Abstract
The development of anti-donor humoral responses after transplantation associates with higher risks for acute rejection and 1-year graft survival in adults, but the influence of humoral immunity on transplant outcomes in children is not well understood. Here, we studied the evolution of humoral immunity in low-risk pediatric patients during the first 2 years after renal transplantation. Using data from 130 pediatric renal transplant patients randomized to steroid-free (SF) or steroid-based (SB) immunosuppression in the NIH-SNSO1 trial, we correlated the presence of serum anti-HLA antibodies to donor HLA antigens (donor-specific antibodies) and serum MHC class 1-related chain A (MICA) antibody with both clinical outcomes and histology identified on protocol biopsies at 0, 6, 12, and 24 months. We detected de novo antibodies after transplant in 24% (23% of SF group and 25% of SB group), most often after the first year. Overall, 22% developed anti-HLA antibodies, of which 6% were donor-specific antibodies, and 6% developed anti-MICA antibody. Presence of these antibodies de novo associated with significantly higher risks for acute rejection (P=0.02), chronic graft injury (P=0.02), and decline in graft function (P=0.02). In summary, antibodies to HLA and MICA antigens appear in approximately 25% of unsensitized pediatric patients, placing them at greater risk for acute and chronic rejection with accelerated loss of graft function. Avoiding steroids does not seem to modify this incidence. Whether serial assessments of these antibodies after transplant could guide individual tailoring of immunosuppression requires additional study.
Kidney transplantation is the modality of choice for children with ESRD. Despite an improvement in early outcomes, long-term allograft survival remains restricted1,2; chronic graft injury is the prime limiting survival factor and largely driven by the development of antidonor humoral responses.3,4 Anti-human leukocyte antigen (anti-HLA) antibodies (Abs) are a major cause of acute antibody-mediated rejection and also may damage the kidney in a more indolent process, leading to chronic antibody-mediated rejection and eventual allograft loss.5 Nevertheless, ongoing allograft rejection in HLA identical transplant recipients and patients without detectable anti-HLA Abs to donor HLA antigens (DSA) supports an additional pathogenic role for graft injury by non-HLA antigens, such as protein kinase-ζ, and MHC class 1-related chain A (MICA).6–9
In adult renal transplantation, Terasaki10 and Terasaki and Cai11 have shown that preformed and de novo post-transplant production of DSAs correlates with a higher risk of acute rejection (AR) and reduced 1-year graft survival. The impact of the humoral response has not been well studied in the pediatric transplantation. We performed quantitative analysis of post-transplant de novo Abs to HLA and MICA in children undergoing kidney transplants and questioned differences in Ab profiles with steroid avoidance.12–14 To this end, we compared the measured humoral immune responses of pediatric kidney transplant patients in a randomized, multicenter, open-labeled study for a steroid-based (SB) or steroid-free (SF) immunosuppression protocol (SNSO1).15,16 We conducted serial monitoring of quantitative titers of circulating for MHC classes I and II and Abs to MICA in patients with stable graft function, acute graft rejection, and chronic graft injury as evaluated by matched protocol or indicated renal allograft biopsies (Figure 1). We intended to find if there were differences in the detection of these Abs with steroid avoidance, the average time for de novo Ab detection post-transplantation, and the correlation of Ab levels with graft injury and function. Correlation of the negative impact of the peripheral and intragraft humoral responses and their specificities with adverse graft outcomes in children could develop a novel means of monitoring and titrating immunosuppression in pediatric renal transplantation.
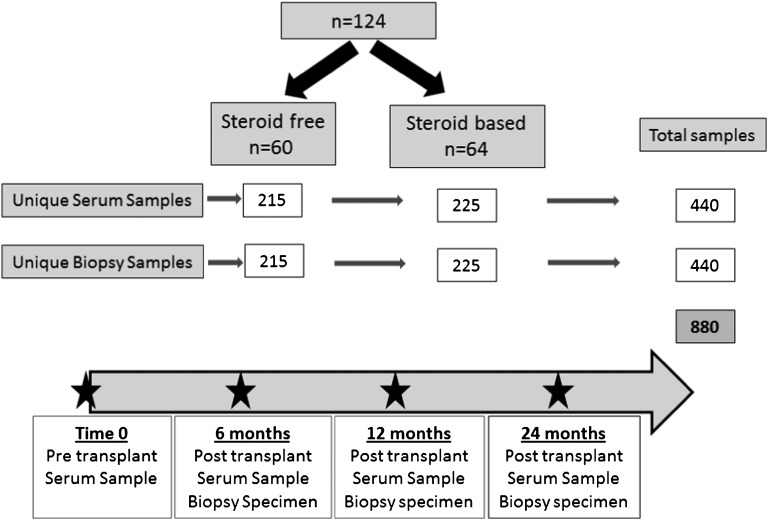
Figure 1.
Study outline. This study used 440 serum samples and 440 matched blinded biopsy scores for CADI, CNIT, Banff rejection grading, and C4d stains on 440 matched protocol biopsies from the SNSO1 multicenter randomized trial of SF and SB immunosuppression in pediatric renal transplantation.15,16 Samples and biopsies were assayed at 0 (pretransplant), 6, 12, and 24 months post-transplantation. Of 130 patients in the trial, 124 patients had at least three of four sera samples collected and were included in the analysis.
