Abstract
Little information exists regarding the efficacy, modifiers, and outcomes of anemia management in children with CKD or ESRD. We assessed practices, effectors, and outcomes of anemia management in 1394 pediatric patients undergoing peritoneal dialysis (PD) who were prospectively followed in 30 countries. We noted that 25% of patients had hemoglobin levels below target (<10 g/dl or <9.5 g/dl in children older or younger than 2 years, respectively), with significant regional variation; levels were highest in North America and Europe and lowest in Asia and Turkey. Low hemoglobin levels were associated with low urine output, low serum albumin, high parathyroid hormone, high ferritin, and the use of bioincompatible PD fluid. Erythropoiesis-stimulating agents (ESAs) were prescribed to 92% of patients, and neither the type of ESA nor the dosing interval appeared to affect efficacy. The weekly ESA dose inversely correlated with age when scaled to weight but did not correlate with age when normalized to body surface area. ESA sensitivity was positively associated with residual diuresis and serum albumin and inversely associated with serum parathyroid hormone and ferritin. The prevalence of hypertension and left ventricular hypertrophy increased with the degree of anemia. Patient survival was positively associated with achieved hemoglobin and serum albumin and was inversely associated with ESA dose. In conclusion, control of anemia in children receiving long-term PD varies by region. ESA requirements are independent of age when dose is scaled to body surface area, and ESA resistance is associated with inflammation, fluid retention, and hyperparathyroidism. Anemia and high ESA dose requirements independently predict mortality.
Almost three decades after the advent of recombinant erythropoietin, the management of renal anemia has become a recent focus of attention and changing paradigms. Whereas correction of hemoglobin (Hb) levels to near-normal has previously been recommended on the basis of association studies linking more severe anemia to morbidity and mortality with dialysis,1–3 interventional clinical trials consistently demonstrate that near-normalization of Hb increases the risk of vascular events and mortality in adults receiving maintenance hemodialysis and in those with CKD who are not undergoing dialysis.4–6 This has prompted ongoing reevaluation and revisions of treatment targets in patients exposed to erythropoiesis-stimulating agents (ESAs).7
The appropriateness of applying treatment recommendations established in adult hemodialysis populations at high cardiovascular risk and adults with CKD to children undergoing dialysis is questionable because cardiovascular events are far less common in children with CKD. Furthermore, two thirds of children requiring dialysis initially opt for peritoneal dialysis (PD), and there are no systematic studies in the adult PD population to inform the optimal Hb target range in these patients. The risk profile of patients receiving PD may differ from that of the hemodialysis setting because of the absence of dialysis-induced intermittent hemoconcentration and lack of contact activation of the complement and coagulation systems.
Further aspects to consider in pediatric anemia management are the greater physical activity of children and the need for optimal cognitive functioning at school.8,9 The significant physiologic variation of the normal Hb range with age10 and the relative ESA sensitivity that reportedly increases with age during early childhood are also noteworthy.11
The registry of the International Pediatric Peritoneal Dialysis Network (IPPN) prospectively collects detailed clinical, biochemical, dialysis, and medication-related information (including ESA types and doses and modalities of iron supplementation) from a substantial number of children undergoing long-term PD around the world. In-depth analysis of this unique database has allowed us to (1) gain insight into the demographic characteristics of renal anemia and its treatment in the pediatric PD population worldwide, (2) explore the relationship between ESA dose requirements and body dimensions, (3) identify factors contributing to ESA resistance in children, and (4) associate anemia control with patient outcomes.
Results
Patient Characteristics
Between April 2007 and April 2011, a total of 1394 pediatric patients age 1 month to 20 years (median age, 10.2 years; interquartile range [IQR], 3.9–14.4 years) were enrolled into the IPPN registry from 81 pediatric dialysis centers in 30 countries. Patient characteristics are given in Table 1. The final data set contained 1394+924+597+404+219+110+51+25+7 observations at 0, 6, 12, 18, 24, 30, 36, 42, and 48 months, respectively. The median follow-up time was 0.8 year (IQR, 0.22–1.56 years).
Table 1.
Characteristics of 1394 children enrolled in the IPPN registry
| Characteristic | n (%) |
|---|---|
| Male patients | 756 (54.2) |
| Primary renal diagnosis | |
| CAKUT | 646 (46.3) |
| Glomerulopathy | 384 (27.6) |
| Vasculitis | 127 (9.1) |
| Metabolic disease | 28 (2.0) |
| Ischemia | 29 (2.1) |
| Other/unknown | 180 (12.9) |
| Age at RRT start | |
| <1 yr | 221 (15.9) |
| 1–5 yr | 303 (21.7) |
| 6–11 yr | 397 (28.5) |
| 12–18 yr | 456 (32.7) |
| >18 yr | 17 (1.2) |
| Age at first observation | |
| <1 yr | 117 (8.4) |
| 1–5 yr | 342 (24.5) |
| 5–12 yr | 371 (26.6) |
| 12–18 yr | 510 (36.6) |
| >18 yr | 54 (3.9) |
| PD modality | |
| CAPD | 341 (24.5) |
| CCPD | 492 (35.3) |
| NIPD | 538 (38.6) |
| IPD | 23 (1.6) |
| Biocompatible PD fluid type | 458 (32.9) |
| ESA use | |
| No ESA | 116 (8.3) |
| Epoetin-α | 579 (41.5) |
| Epoetin-β | 482 (34.6) |
| Darbepoetin | 208 (14.9) |
| CERA | 7 (0.5) |
| Epoetin-δ | 2 (0.2) |
| Iron | |
| No iron | 225 (16.2) |
| Oral | 975 (69.9) |
| Intravenous | 154 (11.0) |
| Intravenous + oral | 40 (2.9) |
CAKUT, congenital anomalies of the kidney and urinary tract; RRT, renal replacement therapy; CAPD, continuous ambulatory PD; CCPD, continuous cycling PD; NIPD, nocturnal intermittent PD; IPD, intermittent PD: CERA, continuous erythropoietin receptor activator.
Follow-up was discontinued during the observation period in 684 patients because of kidney transplantation (453 patients [66% of all dropouts]), technique failure and switch to hemodialysis (131 patients [19%]), partial recovery of renal function (15 patients [2%]), transfer to adult care (39 patients [6%]), or death (46 patients [7%]). A total of 615 peritonitis episodes were reported in 340 of the 1394 patients. The mean peritonitis incidence rate was 0.43 episode per year.
Demographics of Anemia and Its Management
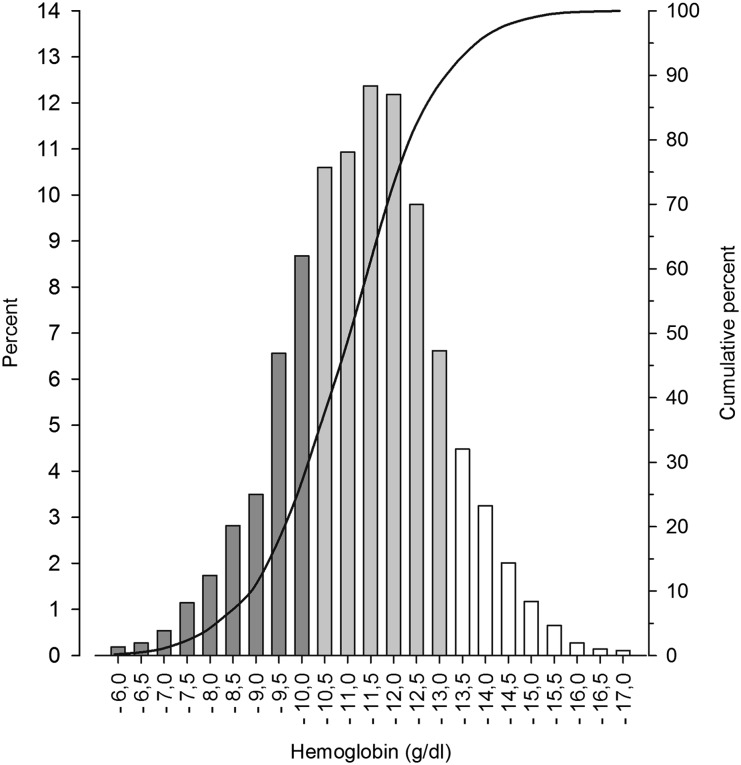
The mean Hb level ± SD was 11.0±1.1 g/dl; levels were <10 g/dl in 25.5% of measurements and >13 g/dl in 10.5% of measurements (Figure 1). Hb distribution was largely independent of age and sex, with the exception of slightly higher levels in pubertal boys (11.4±1.1 versus 10.9±1.1 g/dl, P<0.001).
