 and CD163 or CD206, anti-IL-10 antibody was detected by staining with biotinylated donkey anti-goat IgG followed by FITC-conjugated avidin (Vector Laboratories) and anti-CD163 antibody or anti-CD206 antibody was detected by staining with Alexa555 streptavidin (Molecular Probes) or Alexa555 goat anti-rabbit IgG, respectively as described above. Biotinylated CD4 mAb (LifeSpan Biosciences, Seattle, WA) was used to stain CD4+ T cells according to the same method used for detection of CD163.
Determination of Growth Factor and Cytokine Concentrations
The concentrations of growth factors and cytokines were determined using ELISA kits, each used according to the manufacturer’s instructions. ELISA analyses of VEGF (IBL, Gunma, Japan), HGF (Institute of Immunology Co. Ltd., Tokyo, Japan), and IL-6 (Thermo Scientific, Rockford, IL) were performed on cultured MSCs at the fifth passage.14,15 For cytokine profiles in the kidney, the concentrations of IL-1β, IL-12 p70, and IL-10 were measured by ELISA (Invitrogen) in renal cortex homogenates. Secretion of IL-10 from 2×106 peritoneal macrophages cocultured with 0.1×106 MSCs was assessed for functional evaluation of M2 cells. Generation of PGE2 and 15d-PGJ2 by MSCs cultured with peritoneal macrophages at a 2:1 ratio or without peritoneal macrophages were determined by ELISA (Enzo Life Sciences, Farmingdale, NY).
Tracking of Intravenously Injected ASCs in Rat Organs
Rats were administered 2×106 LASCs stained with CFSE (Molecular Probes) on days 0–4 via the tail vein, and tissue samples were taken on days 5, 14, and 28. Uniform CFSE staining of LASCs was confirmed by flow cytometry analysis before injection (Supplemental Figure 5J). Cryostat tissue sections were stained with goat polyclonal anti-CFSE IgG (Molecular Probes) followed by a conjugate of rabbit anti-goat IgG and horseradish peroxidase–labeled polymer (Histofine Simple Stain; Nichirei, Tokyo, Japan) as a secondary reagent. For each animal, CFSE+ cells were counted in at least 100 glomeruli per renal cross-section.
Time-Lapse Recording of MSC-Mediated Polarization of Macrophages to CD163-Presenting Cells
BM-MSCs or LASCs from GFP transgenic rats were plated with GFP-negative macrophages from WKY/NCrj rats at a 1:2 ratio. Cells were harvested with culture medium containing mouse Alexa568-conjugated anti-rat CD163 IgG and maintained on a microscope slide for 30 hours at 37°C in a 5% CO2 atmosphere in a recording chamber. Isotype mouse IgG (BD Pharmingen) was used as a negative control. Both phase and fluorescent images were captured every 15 minutes using an LCV110 incubator microscope system (Olympus, Tokyo, Japan).
Western Blot Analyses
The concentration of each purified protein was measured using a protein quantification kit (Thermo Scientific). Protein from each sample (3 μg) was separated by SDS-PAGE on a NuPAGE 4%–12% Bis-Tris gel electrophoresed at 200 V using a mini-cell gel apparatus (Invitrogen). Separated proteins were then electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore, Bedford, MA) at 30 V for 720 minutes using a semidry transfer module. Nonspecific binding was blocked with Blocking One (Nacalai Tesque, Kyoto, Japan). Blots were then incubated for 60 minutes at room temperature with the appropriate primary antibody in antibody buffer containing 5% Blocking One in PBST (0.05% v/v Tween 20 in PBS), washed three times with PBST, and then incubated for 1 hour with a peroxidase-conjugated secondary antibody in the antibody buffer. After washing, the blots were developed for visualization using an enhanced chemiluminescence detection kit (ImmunoStar LD; Wako, Osaka, Japan). The primary antibodies and their titers were as follows: CD163, 1:10,000 (AbD Serotec); and β-actin, 1:500,000 (Sigma-Aldrich).