Results
Detection of Preformed Anti-HLA and Anti-MICA Antibodies before Transplantation
Eleven percent of the patients had preformed anti-HLA Abs. Of these patients, 7% had non-DSA or nondonor-specific antibody (NDSA), and 4% had DSA (3% was to class I and 1% was to class II). Additionally, 6% of patients had preformed anti-MICA Abs (Table 1); 1.6% (2/124) of patients had both anti-HLA and anti-MICA Abs in the pretransplant sera. Both these patients had been on chronic hemodialysis. There was no difference in the incidence or titer of preformed HLA Abs between the SF and SB groups.
Table 1.
The incidence of preformed Abs in the SF and SB groups of patients in the SNSO1 study
| Preformed Abs | SF (n=60) | SB (n=64) | P Value | Total (n=124) | |||
|---|---|---|---|---|---|---|---|
| Number (%) | Average Titer | Number (%) | Average Titer | Number | Titer | ||
| NDSA | 4 (7%) | 3688 | 5 (10%) | 3976 | 1.00 | 0.83 | 9 (7%) |
| DSA | 2 (3%) | 10,154 | 3 (5%) | 1857 | 0.81 | 0.12 | 5 (4%) |
| Class 1 | 1 (2%) | 3751 | 3 (5%) | 1857 | 0.62 | NA | 4 (3%) |
| Class 2 | 1 (2%) | 16,558 | 0 (0%) | NA | 0.49 | NA | 1 (1%) |
| MICA | 2 (3%) | 4810 | 6 (10%) | 5377 | 0.27 | 0.79 | 8 (6%) |
Detection of Newly Formed or De Novo Antibodies to HLA and MICA after Transplantation
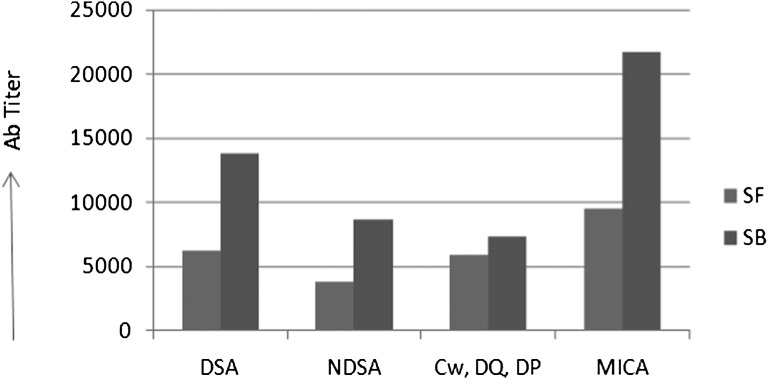
Twenty-two percent of overall patients developed newly formed anti-HLA Abs; 6% of all patients developed anti-MICA Abs (Table 2), and 3% of patients developed de novo Abs to both HLA and MICA. Among the de novo anti-HLA Abs, 6% were to DSA, and 7% were to NDSA. Because the donors were not typed for Cw, DP, and DQ, 13% of the anti-HLA Abs noted to the Cw, DP, and DQ antigens could not be typed for their donor specificity. Between the SF and SB groups, there was no difference in the number of patients developing de novo anti-HLA DSA (5% for SF and 6% for SB, P=1.00), anti-HLA NDSA (7% for SF and 8% for SB, P=1.00), or anti-MICA Abs (7% for SF and 5% for SB, P=0.70), and the median time to first detection of de novo anti-HLA Abs (12 months for SF and 14 months for SB, P=0.10) and anti-MICA Abs (12 months for SF and 12 months for SB, P=1.00) was not statistically different. The highest mean signal intensity of de novo Abs as measured by the mean fluorescence intensity (MFI) was higher in the SB group, but because of small sample numbers in the Ab-positive groups, the numbers were only significant for overall HLA Abs and anti-MICA Ab titers. The values for anti-HLA Abs were 9322±5781 MFI in the SB group compared with 4526±3014 MFI in the SF group (P=0.01) (Figure 2). The difference in titer was statistically significant only in the case of de novo MICA Abs, but it did not reach statistical significance in the case of HLA Abs, likely because of the smaller number of Ab-positive patients (DSA P=0.08, MICA P=0.04). One patient who developed MICA and DSA as well as NDSA Abs developed AR at 14 months and had marked decline in creatinine clearance by 20 ml/min per 1.73 m2 body surface area at 12 months post-transplant.
Table 2.
The incidence of different groups of de novo antibodies in patients in SF and SB immunosuppression
| De novo Abs | SF (n=60) | SB (n=64) | P Value (Titer) | Total (n=124) | ||
|---|---|---|---|---|---|---|
| Number (%) | Average Titer | Number (%) | Average Titer | |||
| Class 1 | 2 (3%) | 6130 | 3 (5%) | 12,970 | 0.44 | 5 (4%) |
| Class 2 | 1 (2%) | 6383 | 1 (2%) | 13,835 | 2 (2%) | |
| Cw, DQ, and DP | 6 (10%) | 5894 | 10 (16%) | 7348 | 0.51 | 16 (13%) |
| NDSA | 4 (7%) | 3823 | 5 (8%) | 8705 | 0.15 | 9 (7%) |
| DSA | 3 (5%) | 6214 | 4 (6%) | 13,829 | 0.08 | 7 (6%) |
| MICA | 4 (7%) | 9512 | 3 (5%) | 21,762 | 0.05 | 7 (6%) |
There are some trend differences in DSA and MICA in the SF and SB groups, but the number of events is too small in each arm to reach significance.
Figure 2.