Figure 1.
Distribution of mean Hb concentrations in 1394 pediatric patients undergoing long-term PD. Bars indicate the fraction of patients with mean HB within certain HB ranges.
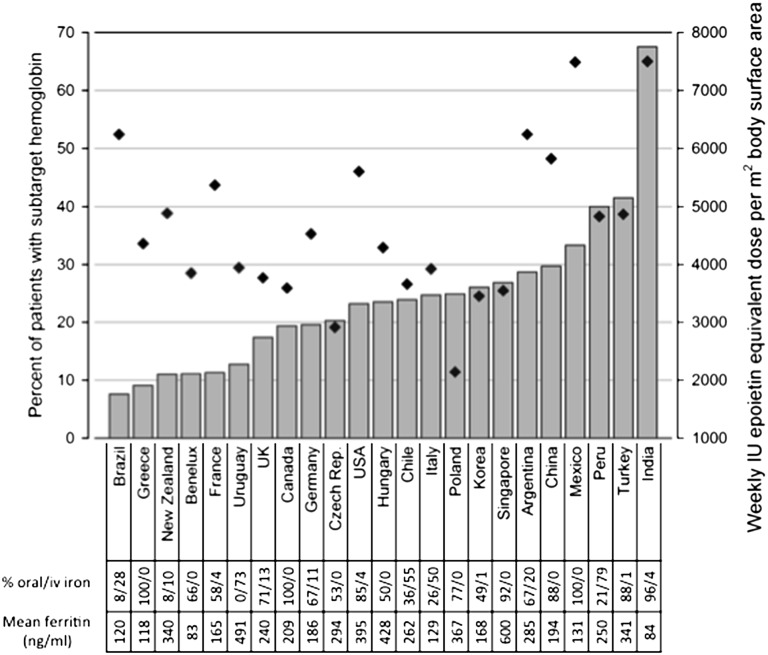
Anemia control varied regionally, with higher Hb levels in North America/Europe (11.2±1.1 g/dl) and Latin America (11.0±1.3 g/dl) than in Asian countries (10.8±1.2 g/dl) and Turkey (10.4±0.9 g/dl). Figure 2 shows the patient fractions with subtarget Hb by country of residence.
Figure 2.
Regional variation of anemia control. Bars indicate percentage of patients with subtarget Hb (<10 g/dl if 2 years and older, <9.5 g/dl if younger than 2 years). Diamonds indicate median weekly epoetin equivalent dose per country. iv, intravenous; UK, United Kingdom.
ESAs were used in 91.7% of children (Table 1). ESAs were exclusively administered via the subcutaneous route. The mean dosing intervals were 4.8±2.0 days with α-epoetin, 6.2±2.0 days with β-epoetin, 12.1±4.3 days with darbepoetin-α, and 25.1±5.0 days in the few children receiving a continuous erythropoietin receptor activator. Achieved Hb levels did not differ between ESA types. ESAs were used more commonly in Asia (97%) and Europe (95%) than in Turkey (86%) and North America (91%). The mean weekly ESA dose differed between countries, with dosing being lowest in Poland and the Czech Republic and highest in Mexico and India (Figure 2).
Iron was supplemented in 84% of patients, usually by the oral route (70%) (Table 1). Intravenous iron administration was preferred in most Latin American countries (Peru, 76% of all patients; Uruguay, 76%; Chile, 50%; Brazil, 32%) and Italy (52% of all patients).
ESA Dosing and Body Dimensions
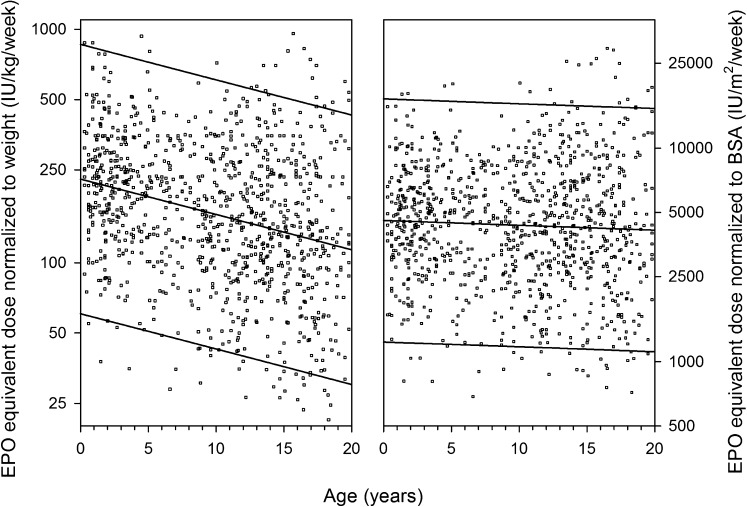
The median weekly ESA dosage was 154 (IQR, 94–260) IU per kg body weight or 4208 (IQR, 2582–6863) IU per m2 body surface area. The weekly ESA dose scaled to body weight was inversely correlated with age (r=−0.22; P<0.001), whereas ESA dose was independent of age when normalized to body surface area (r=−0.002; P=0.91) (Figure 3).
Figure 3.
ESA dose distribution scaled to body weight (left panel) vs. body surface area (right panel) in 1004 patients with available ESA dose information. Each dot represents patient-specific mean age and weekly erythropoietin (EPO) dose during observation period.
Risk Factors for Anemia and ESA Resistance
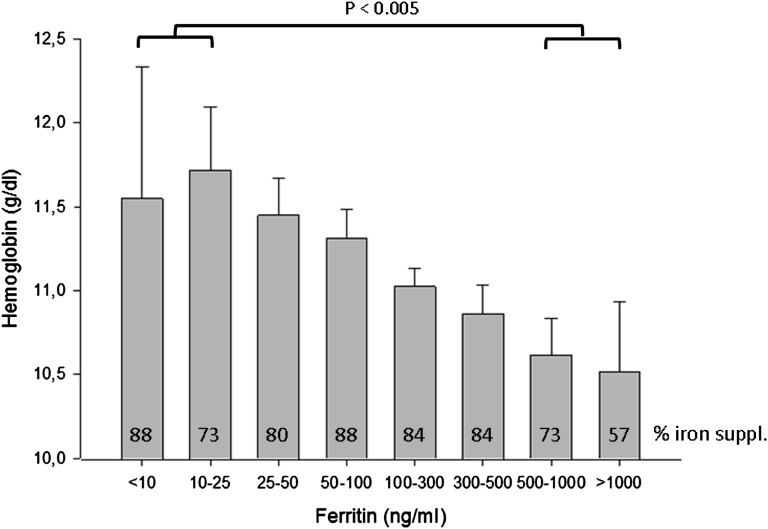
A detailed analysis of clinical, biochemical, and dialysis- and medication-related features associated with Hb distribution is given in Table 2. Patients with lower Hb levels tended to have less residual urine production, more fluid overload by clinical judgment, lower serum albumin, higher serum ferritin (Figure 4) and parathyroid hormone (PTH) (Figure 5), and higher BP; received higher ESA doses; and were more often treated with intravenous iron. Hb was significantly lower in anuric patients (10.7 versus 11.1 g/dl, P<0.001). The prevalence of left ventricular hypertrophy showed a curvilinear association with Hb levels, being highest in patients with Hb level < 8.5 g/dl.