Pharmaceutical Ablation, Blocking, and Stimulation of PGE2 or IL-6
For the pharmaceutical ablation of PGE2 synthesis in LASCs, 1–100 μM selective COX-2 inhibitor (CAY10404; Cayman Laboratories, Ann Arbor, MI) or 10–1000 μM of aspirin (Cayman Laboratories) dissolved in ethanol was added to the cell culture medium. The duration of the effect of CAY10404 on LASCs is shown in Supplemental Figure 8. For in vivo analysis of PGE2-ablated LASCs, LASCs were incubated with 50 μM CAY10404 or 1 mM aspirin for 48 hours and then injected into anti-GBM GN rats as described above. For the evaluation of CD163 expression by macrophages induced by LASC-derived IL-6 and PGE2, IL-6 present in coculture medium along with LASCs and macrophages was neutralized by addition of 0.05–5 mg/ml of anti-IL-6 antibody (R&D Systems), or 0.5–50 ng/ml IL-6 (R&D Systems) and 0–1 μg/ml of synthetic PGE2 (Sigma-Aldrich) were added to the culture medium containing macrophages only and incubated for 48 hours.
Statistical Analyses
Statistical analyses were performed using SPSS 18.0 statistical software (SPSS Inc, Chicago, IL). The means and SDs were calculated for all parameters determined in this study. Statistical significance was evaluated by ANOVA to determine the significance of differences between experimental groups. When a statistically significant difference was indicated by ANOVA, further analysis was performed using Tukey’s test to determine the significance of differences between any pair of groups. A significant difference was defined as a P value <0.05.
Disclosures
None.
Acknowledgments
The authors thank Ms. N. Asano, Ms. Y. Sawa, and Mr. N. Suzuki for their excellent technical assistance. We also thank Dr. Tanya N. Mayadas (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) for insightful suggestions and for editing the manuscript. The authors thank Ono Pharmaceutical Co., Ltd. (Osaka, Japan) for supplying the EP1, EP3, and EP4 receptor antagonists.
This work was supported by a grant-in-aid for scientific research (C) from the Ministry of Education, Culture, Sports, Science, and Technology (T.O., N.T., and S.M.), and by a grant-in-aid for progressive renal diseases research, research on intractable diseases, from the Ministry of Health, Labour, and Welfare of Japan (Seiichi Matsuo and Shoichi Maruyama). This work was also supported by the Aichi Kidney Foundation (K.F., N.T., T.O., and S.M.) and the NAGONO Medical Promotion Foundation (K.F.), as well as a Molecular Nephrology Forum Award Japan (K.F.) and a Study for Renal Disease and Hypertension Award supported by the Research Foundation for Community Medicine, Japan (K.F.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012030264/-/DCSupplemental.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA: Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 2: 83–92, 1974 [PubMed] [Google Scholar]
- 3.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM: Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R: Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A: Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755–1761, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Parekkadan B, Tilles AW, Yarmush ML: Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells 26: 1913–1919, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O: Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363: 1439–1441, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C: Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 307: 1169–1177, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI, Dennis JE: Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076–1084, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F: Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105: 2821–2827, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V: Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 26: 151–162, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E: Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP: Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156: 149–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saka Y, Furuhashi K, Katsuno T, Kim H, Ozaki T, Iwasaki K, Haneda M, Sato W, Tsuboi N, Ito Y, Matsuo S, Kobayashi T, Maruyama S: Adipose-derived stromal cells cultured in a low-serum medium, but not bone marrow-derived stromal cells, impede xenoantibody production. Xenotransplantation 18: 196–208, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Iwashima S, Ozaki T, Maruyama S, Saka Y, Kobori M, Omae K, Yamaguchi H, Niimi T, Toriyama K, Kamei Y, Torii S, Murohara T, Yuzawa Y, Kitagawa Y, Matsuo S: Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cells Dev 18: 533–543, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH: Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 7: 211–228, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA: Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 24: 2582–2591, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS: Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett 126: 37–42, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Fang B, Song Y, Liao L, Zhang Y, Zhao RC: Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc 39: 3358–3362, 2007 [DOI] [PubMed] [Google Scholar]
- 20.García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA: A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 48: 1416–1423, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Casteilla L, Planat-Benard V, Laharrague P, Cousin B: Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells 3: 25–33, 2011 [DOI] [PMC free article] [PubMed]