De novo antibody titers in SF versus SB immunosuppression. Antibody titers. The highest MFIs are shown for DSA, NDSA, Cw, DQ, and DP (where donor typing for these antigens were not available), and MICA antigens.
AR
The prevalence of AR was 31% (38/124) in patients, with three patients having three AR episodes within the follow-up period; 66% (25/38) of AR episodes were early within the first 6 months of the post-transplant period. There was no difference in the prevalence of AR between the SF and SB groups.15,16 Based on biopsy, 95% of patients had T cell-mediated acute cellular rejection (IA and IB Banff criteria). However, 34% (13/38) of patients had positive de novo HLA Abs associated with an AR episode. Of these patients, 38% (5/13) had de novo DSA, 23% (3/13) had de novo NDSA, and 62% (8/13) had Cw, DP, and DQ Abs.
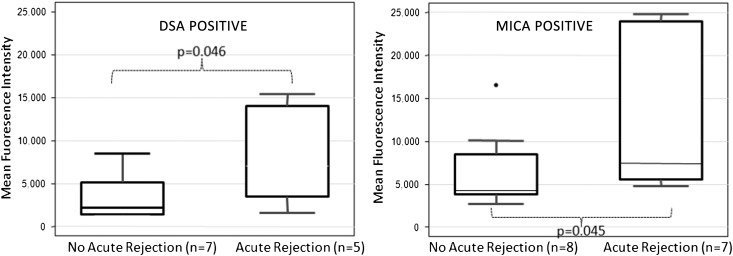
There was no association between the presence or titer of preformed Abs (DSA, NDSA, and MICA) and development of AR in the post-transplant period (P=0.40, P=0.08, and P=0.20, respectively). However, development of de novo DSA Abs was significantly associated with the development of AR in the post-transplant period (P=0.03), regardless of the immunosuppression used. De novo NDSA showed no association with AR (P=0.30). Particularly, development of DQ Abs was significantly associated with AR (P=0.04). It is unknown if these DQ Abs were donor-specific. Moreover, studying the development of Abs longitudinally over time, the de novo HLA Abs appeared before the AR episode in 85% (11/13) of patients. DSA development at 6 months was significantly associated with AR at 6 months (P=0.003); because the Abs were first checked at 6 months, DSA development may have preceded the development of AR. Development of de novo MICA Abs also showed significant association with AR (P=0.03), regardless of the immunosuppression used. Remarkably, the titer of MICA Abs in patients who had AR was significantly higher (12,858.8±7915.5) compared with the titer of MICA Abs in patients who did not have AR (6049±4796.5, P=0.04) (Figure 3); 29% (11/38) of the patients had subclinical rejection (rejection diagnosed at protocol biopsy with normal creatinine clearance at the time of biopsy), and 45% (5/11) of the patients with subclinical rejection developed de novo HLA Abs, with 60% (3/5) of them developing them after the rejection episode.
Figure 3.
Significant association of Ab positivity and biopsy-confirmed AR. Anti-HLA DSA and MICA Ab titers in patients in the entire study with and without AR.
By logistic regression model, related clinical parameters, such as immunosuppression, donor source, de novo NDSA, de novo DSA, and de novo MICA, were used to predict potential risk factor for AR. De novo DSA (P=0.08, odds ratio=4.90, 95% confidence interval=0.80–29.50) and de novo MICA (P=0.07, odds ratio=5.20; 95% confidence interval=0.90–30.80) proved to be a potential risk factor with a trend to statistical significance.
Graft Function/Creatinine Clearance
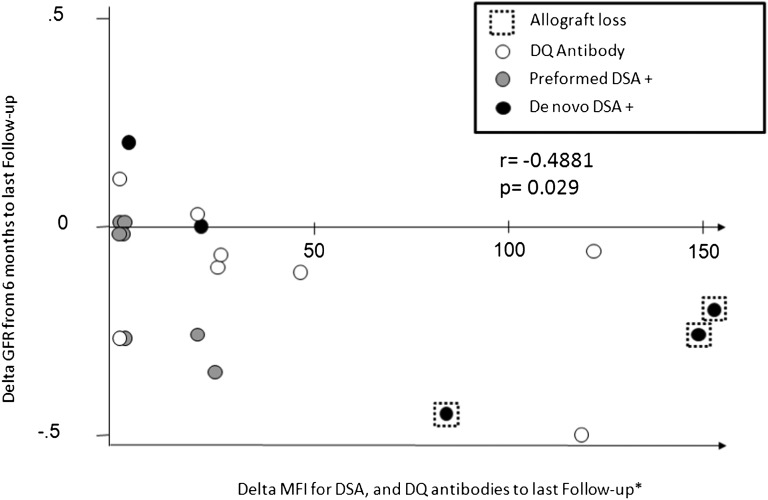
Decline in graft function over time was assessed by the difference between creatinine clearances (estimated by the Schwartz equation) at 6 and 12/24 months. There was no difference in decline in graft function at 12 or 24 months between SF and SB groups (P=0.44 and P=0.76, respectively). Patients with de novo DSA development had greater decline in creatinine clearance at 24 months (P=0.02) compared with patients who did not. Preexisting HLA Abs were also associated with greater decline in creatinine clearance at 24 months (P=0.005) (Figure 4).
Figure 4.
Correlation between the intensity of DSA titers and decline in graft function. The x axis represents ΔMFI; the y axis represents ΔGFR between 6 months post-transplantation (taken as the baseline serum creatinine in milligrams per deciliter) and the last patient follow-up at 24 months post-transplantation.