Table 2.
Characteristics of all patients.
| Variable | Hb Range | Group Effect P Value | Time Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <8.5 g/dl (n=249) | 8.5–9.99 g/dl (n=697) | 10.0–11.49 g/dl (n=1272) | 11.5–12.99 g/dl (n=1067) | 13–14.49 g/dl (n=359) | >14.5 g/dl (n=87) | Unit Change | Estimated P Value | ||
| Age (yr) | 9.9±5.4 | 9.2±4.9 | 9.8±4.6 | 10.1±4.4 | 10.1±5.1 | 10.6±5.4 | 0.18 | – | – |
| Pubertal male (%) | 11.2 | 10.3 | 16.4 | 19.2 | 18.7 | 19.5 | 0.02 | 0.114 | 0.19 |
| PD vintage (yr) | 1.7±1.6 | 1.8±1.6 | 1.7.±1.3 | 1.7±1.3 | 1.6±1.5 | 1.3±1.7 | 0.03 | – | – |
| Use of biocompatible PD fluids (%) | 22.1 | 26.8 | 31.8 | 35.3 | 30.9 | 35.6 | 0.42 | −0.120 | 0.11 |
| PET 4h-D/Pcreatinine | 0.68±0.16 (n=61) | 0.68±0.14 (n=158) | 0.66±0.12 (n=247) | 0.64±0.13 (n=244) | 0.61±0.16 (n=70) | 0.63±0.13 (n=22) | 0.59 | −0.0032 | 0.55 |
| Estimated deviation from dry weight (%) | 2.02±3.71 | 2.09±2.90 | 1.41±2.46 | 1.32±2.04 | 1.49±3.29 | 1.51±2.35 | <0.001 | −0.0502 | 0.43 |
| PD fluid turnover (L/m2 per day) | 6.33±2.75 | 6.65±2.55 | 6.98±2.60 | 6.76±2.53 | 6.85±2.91 | 6.44±2.63 | 0.07 | 0.587 | <0.001 |
| Ultrafiltration volume (L/m2 per day) | 0.65±0.41 | 0.56±0.35 | 0.55±0.37 | 0.50±0.32 | 0.57±0.41 | 0.53±0.45 | 0.008 | 0.0353 | <0.001 |
| Urine volume (L/m2 per day) | 0.40±0.52 | 0.55±0.57 | 0.61±0.53 | 0.71±0.59 | 0.67±0.66 | 0.73±0.68 | 0.003 | −0.088 | <0.001 |
| Total fluid output (L/m2 per day) | 1.07±0.55 (n=225) | 1.13±0.55 (n=599) | 1.18±0.54 (n=1097) | 1.23±0.53 (n=939) | 1.26±0.64 (n=300) | 1.27±0.68 (n=68) | 0.20 | 0.0423 | <0.001 |
| ESA use (%) | |||||||||
| None | 4.8 | 4.2 | 3.9 | 7.7 | 16.4 | 31.0 | <0.001 | −0.212 | 0.03 |
| Epoetin | 79.5 | 80.2 | 77.3 | 73.5 | 71.9 | 57.5 | 0.003 | −0.154 | 0.02 |
| Darbepoetin | 14.9 | 14.6 | 17.3 | 17.2 | 10.6 | 11.5 | 0.21 | 0.225 | 0.004 |
| ESA dose (1000 U/m2 per week) | 7.12±4.53 (n=191) | 6.12±3.22 (n=545) | 5.26±3.12 (n=1002) | 4.89±3.25 (n=769) | 4.19±3.13 (n=240) | 5.83±4.81 (n=52) | <0.001 | 0.0036 | 0.96 |
| Iron use (%) | |||||||||
| None | 18.1 | 20.1 | 17.4 | 17.2 | 15.3 | 23.0 | 0.44 | 0.288 | <0.001 |
| Oral | 60.6 | 67.0 | 65.3 | 68.6 | 66.3 | 70.1 | 0.20 | −0.361 | <0.001 |
| Intravenous (with or without oral) | 21.3 | 12.9 | 17.3 | 14.2 | 18.4 | 6.9 | 0.07 | 0.0808 | 0.29 |
| Biochemistry | |||||||||
| Hemoglobin (g/dl) | 7.6±0.6 | 9.3±0.3 | 10.7±0.3 | 12.1±0.3 | 13.5±0.4 | 15.2±0.7 | 0.02 | 0.0223 | <0.001 |
| Serum ferritin (ng/ml) | 262 (125–486) (n=130) | 234 (121–402) (n=429) | 188 (85–356) (n=768) | 160 (75–312) (n=625) | 164 (66–336) (n=211) | 128 (59–304) (n=56) | 0.04 | 15.3 | <0.001 |
| Serum albumin (g/l) | 33.1±6.6 | 35.4±5.1 | 36.3±4.9 | 37.2±4.7 | 38.6±4.9 | 39.1±4.9 | <0.001 | 0.0884 | 0.34 |
| Plasma PTH (pg/ml) | 452 (177–740) | 325 (122–675) | 218 (99–494) | 213 (104–436) | 215 (95–465) | 197 (79–412) | <0.001 | 15.6 | 0.06 |
| Cardiovascular variables | |||||||||
| Systolic BP SDS | 1.34±1.63 | 1.12±1.39 | 0.92±1.20 | 0.87±1.13 | 0.72±1.36 | 0.66±1.56 | <0.001 | −0.109 | <0.001 |
| Diastolic BP SDS | 1.19±1.27 | 1.03±1.25 | 0.90±1.07 | 0.85±1.03 | 0.74±1.23 | 0.74±1.44 | 0.002 | −0.153 | <0.001 |
| Hypertensive (%) | 49.8 | 43.5 | 36.9 | 36.4 | 32.1 | 42.7 | 0.002 | −0.146 | 0.005 |
| LV mass index | 66.8±49.4 (n=80) | 48.9±22.5 (n=263) | 48.4±27.5 (n=489) | 48.6±33.1 (n=414) | 44.2±26.1 (n=134) | 53.1±30.2 (n=32) | <0.001 | −2.45 | 0.01 |
| LV hypertrophy (%) | 62.5 | 43.0 | 41.5 | 39.4 | 37.3 | 43.8 | 0.05 | −0.135 | 0.08 |
Data are given as mean ± SD, median (IQR), or percentage as appropriate. Group effect column denotes differences between Hb group means. Time effect column shows the unit change per observation year. Numbers in parentheses denote available observations. PET 4h-D/Pcreatinine, peritoneal equilibration test 4 hour dialysate plasma to creatinine ratio; SDS, standard deviation score; LV, left ventricular.
Figure 4.
Hb grouped by concomitant serum ferritin levels. Bars indicate group means, error bars + 2 SEM. Numbers in bars indicate percentage of measurements obtained in patients currently receiving iron medication.
Figure 5.