- 22.Levy JB, Turner AN, Rees AJ, Pusey CD: Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 134: 1033–1042, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Tsuboi N, Asano K, Lauterbach M, Mayadas TN: Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity 28: 833–846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirahashi J, Hishikawa K, Kaname S, Tsuboi N, Wang Y, Simon DI, Stavrakis G, Shimosawa T, Xiao L, Nagahama Y, Suzuki K, Fujita T, Mayadas TN: Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation 120: 1255–1265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujinaka H, Yamamoto T, Feng L, Nameta M, Garcia G, Chen S, El-shemi AA, Ohshiro K, Katsuyama K, Yoshida Y, Yaoita E, Wilson CB: Anti-perforin antibody treatment ameliorates experimental crescentic glomerulonephritis in WKY rats. Kidney Int 72: 823–830, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J: Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 167: 1207–1219, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, Mayer G, Gunsilius E, Rosenkranz AR: CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol 16: 1360–1370, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Aki K, Shimizu A, Masuda Y, Kuwahara N, Arai T, Ishikawa A, Fujita E, Mii A, Natori Y, Fukunaga Y, Fukuda Y: ANG II receptor blockade enhances anti-inflammatory macrophages in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol 298: F870–F882, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Fujita E, Shimizu A, Masuda Y, Kuwahara N, Arai T, Nagasaka S, Aki K, Mii A, Natori Y, Iino Y, Katayama Y, Fukuda Y: Statin attenuates experimental anti-glomerular basement membrane glomerulonephritis together with the augmentation of alternatively activated macrophages. Am J Pathol 177: 1143–1154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anders HJ, Ryu M: Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsuno T, Ozaki T, Saka Y, Furuhashi K, Kim H, Yasuda K, Yamamoto T, Sato W, Tsuboi N, Mizuno M, Ito Y, Imai E, Matsuo S, Maruyama S: Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats [published online ahead of print September 7, 2012]. Cell Transplant doi:10.3727/096368912X655019 [DOI] [PubMed] [Google Scholar]
- 33.David S, Kroner A: Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12: 388–399, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki K, Yaoita E, Yamamoto T, Kihara I: Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int 41: 1517–1526, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Kovalenko P, Fujinaka H, Yoshida Y, Kawamura H, Qu Z, El-Shemi AG, Li H, Matsuki A, Bilim V, Yaoita E, Abo T, Uchiyama M, Yamamoto T: Fc receptor-mediated accumulation of macrophages in crescentic glomerulonephritis induced by anti-glomerular basement membrane antibody administration in WKY rats. Int Immunol 16: 625–634, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Gordon S, Martinez FO: Alternative activation of macrophages: Mechanism and functions. Immunity 32: 593–604, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG: Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK: Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sulahian TH, Högger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM, Guyre PM: Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12: 1312–1321, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Zhang ZY, Schluesener HJ: Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J Immunol 183: 3081–3091, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC: Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int 72: 290–299, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zheng D, Wang Y, Cao Q, Lee VW, Zheng G, Sun Y, Tan TK, Wang Y, Alexander SI, Harris DC: Transfused macrophages ameliorate pancreatic and renal injury in murine diabetes mellitus. Nephron, Exp Nephrol 118: e87–e99, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC: IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol 21: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, Ferrone S, Barnaba V, Pistoia V: Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells 26: 1275–1287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds J: Strain differences and the genetic basis of experimental autoimmune anti-glomerular basement membrane glomerulonephritis. Int J Exp Pathol 92: 211–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D: Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25: 2025–2032, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH: M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 187: 1157–1165, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A: IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 38: 1745–1755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S: Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol 11: 150–156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H: Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12: 459–465, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Maruyama S, Yamamoto T, Kamo I, Yasuda K, Saka Y, Ozaki T, Yuzawa Y, Matsuo S, Gotoh M: Increased urethral resistance by periurethral injection of low serum cultured adipose-derived mesenchymal stromal cells in rats. Int J Urol 18: 659–666, 2011 [DOI] [PubMed]
- 53.Kohda T, Okada S, Hayashi A, Kanzaki S, Ninomiya Y, Taki M, Sado Y: High nephritogenicity of monoclonal antibodies belonging to IgG2a and IgG2b subclasses in rat anti-GBM nephritis. Kidney Int 66: 177–186, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Kitching AR, Turner AL, Semple T, Li M, Edgtton KL, Wilson GR, Timoshanko JR, Hudson BG, Holdsworth SR: Experimental autoimmune anti-glomerular basement membrane glomerulonephritis: A protective role for IFN-gamma. J Am Soc Nephrol 15: 1764–1774, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Zhou A, Ueno H, Shimomura M, Tanaka R, Shirakawa T, Nakamura H, Matsuo M, Iijima K: Blockade of TGF-beta action ameliorates renal dysfunction and histologic progression in anti-GBM nephritis. Kidney Int 64: 92–101, 2003 [DOI] [PubMed] [Google Scholar]