Chronic Graft Injury
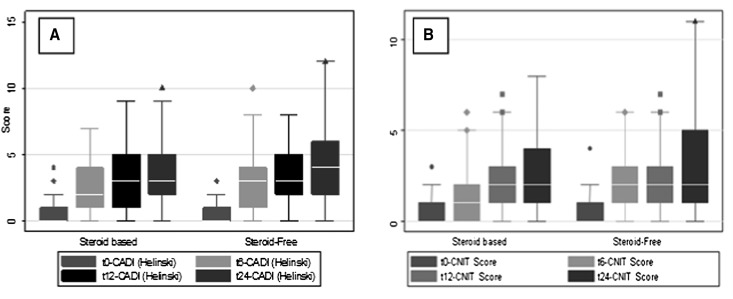
A substantial 48% (60/124) of patients had some element of chronic allograft nephropathy, and 30% (37/124) of patients had some evidence of calcineurin inhibitor toxicity (CNIT) on biopsy. Chronic allograft damage estimated by the chronic allograft damage index (CADI) score progressed similarly over time in the entire cohort of patients (both SF and SB groups) as evidenced in the biopsies obtained at 0, 6, 12, and 24 months.15 There was also progression of CNIT score over time irrespective of immunosuppression used in biopsies obtained at 0, 6, 12, and 24 months related to length of calcineurin inhibitor exposure (Figure 5). De novo HLA antibody development (DSA, Cw, DP, DQ, and NDSA) was associated with peak CADI (P=0.02), and CNIT scores (P=0.02), suggesting a role of humoral responses in chronic graft injury. Also, patients developing de novo DSA Abs had more severe interstitial inflammation than patients with negative DSA Abs (57% versus 10%, P=0.005). This significant difference existed in cases of positive preformed DSA compared with patients with no preformed DSA (33% versus 10%, P=0.05).
Figure 5.
There was an increase of CADI/CNIT scores due to time post-transplantation. Progression of the (A) CADI and (B) CNIT scores in the protocol biopsies over time after transplantation. Both CADI and CNIT scores increased significantly with time post-transplantation (significance shown by highlights for P<0.001; protocol biopsies at times 0, 6, 12, and 24 months post-transplantation marked as t0, t6, t12, and t24, respectively).
Graft Loss
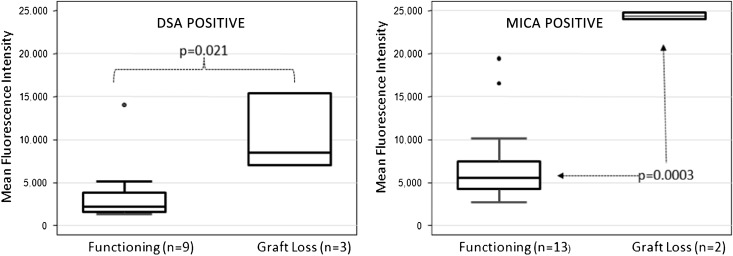
Patient survival was 100% at 3 years in both the SF and SB cohorts15,16; 5 of 124 (4%) patients lost their graft by 2 years after transplantation. Patients contributed samples up to and beyond graft failure. One of five patients had SF immunosuppression, and four of five patients had SB immunosuppression, which is not a significant difference (P=0.30). However, all graft losses were secondary to noncompliance of immunosuppressive agents. These patients also had a higher degree of donor–recipient HLA antigen mismatch (6, 6, 5, 5, and 3) at the time of transplant. Presence of preformed MICA or DSA Abs was not associated with graft loss. There was a strong association with development of de novo DSA before graft loss (P=0.02) (Figure 6). No such association was seen with NDSA Abs. Development of de novo MICA Abs also had strong association with graft loss (P<0.001) (Figure 6). Moreover, titers of DSA (mean=13,258 versus 7001) as well as MICA (mean=243,815 versus 7419) Abs were much higher in patients who had graft loss (P=0.06 and P<0.001, respectively).
Figure 6.
Significant association of Ab positivity and graft loss. Anti-HLA DSA and anti-MICA Ab positivity in patients associates with greater risk of graft loss over the course of the SNSO1 study, even in low-risk pediatric renal transplant recipients.
Discussion
DSA produced de novo after transplantation can initiate a process of damage and repair in the endothelium, resulting ultimately in the characteristic constriction of the blood vessels commonly found in chronic rejection. Our study applied the single-antigen microsphere technology to the retrospective analysis of sequential post-transplant serum samples to study the development of anti-HLA and non-HLA MICA Abs longitudinally in a large group of low-risk pediatric renal transplant recipients in the context of the patients’ clinical and histologic course and immunosuppression usage (SF versus SB). We studied the correlation of the negative impact of the peripheral and intragraft humoral responses with adverse graft outcomes in children. The main objective of this study is to understand the evolution of humoral immunity against HLA specifically in the pediatric population so that this knowledge can be applied for clinical monitoring and risk prediction.