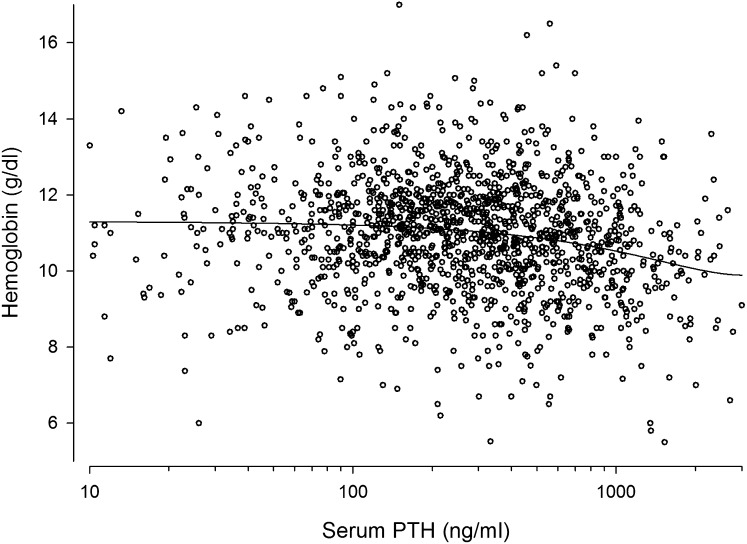
Association between serum PTH and Hb levels in 1394 pediatric PD patients. Each dot represents patient-specific mean PTH and Hb levels during observation period. A significant inverse correlation was observed (r=−0.157; P<0.0001).
Conversely, patients with high Hb levels (>13 g/dl) usually appeared in fluid balance, with well controlled BP and high serum albumin; were less likely to receive ESA and intravenous iron; and required significantly lower ESA doses if treated. Of note in this context, the use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers was not associated with Hb levels.
Furthermore, patients with underlying metabolic disorders (oxalosis, cystinosis) were more markedly anemic than patients with other diagnoses (10.2±1.3 vs. 11.0±1.1 g/dl; P<0.005). In addition, patients dialyzed with “biocompatible” PD solutions (neutral pH, low content of glucose degradation products) displayed slightly higher Hb levels than those receiving conventional fluids (11.1±1.1 versus 10.9±1.1 g/dl) (P<0.001). The results of the sensitivity analysis using the complete data confirmed these findings.
Logistic regression analysis was performed to identify independent predictors of subtarget Hb (Table 3). High ferritin, high PTH, and low serum albumin were independent risk factors of subtarget Hb, whereas significant residual urine output, high PD fluid turnover, and the use of neutral-pH biocompatible PD solutions were associated with reduced risk of subtarget Hb. In addition to these factors, postpubertal males were less likely to have subtarget Hb levels.
Table 3.
Multivariate mixed logistic regression analysis of factors predicting subtarget Hb levels.
| Variable | Model 1 (Excluding Serum Ferritin; n=3073) | Model 2 (Including Serum Ferritin; n=1781) | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age at study entry (yr) | 1.03 (1.01–1.05) | 0.01 | 1.02 (0.996–1.05) | 0.10 |
| Time on PD before study entry (yr) | 1.04 (0.98–1.11) | 0.21 | 1.05 (0.96–1.14) | 0.27 |
| Study time (yr) | 0.96 (0.86–1.08) | 0.54 | 0.93 (0.81–1.07) | 0.33 |
| Pubertal male | 0.51 (0.38–0.70) | <0.001 | 0.50 (0.32–0.74) | <0.001 |
| Residual urine output (L/m2 per day) | 0.73 (0.62–0.85) | <0.001 | 0.81 (0.66–0.99) | 0.04 |
| PD fluid turnover (L/m2 per day) | 0.96 (0.93–0.99) | 0.01 | 0.94 (0.90–0.98) | 0.01 |
| Use of biocompatible PD fluids | 0.73 (0.59–0.91) | 0.005 | 0.70 (0.52–0.93) | 0.01 |
| Use of long-acting ESA | 0.995 (0.78–1.28) | 0.97 | 0.97 (0.71–1.32) | 0.84 |
| Use of intravenous iron | 0.87 (0.67–1.13) | 0.30 | 0.85 (0.62–1.17) | 0.31 |
| ACE/ARB treatment | 1.05 (0.85–1.29) | 0.66 | 1.17 (0.89–1.53) | 0.27 |
| Serum PTH (log; pg/ml) | 1.33 (1.22–1.44) | <0.001 | 1.35 (1.22–1.50) | <0.001 |
| Serum albumin (g/L) | 0.94 (0.92–0.95) | <0.001 | 0.93 (0.91– 0.95) | <0.001 |
| Serum ferritin (log; ng/ml) | – | – | 1.43 (1.26–1.61) | <0.001 |
| Time since last peritonitisa (mo) | 1.00 (0.99–1.01) | 0.998 | 1.00 (0.99–1.01) | 0.64 |
For variables with units given in parentheses, odds ratios refer to change in likelihood per unit change (e.g., an odds ratio of 0.73 indicates a 27% risk decrease per 1 L/m2 per day urine output). CI, confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker.
For observations in patients without previous peritonitis episodes, the study entry date minus 1 year was arbitrarily entered.
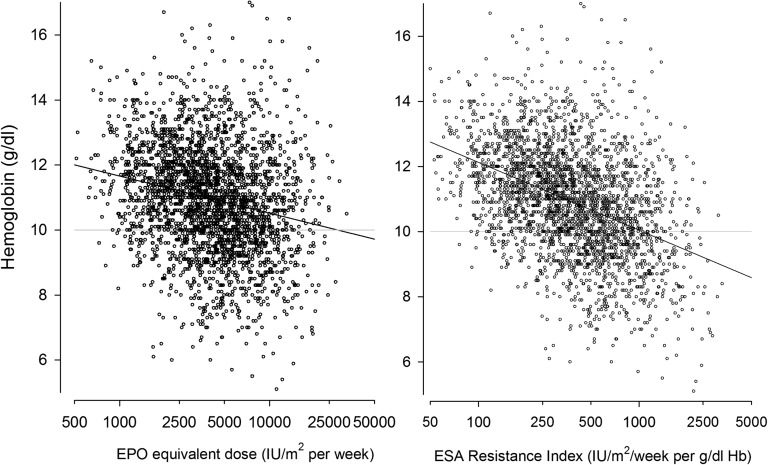
Because the relationship between ESA dose and the achieved Hb level is affected by therapeutic practice, as well as by factors modulating ESA responsiveness, the relative sensitivity to ESA therapy was expressed by the ratio of the Hb level achieved per 1000 IU ESA equivalent/m2 per week (Table 4) or its reciprocal value, the ESA resistance index (Figure 6). After adjustment for ESA type and dose, ESA sensitivity was independently positively associated with residual diuresis and serum albumin levels, and inversely with serum PTH (Table 4). These results were consistent when only patients with serum ferritin levels were considered. High serum ferritin levels were associated with an increased risk of subtarget hemoglobin, and ferritin showed a weak inverse relationship with ESA sensitivity of borderline significance. Sensitivity analysis showed similar results.
Table 4.