Twenty-two percent and six percent of recipients developed de novo post-transplant HLA Abs and MICA Abs in this study. Among the treatment groups of SF and SB, there was no difference in the incidence or time to development of de novo HLA Abs and MICA Abs between the SF and SB groups, but the intensity of the HLA Abs response was higher in the SB group. Clinical outcomes from this randomized study has shown benefits in reduced morbidity and fewer drug-related side effects and no adverse impact on long-term outcome with SF immunosuppression.14,15 Our study suggests the possibility of an added benefit of steroid avoidance in reducing the intensity of the humoral response, although this finding can only be confirmed in a larger-scale trial, because the number of patients with positive HLA Abs in our study is small. Interestingly, one patient developed MICA, DSA, and NDSA Abs; this patient developed an aggressive AR at 14 months with loss of graft function 1 year later. Although our data suggest that patients who develop both HLA and non-HLA Abs in the post-transplant period may have poorer outcome, we do not have a sufficient number of these dual antibody developers in our study to confirm this assumption. Significant anti-HLA DSA is most likely to develop in noncompliant patients; however, with consistent measure of noncompliance not available in this study group, we were unable to quantify how many of the de novo Abs developers were actually noncompliant.
The de novo occurrence of Abs against HLA is known to play an important role in solid organ AR.3,10 Consistent with other studies, we found a clear association between anti-HLA Abs and AR. DSA anti-HLA Abs were often detected before AR; however, sometimes, they peaked at or even after clinical detection of AR. In particular, the development of DQ Abs was the most significantly associated with subsequent AR, suggesting that these patients may benefit from serial analysis of Ab titers and a lower threshold to biopsy the graft for earlier AR diagnosis and treatment. However, more studies are required on HLA specificities, because not all patients who developed de novo anti-HLA Abs in our study experienced AR. Development of NDSA Abs post-transplant has been associated with adverse outcomes in previous studies, which is in contrast to patients who do not produce Abs17; however, we did not find significant association of NDSA Abs with AR in our group of patients, but this result may be because of the small number of patients with positive NDSA Abs.
Clinical heterogeneity of rejection outcomes may be driven by the quantity and composition of different infiltrating cells: T cells, B cells, macrophages, natural killer (NK) cells, and plasma cells; additionally, rejection episodes may vary from being cellular, humoral (antibody-mediated), or mixed with varying prognoses.18,19 Ab-mediated rejection is partly caused by circulating DSA, which is produced by recipient plasmablasts and plasma cells, primed against donor antigen.20 Humoral rejection is refractory to conventional T cell-based immunosuppressive therapy, and it is a strong harbinger of progressive chronic graft injury. Peritubular C4d deposition is a recognized marker for humoral injury,21 but many humoral rejections are C4d-negative because of the short half-life of C4d.5 This finding results in an incomplete diagnosis of humoral rejection. Recent data now suggest that Ab-mediated rejection can occur in the absence of C4D deposition.22 Given these logistical issues of diagnosing humoral rejection, in our study group, 95% of patients were diagnosed by central histology reads to have T cell-mediated acute cellular rejection (IA and IB Banff criteria) with negative C4D; however, 34% (13/38) of these patients are now found to have positive de novo HLA Abs, suggesting a diagnosis of mixed rejection. Thus, detection of DSA associated with a histologic diagnosis of AR could prompt a diagnosis of Ab-mediated humoral rejection, even in the absence of C4D positivity. This finding is especially important given that the most common agents used to treat rejection routinely lack effect on Abs production. After treatment ensues, evidence that the acute process has subsided is shown in histologic resolution. However, in many cases, despite histologic reversal, Ab production is unaffected by treatment, and Ab levels may persist. With highly sensitive testing for HLA Ab production now available, it is possible to clearly define the long-term risk of persisting Ab production after AR resolution. Early evidence suggests that those patients who do persist have poorer outcomes compared with those patients who have Ab reduction/removal after AR treatment.18,23–25 Our data support additional studies to evaluate the clinical impact of prospective monitoring of anti-HLA Abs and the role of strategies to modify the Ab response as a critical factor to extend the longevity of transplanted organs. The recent finding of a blood-based gene panel for diagnosis of both T cell- and antibody-mediated AR in the same cohort group from the SNSO1 trial19 supports that a combination of transcriptional and antibody monitoring techniques for noninvasive diagnosis and prediction of biopsy-confirmed acute renal transplant rejection will be a significant advance in the clinical care of transplant patients, because early detection and prompt treatment of AR can limit chronic injury and extend graft survival.
Anti-MICA Abs have been associated with renal allograft dysfunction, rejection, and graft failure in adults26–29; however, anti-MICA Abs are not routinely measured in clinical practice. We found similar association of de novo MICA Ab development with AR in our single-center cohort (P=0.03), and patients who developed graft loss also had increased titers of MICA Abs detected before graft loss. Additional prospective trials are needed to determine the clinical relevance of the MICA antigen, routine MICA Ab screening, and serial quantification and monitoring of MICA antibody titers levels that could prevent allograft rejection, alter the clinical management of allograft rejection, and enhance graft survival.
Chronic allograft nephropathy and CNIT are major determinants for graft function and longevity. The incidence of chronic allograft injury is underestimated; we found that 48% of our patient group developed interstitial fibrosis and tubular atrophy within 2 years post-transplant and 30% developed lesions of CNIT based on biopsy findings. We found an association between the development of de novo HLA Ab and both CADI and CNIT scores in our patient population, suggesting the role of humoral response in progressive chronic histologic damage, a strong predictor of transplant outcome. Association between the presence of HLA-specific and non-HLA–specific allo-Abs and diminished renal graft function is supported by several studies.30 Longer-time allograft survival in DSA-positive kidney transplants seems to be shorter than the time seen in DSA-negative transplants.31,32 Our study confirmed that patients with de novo DSA Ab development have a greater decline in graft function over time compared with patients who do not. We found similar association with preexisting HLA Abs as well; 4% of patients developed graft failure within the follow-up period of 2 years, and not unexpectedly, all of them were secondary to noncompliance. Increasing titers of HLA and MICA Abs were noted in these patients before graft failure, indicating the prognostic importance of these Abs.
Our study group comprised of patients with low immunologic risk with a low rate of development of clinical events, but nevertheless, it is representative of the pediatric renal transplant recipient population. Donor specificities were not available for MICA as well as Cw, DP, and DQ Abs; however, irrespective of donor specificity, de novo development of these Abs was associated with poor graft outcomes.
In summary, the de novo occurrence of Abs to HLA plays an important role in all types of solid organ rejection, both acute and chronic. It may, therefore, be useful to distinguish patients with a high immunologic risk from those patients with a low immunologic risk. Immunization minimization protocols may incorporate Ab screening as a part of post-transplant monitoring, with a goal of identifying individuals in whom additional immunosuppression reduction may not be feasible. Over the last decade, advances in Ab detection have revolutionized our approach to kidney transplantation. Balancing the need for immunosuppression to prevent allograft rejection while minimizing drug toxicity and the risk of infections and malignancy continues to be a challenging task. The target is for patients to display stable renal function with the lowest risk for infection, malignancy, or rejection. Early identification of transplant recipients with newly developed DSA may permit intervention before allograft injury. In this report, we recommend monitoring of the presence of DSA at post-transplantation stages to predict the unwanted immunologic events, such as rejection, and customize the immunosuppressive strategies for enhanced clinical outcome.
Concise Methods
Patients and Samples
Pediatric subjects, ages 0–21 years, who received a primary kidney transplant from a deceased or living donor and were of low immunologic risk (peak panel reactive antibody titer<20% before transplantation) were enrolled in the SNSO1 (ClinicalTrials.gov Number NCT00141037) after institutional review board approval at each site, informed consent, and where appropriate, patient assent. This multicenter study used a randomized, open-label, parallel group design. Details of immunosuppression, inclusion and exclusion criteria, and study design have been published elsewhere16; 130 patients were enrolled in the trial. SF patients received a protocol of 6 months daclizumab induction, tacrolimus (maintenance trough target of 5–7 ng/ml), and mycophenolate mofetil (target maintenance dosing of 300 mg/m2 per dose); SB patients received a protocol of 2 months daclizumab induction, similar levels of tacrolimus and mycophenolate mofetil, and maintenance prednisone at 0.1 mg/kg per day. Protocol surveillance biopsies were also done in this trial at 0, 6, 12, and 24 months. Blinded histology analysis of this large histology dataset has also been published elsewhere.15 Sera samples were targeted for collection longitudinally at similar time points at 0, 6, 12, and 24 months.
For inclusion in this study, patients were selected if at least three of four serial sera and biopsy samples had been collected and archived and if graft function data were available serially for the first 2 years. Thus, 440 unique sera were examined from 124 SNSO1 patients; 215 sera were from 60 SF patients, and 225 sera were from 64 SB patients. Four hundred forty matching biopsy histologies were examined for acute and chronic injury grades15 and correlated with distinct serum antibody measurements (Figure 1). The two groups were demographically comparable (Table 3).33 Clinical outcomes in prospectively transplanted SF patients were recently published from our single-center study with low rates of AR and excellent outcomes at 8 years of follow-up14 and from the multicenter SNSO1 study with 3 years of follow-up.16 Serial protocol biopsy analysis of the SF and SB patients has also recently been published from our single-center study34 and the multicenter SNSO1 study,16 and it showed no impact of steroid avoidance on the evolution of chronic graft injury. They had been transplanted between 2000 and 2008 (for consistency of clinical and immunosuppression management). Exclusion criteria were sensitized patients, patients with delayed graft function or disease recurrence, or SF patients who transitioned in the first 2 years to SB immunosuppression.35 Patients studied had a mean age of 11.7±5.9 years (range=5–20 years) at the time of transplant, and 39% were female. Patients receiving SF and SB immunosuppression were matched for all clinical criteria, including recipients’ sex, age, race, cause of ESRD, donor source, donor sex, and donor age (Table 3). The study was approved by the Stanford Institutional Review Board.
Table 3.
Baseline patient characteristics of the 124 patients included in the analysis of the SNSO1 study patients15,16
| SF | SB | P | |
|---|---|---|---|
| Total number | 60 | 64 | |
| Recipient | |||
| Mean age (yr) | 11.8±5.8 | 11.6±6.0 | 0.80 |
| Infant≤5 yr (%) | 20% | 22% | 0.73 |
| Mean age (yr) male | 11.8±6.0 | 11.7±6.6 | 0.91 |
| Mean age (yr) female | 11.8±6.0 | 11.4±5.3 | 0.79 |
| Sex: female (%) | 34 | 43 | 0.43 |
| Race (1, 2, 3, 4)a | (62%, 13%, 8%, 16%) | (49%, 26%, 5%, 21%) | 0.25 |
| Cause of ESRD (1, 2, 3, 4, 5, 6, 7, 8)b | (7%, 2%, 15%, 0%, 10%, 11%, 5%, 51%) | (11%, 25, 16%, 11%, 10%, 8%, 10%, 36%) | 0.13 |
| Mean calculated creatinine clearance (ml/min per 1.73 m2) | 13.7±10.6 | 12.1±8.4 | 0.65 |
| Donor | |||
| Mean age (yr) | 30±10.1 | 27.3±9.7 | 0.13 |
| Sex: female (%) | 47 | 40 | 0.97 |
| Donor type (LRD) | 47% | 38% | 0.52 |
| Mean HLA mismatch | 3.85±1.6 | 4.16±1.5 | 0.21 |
LRD, Living Related Donor.