Mixed linear model analyses of factors predicting ESA sensitivity (Hb level achieved per 1000 IU/m2 weekly erythropoietin equivalent dose) excluding and including serum ferritin values
| Variable | Model 1 (Excluding Serum Ferritin, n=2798) | Model 2 (Including Serum Ferritin, n=1880) | ||
|---|---|---|---|---|
| Parameter Estimate ± SEM | P Value | Parameter Estimate ± SEM | P Value | |
| Intercept | 2.20±0.46 | <0.001 | 2.62±0.67 | <0.001 |
| Use of long-acting ESA | 0.03±0.18 | 0.86 | 0.06±0.21 | 0.76 |
| Age at study entry (yr) | 0.02±0.01 | 0.25 | 0.03±0.02 | 0.09 |
| Study time (yr) | 0.01±0.06 | 0.83 | 0.08±0.07 | 0.24 |
| Time on PD before study entry (yr) | −0.16±0.05 | 0.001 | −0.16±0.06 | 0.01 |
| Pubertal male | 0.20±0.19 | 0.31 | 0.12±0.23 | 0.62 |
| Residual urine output (L/m2 per day) | 0.42±0.09 | <0.001 | 0.46±0.12 | <0.001 |
| PD fluid turnover (L/m2 per day) | −0.01±0.02 | 0.62 | −0.02±0.03 | 0.49 |
| Use of biocompatible PD fluid | −0.09±0.15 | 0.56 | −0.18±0.20 | 0.36 |
| Serum albumin (g/L) | 0.05±0.01 | <0.001 | 0.06±0.01 | <0.001 |
| Serum PTH (log; pg/ml) | −0.15±0.04 | <0.001 | −0.18±0.05 | <0.001 |
| Serum ferritin (log; ng/ml) | – | – | −0.11±0.07 | 0.08 |
| Time since last peritonitisa (mo) | −0.002±0.01 | 0.76 | −0.001±0.006 | 0.85 |
For observations in patients without previous peritonitis episodes, the study entry date minus 1 year was arbitrarily entered.
Figure 6.
Relationship between Hb levels and ESA dose (left panel) and ESA resistance index (right panel). EPO, erythropoietin.
Anemia Control and Mortality
A total of 46 of 1394 children died during the observation period, most commonly of infections (n=17) and cardiovascular causes (n=13).
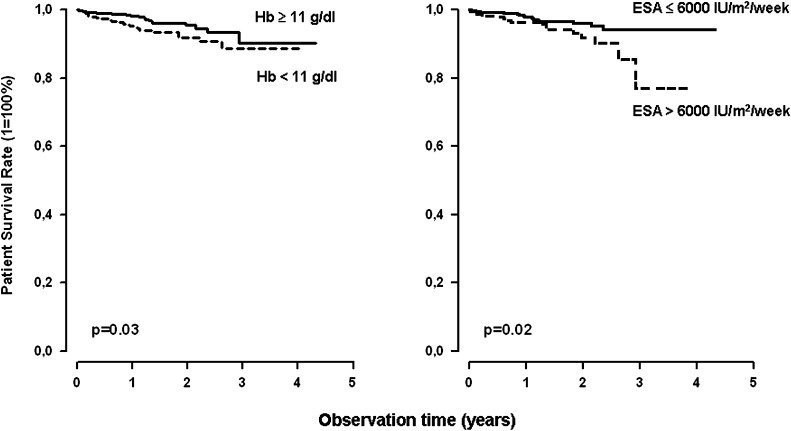
Death on PD occurred in 26 of 617 patients (4.2%) with a mean achieved Hb of <11 g/dl and in 20 of 777 patients (2.6%) with a mean Hb >11 g/dl. Actuarial survival rates were 95%, 92%, and 89% at 12, 24, and 36 months of observation, respectively, with mean Hb <11 g/dl, compared with 98%, 96%, and 90% in those with higher mean Hb (P=0.03) (Figure 7, left).
Figure 7.
Kaplan-Meier actuarial survival curves for patients with mean Hb < or >11 g/dl (left panel) and those with mean administered ESA equivalent dose < or >6000 IU/m2 per week.
Among the 1147 patients with available ESA doses, 17 of 791 patients receiving an average ESA equivalent dose of <6000 IU/m2 per week died during follow-up compared with 19 of 356 patients receiving higher ESA doses. Actuarial patient survival rates at 1, 2, and 3 years of observation were 98%, 96%, and 94% in the low-dose group and 96%, 92%, and 77% in the high-dose group (P=0.02) (Figure 7, right).
According to extended Cox regression analysis, the risk of patient death on dialysis was independently inversely associated with Hb (hazard ratio, 0.23; P<0.003) and serum albumin levels (hazard ratio per g/L, 0.87; P<0.0001) and was positively associated with the use of high ESA doses (hazard ratio per 1000 IU/m2 per week, 1.33; P<0.01).
No associations were noted between anemia or ESA doses and surrogate markers of patient morbidity, such as hospitalization rates or statural growth.
Discussion
This analysis of current anemia management and outcomes in children undergoing PD from around the world provides novel insights that have the potential to affect treatment paradigms in this population.
First, HB concentrations varied widely. Even allowing for a liberal target range of 10–13 g/dl, not only were subtarget Hb levels observed in more than a quarter of the patients, but supratarget levels were noted in one of nine children at any given time. Significant regional variation was observed, with European and North American centers generally achieving higher Hb ranges than centers in Asia, Turkey, and some Latin American countries. No effect of ESA availability was obvious; in fact, the countries with the lowest achieved Hb levels tend to administer the highest ESA doses. Likewise, no consistent associations of Hb with the modalities of iron supplementation was apparent. Hence, our findings suggest that factors unrelated to anemia management may underlie the regional variation of anemia prevalence.
A detailed risk factor analysis at the patient level identified several conditions that might affect anemia control. Patients with subtarget Hb levels generally tended to receive higher ESA doses, confirming ESA resistance rather than underdosing as the primary problem.
The role of iron deficiency as a cause of ESA-resistant anemia was difficult to address in this study because the registry does not record transferrin saturation as a direct measure of iron status. Hb levels showed no association with iron supplementation; severely anemic patients were even more likely to receive intravenous iron. Hb was inversely associated with serum ferritin throughout the entire ferritin range; no cutoff level defining a critical lower ferritin concentration could be established. This observation may reflect the limited usefulness of serum ferritin as a marker of iron status in children undergoing PD and is compatible with the view that ferritin might predominantly reflect inflammatory processes in this patient group.
Severe anemia tended to be associated with risk factors and manifestations of fluid overload, including low urine output, high ultrafiltration requirements, a high peritoneal transporter state in the peritoneal equilibration test, hypertension, and left ventricular hypertrophy. The link of anemia with hypertension was independent of the use of renin-angiotensin system antagonists. The consistent inverse association between Hb and BP is at variance with the common notion that higher Hb levels predispose to hypertension and points to a more important causative role of fluid retention in the pathogenesis of both PD-related hypertension and treatment-refractory anemia. A close association was also noted between serum albumin and Hb levels, which may in part be explained by fluid overload leading to dilution of both markers. Residual diuresis and serum albumin emerged as the two most significant independent predictors of ESA sensitivity in the mixed linear model analysis. Hence, fluid overload may be an underrecognized cause of “ESA-resistant” anemia that deserves closer attention in clinical patient management. Our findings point to the possibility that some patients with apparent ESA resistance may actually have an appropriate erythropoietic response, which is, however, masked by dilution of the red cell mass in a concomitantly increased extracellular fluid space.
An alternative and potentially complementary explanation of the observed association of Hb and serum albumin could be given by inflammatory conditions leading to both hypoalbuminemia and ESA-refractory anemia. The observed inverse association of serum ferritin with Hb levels is also consistent with a causative role of inflammation in ESA resistance. In this context, the reduced risk of subtarget Hb levels associated with the use of “biocompatible” PD fluids. PD fluid toxicity (“bioincompatibility”) is considered the major cause of the gradual degeneration of the peritoneal membrane, characterized by sclerosis and neovascularization and leading to ultrafiltration failure. A new generation of biocompatible PD fluids with neutral pH and low content of toxic glucose degradation products has recently been introduced. These products are highly reactive, rapidly resorbed substances eliciting a local and possibly systemic inflammatory response.12–14 Low-glucose degradation product PD fluids cause less release of proinflammatory cytokines, such as IL-6, from mesothelial cells.15 It is tempting to speculate that less chronic inflammation brought about by the use of biocompatible PD fluids leads to better ESA responsiveness. However, center effects cannot be excluded because usually either no or all patients within a dialysis center receive such fluids. The observation in the multivariate analyses that compatible PD fluid use was associated with higher Hb levels but not with increased ESA sensitivity points to this possibility.