1, white; 2, black/African American; 3, Asian; 4, other.
1, GN; 2, polycystic kidney disease; 3, dysplasia; 4, reflux nephropathy; 5, obstructive uropathy; 6, FSGS; 7, congenital nephrotic syndrome; 8, others.
Detection of Anti-HLA–Specific IgG Abs from Serum Samples
All sera samples were blindly tested at the Terasaki Laboratory, Los Angeles, CA. Each serum sample was screened first with beads coated with pooled HLA class I, HLA class II, or MICA antigen beads (LABScreen Mixed; One Lambda, Inc., Canoga Park, CA). If at least one sample from the patient tested positive by the screening beads, then the entire serial sera set of the patient was next tested by beads individually coated with recombinant rHLA single antigens (LABScreen Single Antigen; One Lambda, Inc.). All LabScreen tests were performed according to the manufacturer’s instructions. Briefly, 20 µL test serum were added to 5 µL beads, incubated in the dark for 30 minutes at room temperature, and washed with wash buffer; 100 μL goat anti-human IgG secondary antibody conjugated with R-Phycoerythrin were added to the beads, incubated for 30 minutes in the dark at room temperature, and then washed and read on the LABScan 100 flow cytometer (One Lambda, Inc.). Every assay included a negative control serum (LSNC; One Lambda, Inc.), which was used to normalize the MFI of test samples. A normalized MFI value of 1000 or greater was considered to be positive. DSAs were positive if Abs were detected against donor HLA-A, -B, or -DR. NDSAs were HLA Abs not directed against the donor HLA type. Donor typing for Cw, DP, and DQ was not available, and hence, they were categorized separately and not as DSA or NDSA.
Diagnosis of AR and Chronic Injury from Biopsy Specimens
Protocol biopsies were obtained in all patients at 0, 6, 12, and 24 months post-transplantation. All biopsies were blindly scored by the single centralized pathologist for the SNSO1 study.16 Diagnosis of AR was made according to the Banff ’97 criteria36 and included grade 1A and above; borderline AR was also noted but not used for the correlation analysis. Evidence of chronic injury was diagnosed and scored semiquantitatively by CADI37 and CNIT scores.38
Serum Creatinine to Assess Graft Function
Graft function was assessed by the calculated creatinine clearance by the Schwartz equation39 at time points 0, 6, 12, and 24 months post-transplant.
Statistical Analyses
Descriptive statistics are presented as percentages, means, and SD; t or chi-squared test and Fisher exact test were used for analysis of continuous or categorical types of data. Multivariate analysis and Pearson/Spearman correlation were used for examining relationships for all parametric or nonparametric variables. P≤0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.1.2 (SAS Institute Inc., Cary, NC).
Disclosures
None.
Acknowledgments
We appreciate the participation of patients in the Stanford University and the National Institutes of Health-funded multicenter randomized study of SNSO1. We also are indebted to transplant patients in the SNSO1 multicenter study centers for support with patient recruitment and sample collections. We thank Nancy Bridges, Daniel Rotrosen, and Nikki Williams from the National Institutes of Health/National Institute of Allergy and Infectious Diseases for their continuous support. The authors are grateful to Dr. Neeraja Kambham from Stanford University for her centralized, blinded reads of graft pathology. We thank the Terasaki Laboratory for conduct and support of all blinded antibody testing. We thank our patients and families for all their support and participation and Leila Nawbat for the help in manuscript preparation and submission.
This study was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health Grant AI-055795 (to O.S. and M.M.S.) made as a component of the Cooperative Clinical Trials in Pediatric Transplantation Consortium. We are also grateful to Astellas and Roche Pharmaceuticals for their generous financial support for this trial.
Footnotes
A.C., M.O., P.I.T., and M.M.S. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Liang LW, Gjertson DW, Lassman C, Wilkinson AH, Kendrick E, Pham PT, Danovitch GM, Gritsch HA, Reed EF: Development of posttransplant antidonor HLA antibodies is associated with acute humoral rejection and early graft dysfunction. Transplantation 79: 591–598, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Terasaki PI, Ozawa M: Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant 4: 438–443, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Sutherland SM, Li L, Sigdel TK, Wadia PP, Miklos DB, Butte AJ, Sarwal MM: Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int 76: 1277–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Buenrostro LE, Alberú J: Anti-major histocompatibility complex class I-related chain A antibodies in organ transplantation. Transplant Rev (Orlando) 22: 27–38, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Sumitran-Holgersson S: Relevance of MICA and other non-HLA antibodies in clinical transplantation. Curr Opin Immunol 20: 607–613, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Sigdel TK, Li L, Tran TQ, Khatri P, Naesens M, Sansanwal P, Dai H, Hsieh SC, Sarwal MM: Non-HLA antibodies to immunogenic epitopes predict the evolution of chronic renal allograft injury. J Am Soc Nephrol 23: 750–763, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Terasaki PI: Humoral theory of transplantation. Am J Transplant 3: 665–673, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Terasaki PI, Cai J: Humoral theory of transplantation: Further evidence. Curr Opin Immunol 17: 541–545, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Sarwal MM, Yorgin PD, Alexander S, Millan MT, Belson A, Belanger N, Granucci L, Major C, Costaglio C, Sanchez J, Orlandi P, Salvatierra O, Jr: Promising early outcomes with a novel, complete steroid avoidance immunosuppression protocol in pediatric renal transplantation. Transplantation 72: 13–21, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Sarwal MM, Vidhun JR, Alexander SR, Satterwhite T, Millan M, Salvatierra O, Jr: Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation 76: 1331–1339, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Li L, Chang A, Naesens M, Kambham N, Waskerwitz J, Martin J, Wong C, Alexander S, Grimm P, Concepcion W, Salvatierra O, Sarwal MM: Steroid-free immunosuppression since 1999: 129 pediatric renal transplants with sustained graft and patient benefits. Am J Transplant 9: 1362–1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naesens M, Salvatierra O, Benfield M, Ettenger RB, Dharnidharka V, Harmon W, Mathias R, Sarwal MM, SNS01-NIH-CCTPT Multicenter Trial : Subclinical inflammation and chronic renal allograft injury in a randomized trial on steroid avoidance in pediatric kidney transplantation. Am J Transplant 12: 2730–2743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarwal MM, Ettenger RB, Dharnidharka V, Benfield M, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Tang L, Liu J, Li L, Naesens M, Sigdel T, Waskerwitz J, Salvatierra O: Complete steroid avoidance is effective and safe in children with renal transplants: A multicenter randomized trial with three-year follow-up. Am J Transplant 12: 2719–2729, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachmann N, Terasaki PI, Schönemann C: Donor-specific HLA antibodies in chronic renal allograft rejection: A prospective trial with a four-year follow-up. Clin Transpl 2006: 171–199, 2006 [PubMed] [Google Scholar]
- 18.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Khatri P, Sigdel TK, Tran T, Ying L, Vitalone MJ, Chen A, Hsieh S, Dai H, Zhang M, Naesens M, Zarkhin V, Sansanwal P, Chen R, Mindrinos M, Xiao W, Benfield M, Ettenger RB, Dharnidharka V, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Davis R, Butte AJ, Salvatierra O, Sarwal MM: A peripheral blood diagnostic test for acute rejection in renal transplantation. Am J Transplant 12: 2710–2718, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Demirci C, Sen S, Sezak M, Sarsik B, Hoşcoşkun C, Töz H: Incidence and importance of c4d deposition in renal allograft dysfunction. Transplant Proc 40: 174–177, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Haas M: C4d-negative antibody-mediated rejection in renal allografts: Evidence for its existence and effect on graft survival. Clin Nephrol 75: 271–278, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Hachem RR, Yusen RD, Meyers BF, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP: Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant 29: 973–980, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Everly MJ: An update on antibody reduction and rejection reversal following bortezomib use: A report of 52 cases across 10 centers. Clin Transpl 2010: 353–362, 2010 [PubMed] [Google Scholar]
- 26.Zou Y, Heinemann FM, Grosse-Wilde H, Sireci G, Wang Z, Lavingia B, Stastny P: Detection of anti-MICA antibodies in patients awaiting kidney transplantation, during the post-transplant course, and in eluates from rejected kidney allografts by Luminex flow cytometry. Hum Immunol 67: 230–237, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Zou Y, Stastny P, Süsal C, Döhler B, Opelz G: Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med 357: 1293–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Amézaga N, Crespo M, Lopez-Cobos M, Millán MA, Viñas O, Solé M, Oppenheimer F, Martorell J, Ercilla MG: Relevance of MICA antibodies in acute humoral rejection in renal transplant patients. Transpl Immunol 17: 39–42, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mizutani K, Terasaki PI, Shih RN, Pei R, Ozawa M, Lee J: Frequency of MIC antibody in rejected renal transplant patients without HLA antibody. Hum Immunol 67: 223–229, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mizutani K, Shibata L, Ozawa M, Esquenazi V, Rosen A, Miller J, Terasaki PI: Detection of HLA and MICA antibodies before kidney graft failure. Clin Transpl 2006: 255–264, 2006 [PubMed] [Google Scholar]
- 31.Terasaki PI, Cai J: Human leukocyte antigen antibodies and chronic rejection: From association to causation. Transplantation 86: 377–383, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff '09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Sarwal M, Ettenger R, Dharnidharka V, Benfield M, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Tang L, Liu J, Li L, Naesens M, Sigdel T, Waskerwitz J, Salvatierra O: Complete steroid avoidance is effective and safe in children with renal transplants: A multicenter randomized trial with three-year follow-up. Am J Transplant 12: 2719–2729, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naesens M, Lerut E, Damme BV, Vanrenterghem Y, Kuypers DR: Tacrolimus exposure and evolution of renal allograft histology in the first year after transplantation. Am J Transplant 7: 2114–2123, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Sutherland S, Li L, Concepcion W, Salvatierra O, Sarwal MM: Steroid-free immunosuppression in pediatric renal transplantation: Rationale for and [corrected] outcomes following conversion to steroid based therapy. Transplantation 87: 1744–1748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou J, Ranshawa P, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Isoniemi H, Taskinen E, Häyry P: Histological chronic allograft damage index accurately predicts chronic renal allograft rejection. Transplantation 58: 1195–1198, 1994 [PubMed] [Google Scholar]
- 38.Kambham N, Nagarajan S, Shah S, Li L, Salvatierra O, Sarwal MM: A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol 2: 135–142, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz GJ, Gauthier B: A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106: 522–526, 1985 [DOI] [PubMed] [Google Scholar]