Another factor consistently associated with ESA resistant anemia was hyperparathyroidism, confirming previous findings in adult and pediatric patients.16–19 Proposed mechanisms include direct effects of PTH on bone marrow progenitor cells, endogenous erythropoietin synthesis and erythrocyte survival, as well as an indirect effect via stimulation of bone marrow fibrosis.20 According to our findings, these effects become clinically relevant at PTH levels >500 pg/ml. Induction of bone marrow fibrosis is also considered the mechanism underlying the marked ESA resistance observed in this and previous studies in patients with ESRD caused by the metabolic storage disorders cystinosis and oxalosis.21,22
Previous studies in pediatric populations have consistently documented an inverse relationship of ESA dose requirements with patient age.11 It has been speculated that the presence of EPO receptors on nonhematopoietic cells in infants and young children might lead to increased clearance of exogenous EPO and hence less hormone available to mediate erythropoietic effects.23 We confirmed that the average weekly prescribed ESA dose decreased by almost 50% from neonatal to young adult age. However, this age dependence was present only when dose was scaled to body weight. When weight was replaced by body surface area, the normalized ESA dose was independent of age, with an average weekly EPO equivalent of 4300 IU/m2 administered throughout the pediatric age range. In children, body surface area is considered a better surrogate marker of basal metabolic rate than is body weight;24 it is conceivable that the erythropoietic capacity (as determined by erythrocyte progenitor cell mass and erythropoietin synthesis), distribution, and receptor expression should change commensurately with the basic oxygen demand given by the basal metabolic rate. Our findings provide a clinical evidence base for scaling ESA dose to body surface area in pediatric patients.
Finally, our study adds important pediatric information to the ongoing discussion about the “optimal” Hb target range in dialyzed patients receiving ESA. Despite the low overall mortality of dialyzed children, our global prospective data collection allowed a valid analysis of patient survival with respect to anemia management. A comparison of different achieved Hb ranges revealed a significant increase in patient mortality associated with a mean achieved Hb <11 g/dl. This finding is in line with previous observational studies in adults and children.1–3 By contrast, interventional trials in adults undergoing hemodialysis and adult diabetic patients with CKD demonstrated an increased risk of cardiovascular events and mortality associated with high hemoglobin targets.4–6 The mechanisms underlying adverse outcomes with full anemia correction may include an increased risk of thrombus formation by hemoconcentration, as well as off-target effects of the high ESA doses required to fully normalize Hb levels. Indeed, high ESA doses independently predicted mortality in observational studies,25 as well as in secondary analyses of the TREAT (Reduce Cardiovascular Events with Aranesp Therapy) and CHOIR (Correction of Hemoglobin in the Outcomes in Renal Insufficiency) trials.26,27 Experimental evidence suggests that high ESA doses may be directly toxic by activating endothelial cells and stimulating production of endothelin and plasminogen activator inhibitor-1. Recombinant erythropoietin has a direct vasoconstrictive effect on isolated renal and mesenteric resistance vessels and induces at high concentrations contractions of rat mesangial and aortic smooth muscle cells. Furthermore, erythropoietin can activate platelets, thereby potentially enhancing thrombosis risks when used in patients with preexisting cardiovascular disease.28–30 Our study describes for the first time an association between mortality and high ESA dose in children and in a PD population. The link of high administered ESA doses with poor patient survival was independent of the Hb level achieved and more discriminative than the association of survival with the mean Hb level. This observation is compatible with the notion that high ESA doses may be harmful even in young patients without major preexisting cardiovascular morbidity, irrespective of whether they are applied because of relative ESA resistance (e.g., in chronic inflammatory conditions) or are administered electively to achieve a high Hb level. In keeping with this line of reasoning, adult hemodialysis patients who spontaneously achieve “high” Hb levels do not show increased mortality.31
Of note in this context, a major fraction of the patients with high Hb in our study actually did not receive any ESA therapy at the time of measurement. In particular, male adolescents tended to have higher Hb levels, probably reflecting the physiologic increase in erythropoiesis in response to rising androgen production during puberty.32
We are aware of the limitations of this global cohort study. The observational nature of the registry largely precludes interpretations of cause-effect relationships because of potential bias by indication. In addition, as a result of the voluntary character of the registry, we cannot entirely exclude enrollment bias. Another specific limitation regarding this anemia analysis was the lack of information on transferrin saturation and more direct markers of inflammation, such as C-reactive protein. Finally, with respect to the observed association of administered ESA doses with patient mortality, we cannot exclude that high ESA doses, rather than exerting direct toxicity, might be a surrogate marker of underlying processes leading to increased mortality, such as infection and inflammation.
Despite the limitations intrinsic to its observational nature, to our knowledge this is the largest study performed to date addressing anemia management in children undergoing long-term PD and provides several conclusions that influence clinical care. These include the preferential dosing of ESA by body surface area, the apparent relative safety of Hb levels near or within the normal range, the potential confounding roles of fluid overload, severe hyperparathyroidism and male puberty in anemia management, and the adverse patient outcomes associated with weekly ESA doses >6000 IU/m2. The observed association of ESA dose with the mortality of children receiving PD is a novel and potentially important finding that deserves further exploration in other pediatric dialysis populations, as well as in the CKD setting.
Concise Methods
Data Collection
The IPPN registry collects information from children and adolescents undergoing long-term PD around the world. Participation in the registry is voluntary. The participating centers are asked to enroll all prevalent and incident consenting patients and follow them until discontinuation of PD. Because of the voluntary nature of the registry, the national coverage of pediatric PD patients varies between countries (e.g., United States, 10%; Turkey, 19%; 12 European countries, 31%; Canada, 59%; Chile, Korea, Argentina, 80%–90%; China, Finland, Singapore, Finland, Macedonia, Nicaragua, Uruguay, 100%).
Data input to the IPPN registry is performed exclusively via an Internet-based Web platform (www.pedpd.org). Data on basic patient and PD modality characteristics, growth and weight gain, nutritional modalities, PD prescription, hematology and serum biochemistry, residual diuresis, renal and dialytic small molecule clearances, medications, CKD- and dialysis-related complications (including PD-related and unrelated infections), and the cause and duration of any intercurrent hospitalizations are submitted every 6 months. The following anemia-related data are recorded: Hb and ferritin levels, use of ESAs (type, dose, administration interval), and use of iron supplements together with their administration mode. Data entries are automatically checked for plausibility and completeness. In addition, results of echocardiography performed according to the guidelines of the American Society of Echocardiography are recorded optionally.33,34 Data protection is ensured by pseudonymized data input. The registry protocol was approved by the ethical committees/institutional review boards as required at each participating center. Written parental consent and, whenever appropriate, assent from patients is obtained.
Because of the mandatory data entry structure of the online case report forms, data collection for the analysis presented here was complete for most variables, with the exception of a few that were included in the database after the registry had already started. Some missing values were imputed by means of multiple imputation for incomplete variables with <10% missing data: i.e., serum albumin, PD fluid turnover, and residual urine output. Imputation was performed with adjustment for age, sex, renal diagnosis group, and time on dialysis. Serum ferritin and ESA dose were missing in 40% (2219 measurements available in 914 patients) and 25% (2799 entries available in 1147 patients) of all observations, respectively, and therefore were not imputed. Hb values were available in all patients at all time points. Echocardiography results were available in 729 patients, and results of standardized peritoneal equilibration tests were available in 505 patients; the latter data were not imputed and were not used in the multivariate statistics.
Calculations and Statistical Analyses
The weekly darbepoetin dose (µg) was converted to epoetin IU equivalent using a conversion factor of 238.35
Subtarget Hb was defined by levels <10 g/dl in children age 2 years and older and <9.5 g/dl in children younger than 24 months, following the most recent guideline of the National Institute for Health and Clinical Excellence.36
Left ventricular mass was calculated according to the formula by Devereux et al.37 Left ventricular mass was indexed to height to the power of 2.7 (height2.7).38 Left ventricular hypertrophy was diagnosed if the left ventricular mass index exceeded the 95th percentile for sex and age of normal children and adolescents.39
The comparative assessment of Hb distribution by country was restricted to countries with >15 registered patients.
Data were checked for normal distribution by the Kolmogorov-Smirnoff test. Data are expressed by mean ± SD for normally distributed variables and by median and IQRs for non-normally distributed variables. Differences in group means (log-transformed in case of non-Gaussian distribution) were assessed by linear mixed models that included Hb group and time. Differences in proportions were assessed by logistic (generalized estimating equation–type) mixed models that included Hb group and time. Associations were assessed by Spearman correlation analysis. For all these procedures, measured values were divided by the number of measurements per patient in order to account for the variable number of repeated measurements available from each patient.
Logistic mixed modeling was applied to analyze subtarget versus on-target Hb. Age at study entry, time on PD before study entry, study time, status pubertal male, residual urine output, PD fluid turnover, use of biocompatible PD fluids, use of long-acting ESA, serum PTH, serum albumin, serum ferritin, and time since last peritonitis were included as independent factors. Linear mixed modeling was used for the multivariate analysis of factors associated with ESA sensitivity, thereby adjusting for multiple measurements in the same patient as well as for possible confounders. In addition to the factors in the logistic model, use of intravenous iron and angiotensin-converting enzyme treatment were included. For the correlation between observations within the same patient, a spatial covariance matrix was applied to take into account variable times between observations. A random intercept at patient level was calculated according to both the imputed and the complete data sets for sensitivity analysis.
Survival analyses were performed using Kaplan-Meier analysis and log-rank significance testing. Extended Cox proportional hazards modeling was used to identify risk factors for death on dialysis, including time-dependent covariates (e.g., serum Hb, serum ferritin, serum PTH [log], and information on peritonitis episodes).
P<0.05 was considered to represent statistically significant differences. Data were analyzed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
Disclosures
None
Acknowledgments
The authors appreciate the continued dedicated support of IPPN by the medical and nursing staff in all collaborating centers.
The authors gratefully acknowledge the support by the International Society for Peritoneal Dialysis, Baxter Health Care, Fresenius Medical Care, Ipsen, Pfizer, and IBM.
Appendix
The following principal investigators are contributing to the IPPN Registry:
Argentina: E. Sojo, Hospital de Pediatria Garrahan, Buenos Aires; P.A. Coccia, Hospital Italiano de Bueanos Aires; A. Suarez, Hospital de Niños Sor. Maria Ludovica La Plata; P.G. Valles, Hospital Pediatrico Humberto Notti, Mendoza; R. Salim, Rennius S.A. Salta, L. Alconcher, Hospital Interzonal General. Belgium: K. van Hoeck, University Hospital Antwerp, Edegem. Brazil: V. Koch, Instituto da Criança–Hospital das Clinicas FMUSP, Sao Paulo. Canada: J. Feber, Children’s Hospital of Eastern Ontario, Ottawa; D.A. Geary, Hospital for Sick Children, Toronto; C. White, BC Children’s Hospital, Vancouver. Chile: M. Valenzuela, Hospital Guillermo Grant Benavente, Concepcion; J. Villagra, Hospital Base, Osorno; F. Cano, Hospital Luis Calvo Mackenna, Santiago; M.A. Contreras, Roberto del Rio Hospital, Santiago; A. Vogel, Pontivicia Universidad Catolica de Chile, Santiago; P. Zambrano, Hospital Dr. Gonzales Cortes, Santiago; P. Berrocal, Hospital Sotero del Rio, Santiago. China: M.C. Chiu, Dept of Pediatric & Adolescent Medicine, Hong Kong; H. Xu, Children’s Hospital of Fudan University, Shanghai. Czech Republic: K. Vondrak, University Hospital Motol, Prague. Finland: K. Rönnholm, Hospital for Children and Adolescents, Helsinki; France: B. Ranchin, Hôpital Femme Mère Enfant, Lyon; G. Roussey, CHU Nantes; T. Ulinski, Armand Trousseau Hospital, Paris; M. Fischbach, Children’s Dialysis Center, Strasbourg; J. Harambat, Hopital de Enfants, Bordeaux. Germany: J. Dotsch, University Hospital, Erlangen; R. Büscher, Children’s Hospital Essen; M. Kemper, University Medical Center, Hamburg; L. Pape, Medical School, Hannover; F. Schaefer, D. Borzych, Center for Pediatrics and Adolescent Medicine, Heidelberg; J. Misselwitz, Kidney Center for Children and Adolescent, Jena; G. Klaus, University Hospital, Marburg; D. Haffner, University Children’s Hospital, Rostock. Greece: F. Papachristou, Aristoteles University, Thessaloniki. Hungary: A. Szabo, Semmelweis University, Budapest. India: A. Bagga, All India Institute of Medical Sciences, New Delhi; M. Kanitkar, Armed Forces Medical College, Pune. Italy: E. Verrina, G. Gasini Institute, Genova; A. Edefonti, Fondazione Ospedale Maggiore Policlinico, Milano; G. Leozappa, Dip. Nefrologia-Urologia, Rome. Israel: D. Landau, Soroka Medical Center, Beer-Sheva. Korea: I.S. Ha, Dialysis Center for Children and Adolescents, Seoul; K.H. Paik, Samsung Medical Center, Seoul. Lebanon: A. Bilal, Rafik Hari University Hospital, Beirut. Macedonia: E. Sahpazova, Pediatric Clinic, Skopje. Mexico: L. Sanchez Barbosa, Pediatric Hospital Medial Center SXXI, Cuahutemoc; Netherlands: J.W. Groothoff, Academic Medical Center, Amsterdam. New Zealand: W. Wong, Starship Children’s Hospital, Aucland. Nicaragua: Y. Silva, Hospital Infantil de Nicaragua, Managua. Peru: R. Loza Munarriz, Cayetano Heredia Hospital, Lima. Poland: A.M. Zurowska, D. Borzych, Medical University, Gdansk; D. Drozdz, Jagellonian University Medical College, Krakow; M. Lipka, Children’s Memorial Health Institute, Warsaw; Z. Wawer, Public Pediatric Teaching Hospital, Warsaw; M. Sczepanska, Dialysis Division for Children, Zabrze. Romania: O. Brumariu, St. Maria Children’s Hospital, Iasi. Saudi Arabia: J. Kari, King Abdul Aziz University Hospital, Jeddah. Singapore: H.K. Yap, Shaw-NKF-NUH Children’s Kidney Center. Spain: G. Ariceta, Hospital de Cruces, Baracaldo. Turkey: A.S. Bakkaloglu, Hacettepe University, Ankara; S. Bakkaloglu, Gazi University, Ankara; I. Bilge, Dept of Pediatric Nephrology, Çapa-Istanbul; L. Sever, Cerrahpasa School of Medicine, Istanbul; E. Serdaroglu, Dr. Behcet Children Research and Educational Hospital, Izmir; A. Bal, Tepecik Children and Research Hospital, Izmir; S. Mir, Ege University Faculty of Medicine, Izmir-Bornova. United Arab Emirates: E. Simkova, Dubai Hospital, Dubai. United Kingdom: L. Rees, Great Ormond Street Hospital, London; A.R. Watson, Children & Young People's Kidney Unit, Notthingham. United States: L. Greenbaum, Children’s Healthcare Pediatric Dialysis Unit, Atlanta, Georgia; A. Neu, Johns Hopkins Hospital, Baltimore, Maryland; D. Askenazi, Children’s Hospital of Alabama, Birmingham, Alabama; D. Gipson, University of North Carolina, Chapel Hill, North Carolina; H. Patel, Cildren’s Hospital, Columbus, Ohio; A. Al-Akash, Driscoll Children’s Hospital, Corpus Christi, Texas; S. Pottoore, Children’s Medical Center, Dallas, Texas; V. Dharnidharka, Division of Pediatric Nephrology, Gainesville, Florida; T. Bunchman, Helen DeVos Children’s Hospital, Grand Rapids, Michigan; A. Chua, Texas Children’s Hospital, Houston, Texas; B.A. Warady, Children’s Mercy Hospital, Kansas City, Missouri; J. Zaritsky, UCLA Medical Center, Los Angeles, California. Uruguay: J. Grünberg, A. Rebori, V. Ramella, SE.N.NI.AD, Montevideo.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.McMahon LP, Mason K, Skinner SL, Burge CM, Grigg LE, Becker GJ: Effects of haemoglobin normalization on quality of life and cardiovascular parameters in end-stage renal failure. Nephrol Dial Transplant 15: 1425–1430, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Fink J, Blahut S, Reddy M, Light P: Use of erythropoietin before the initiation of dialysis and its impact on mortality. Am J Kidney Dis 37: 348–355, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Warady BA, Ho M: Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr Nephrol 18: 1055–1062, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill J, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, Uno H, TREAT Investigators : Baseline characteristics in the trial to reduce cardiovascular events with Aranesp therapy (TREAT). Am J Kidney Dis 54: 59–69, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Chesney RW, Brewer E, Moxey-Mims M, Watkins S, Furth SL, Harmon WE, Fine RN, Portman RJ, Warady BA, Salusky IB, Langman CB, Gipson D, Scheidt P, Feldman H, Kaskel FJ, Siegel NJ: Report of an NIH task force on research priorities in chronic kidney disease in children. Pediatr Nephrol 21: 14–25, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Pattaragarn A, Warady BA, Sabath RJ: Exercise capacity in pediatric patients with end-stage renal disease. Perit Dial Int 24: 274–280, 2004 [PubMed] [Google Scholar]
- 9.Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, Zelikovsky N, Fivush B, Furth S: Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 44: 1017–1023, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Glader B: The Anemias. In: Nelson Textbook of Pediatrics, edited by Kliegman RM, Jenson HB, Behrman RE, Stanton BF, 18th Ed., Philadelphia, Saunders, 2007, pp 2003–2006 [Google Scholar]
- 11.Ho M, Stablein DM: North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) 2003 annual report, Rockville, MD, The EMMES Corporation, 2003 [Google Scholar]
- 12.Lai KN, Leung JC: Inflammation in peritoneal dialysis. Nephron Clin Pract 116: c11–c18, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Witowski J, Wisniewska J, Korybalska K, Bender TO, Breborowicz A, Gahl GM, Frei U, Passlick-Deetjen J, Jörres A: Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J Am Soc Nephrol 12: 2434–2441, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Noh H, Kim JS, Han KH, Lee GT, Song JS, Chung SH, Jeon JS, Ha H, Lee HB: Oxidative stress during peritoneal dialysis: Implications in functional and structural changes in the membrane. Kidney Int 69: 2022–2028, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cooker LA, Luneburg P, Holmes CJ, Jones S, Topley N: Bicarbonate/ Lactate Study Group. Interleukin-6 levels decrease in effluent from patients dialyzed with bicarbonate/lactate based peritoneal solutions. Perit Dial Int 21: S102–S107, 2001 [PubMed] [Google Scholar]
- 16.Rao DS, Shih MS, Mohini R: Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 328: 171–175, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Belsha CW, Berry PL: Effect of hyperparathyroidism on response to erythropoietin in children on dialysis. Pediatr Nephrol 12: 298–303, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gallieni M, Corsi C, Brancaccio D: Hyperparathyroidism and anemia in renal failure. Am J Nephrol 20: 89–96, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Lee GH, Miller JE, Streja E, Jing J, Robertson JA, Kovesdy CP: Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis 53: 823–834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancaccio D, Cozzolino M, Gallieni M: Hyperparathyroidism and anemia in uremic subjects: A combined therapeutic approach. J Am Soc Nephrol 15[Suppl 1]: S21–S24, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Şahin G, Acıkalın MF, Yalcın AU: Erythropoietin resistance as a result of oxalosis in bone marrow. Clin Nephrol 63: 402–404, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Milford DV, Winterborn MH: Resistance to recombinant human erythropoietin in a child with renal failure, cystinosis and beta-thalassaemia minor. Nephron 64: 645–646, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Palis J, Segel GB: Developmental biology of erythropoiesis. Blood Rev 12: 106–114, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez W, Selen A, Avant D, Chaurasia C, Crescenzi T, Gieser G, Di Giacinto J, Huang SM, Lee P, Mathis L, Murphy D, Murphy S, Roberts R, Sachs HC, Suarez S, Tandon V, Uppoor RS: Improving pediatric dosing through pediatric initiatives: What we have learned. Pediatrics 121: 530–539, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ: Epoetin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 44: 866–876, 2004 [PubMed] [Google Scholar]
- 26.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA, Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators : Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 363: 1146–1155, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Smith KJ, Bleyer AJ, Little WC, Sane DC: The cardiovascular effects of erythropoietin. Cardiovasc Res 59: 538–548, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Vaziri ND, Zhou XJ: Potential mechanisms of adverse outcomes in trials of anemia correction with erythropoietin in chronic kidney disease. Nephrol Dial Transplant 24: 1082–1088, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Morakkabati N, Gollnick F, Meyer R, Fandrey J, Jelkmann W: Erythropoietin induces Ca2+ mobilization and contraction in rat mesangial and aortic smooth muscle cultures. Exp Hematol 24: 392–397, 1996 [PubMed] [Google Scholar]
- 31.Goodkin DA, Fuller DS, Robinson BM, Combe C, Fluck R, Mendelssohn D, Akizawa T, Pisoni RL, Port FK: Naturally occurring higher hemoglobin concentration does not increase mortality among hemodialysis patients. J Am Soc Nephrol 22: 358–365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hero M, Wickman S, Hanhijärvi R, Siimes MA, Dunkel L: Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr 146: 245–252, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Borzych D, Bakkaloglu SA, Zaritsky J, Suarez A, Wong W, Ranchin B, Qi C, Szabo AJ, Coccia PA, Harambat J, Mitu F, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network : Defining left ventricular hypertrophy in children on peritoneal dialysis. Clin J Am Soc Nephrol 6: 1934–1943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakkaloglu SA, Borzych D, Soo Ha I, Serdaroglu E, Büscher R, Salas P, Patel H, Drozdz D, Vondrak K, Watanabe A, Villagra J, Yavascan O, Valenzuela M, Gipson D, Ng KH, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network : Cardiac geometry in children receiving chronic peritoneal dialysis: findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol 6: 1926–1933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warady BA, Arar MY, Lerner G, Nakanishi AM, Stehman-Breen C: Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr Nephrol 21: 1144–1152, 2006 [DOI] [PubMed] [Google Scholar]
- 36.The National Clinical Guideline Centre Anaemia management in people with chronic kidney disease: NICE guidance. May 30, 2012. Available at: http://guidance.nice.org.uk/CG114/NICEGuidance/pdf/English
- 37.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N: Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol 57: 450–458, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Lauer MS, Anderson KM, Larson MG, Levy D: A new method for indexing left ventricular mass for differences in body size. Am J Cardiol 74: 487–491, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR: Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009 [DOI] [PubMed] [Google Scholar]