Abstract
Fibroblast growth factor-23 (FGF23) induces phosphaturia through its effects on renal tubules. Higher levels of FGF23 associate with cardiovascular disease (CVD) events and all-cause mortality, but it is unknown whether these associations differ by the degree of phosphaturia. Here, we measured serum FGF23 and 24-hour urine fractional excretion of phosphorus (FePi) in 872 outpatients with stable CVD and a mean estimated GFR of 71 ml/min per 1.73 m2. During an average 7.5 years of follow-up, there were 337 deaths and 199 CVD events. Urinary FePi significantly modified the association of FGF23 with each outcome (P interaction<0.001 for all-cause mortality and P interaction<0.05 for CVD events). In models adjusted for CVD risk factors, kidney function, and PTH, those patients who had FGF23 above the median (≥42.3 relative units [RU]/ml) but FePi below the median (<15.7%) had the highest risks of both all-cause mortality (HR=1.98, 95% CI=1.42–2.77) and CVD events (HR=1.92, 95% CI=1.25–2.94) compared with those patients who had low concentrations of FGF23 and low FePi. In summary, associations of FGF23 with mortality and CVD events are stronger in persons with lower FePi independent of PTH and kidney function. In such individuals, the renal tubular response to FGF23 may be suboptimal.
Fibroblast growth factor-23 (FGF23) is a hormone secreted primarily by bone osteocytes into the circulation. In the kidney tubule, its two major actions are to promote phosphaturia and inhibit the conversion of calcidiol (25[OH] vitamin D) to the active hormone calcitriol (1,25[OH]2 vitamin D).1 In concert, these actions lower serum phosphorus concentrations.2 Prior studies have shown that higher FGF23 concentrations are associated with a greater burden of atherosclerosis in cross-section, and several studies, including a prior study in the Heart and Soul Cohort, have shown that higher FGF23 concentrations are strongly associated with mortality3–5 and cardiovascular disease (CVD) events.4–6
With even very modest decrements in kidney function, FGF23 concentrations are frequently elevated. This finding is often observed at a stage of CKD where parathyroid hormone (PTH) and serum phosphorus concentrations remain normal.7,8 This finding, coupled with the observation from prior studies that associations of FGF23 concentrations with adverse outcomes may be stronger in patients with CKD,9–12 led us to hypothesize that high FGF23 concentrations may identify a novel dimension of kidney function that is not fully captured by estimated GFR (eGFR) or albuminuria. Specifically, we hypothesized that, independent of eGFR and albuminuria, FGF23 concentrations may be particularly high in individuals with kidney tubules that are less capable of responding to a high FGF23 stimulus.
Because FGF23 is known to induce phosphaturia,1 the fractional excretion of phosphorus (FePi) is higher, on average, in individuals with higher FGF23 concentrations.13 However, there is a wide distribution of FePi at any given FGF23 concentration, suggesting considerable biologic variability in the renal response to FGF23. In individuals with relative renal tubule resistance to FGF23, serum FGF23 concentrations may be secondarily increased in a feedback loop in an attempt to further increase phosphorus excretion.14,15
To our knowledge, no prior study has examined the joint relationships of FGF23 concentrations and urine FePi with adverse outcomes. To that end, we evaluated the strength of the associations of serum FGF23 concentrations with mortality and CVD events and determined the extent to which these associations were modified by the degree of renal phosphorus excretion. We hypothesized that associations of FGF23 concentrations with either outcome would be stronger in the subset of individuals with lower urine FePi.
Results
Among the 872 study participants, the mean age was 67±11 years; 82% of participants were male, and 61% of participants were white. The median FGF23 concentration was 42.3 RU/ml (interquartile range [IQR]=28.1, 70.2), and the median FePi was 15.7% (IQR=11.5%, 20.9%). The mean eGFR was 71±22 ml/min per 1.73 m2, and 267 participants had moderate CKD (defined as eGFR<60 ml/min per 1.73 m2). During 7.5 (mean) years of follow-up, there were 337 deaths and 199 CVD events.
Baseline characteristics of the study participants by FGF23 and FePi values below and above the median are shown in Table 1. (Correlates across four mutually exclusive FGF23/FePi categories are shown in Supplemental Table 1.) Compared with participants with FGF23 concentrations below the median, those participants with higher concentrations were more frequently female, were less likely to be using hormone replacement therapy, were more frequently white, had higher body mass index (BMI), were more likely to smoke, and had higher C-reactive protein (CRP), lower eGFR, higher albumin-to-creatinine ratio (ACR), higher PTH, higher serum phosphorus, and higher FePi. Compared with participants with urine FePi below the median, those participants with higher FePi were older, were more frequently male and white, were less likely to smoke, and had lower eGFR, higher PTH, lower serum phosphorus, lower serum calcium, higher urine phosphorus, and higher FGF23 concentrations.
Table 1.
Baseline characteristics by FGF23 and FePi above and below the median
| Variable | Low FGF23 | High FGF23 | P Value | Low FePi | High FePi | P Value |
|---|---|---|---|---|---|---|
| Number | 437 | 435 | 436 | 436 | ||
| Range (RU/ml for FGF23, % for FePi) | <42.3 | ≥42.3 | <15.7 | ≥15.7 | ||
| Age (yr)±SD | 67±11 | 67±11 | 0.77 | 65±11 | 69±11 | <0.001 |
| Sex n (%) | <0.01 | <0.001 | ||||
| Male | 374 (86) | 341 (78) | 330 (76) | 385 (88) | ||
| Female | 63 (14) | 94 (22) | 106 (24) | 51 (12) | ||
| Estrogen use (women) n (%) | 24 (38) | 17 (18) | <0.01 | 29 (27) | 12 (24) | 0.61 |
| Race n (%) | 0.04 | <0.001 | ||||
| White | 253 (58) | 278 (64) | 236 (54) | 295 (68) | ||
| Black | 63 (14) | 68 (16) | 89 (20) | 42 (10) | ||
| Other | 121 (28) | 89 (20) | 111 (25) | 99 (23) | ||
| Diabetes n (%) | 105 (24) | 122 (28) | 0.18 | 121 (28) | 106 (24) | 0.25 |
| Systolic BP (mmHg)±SD | 132±21 | 134±21 | 0.18 | 133±21 | 133±21 | 0.88 |
| BMI (kg/m2)±SD | 28.1±4.6 | 28.8±6.0 | 0.05 | 28.7±5.5 | 28.1±5.2 | 0.07 |
| Smoking n (%) | 67 (15) | 97 (22) | <0.01 | 96 (22) | 68 (16) | 0.02 |
| Total cholesterol (mg/dl)±SD | 175±39 | 180±46 | 0.07 | 179±43 | 176±42 | 0.20 |
| HDL cholesterol (mg/dl)±SD | 46±13 | 45±15 | 0.19 | 46±14 | 45±14 | 0.23 |
| CRP (mg/dl) median (IQR) | 1.8 (0.7–3.5) | 2.8 (1.1–6.3) | <0.001 | 2.3 (0.9–4.6) | 2.1 (0.9–4.8) | 0.95 |
| eGFR (ml/min per 1.73 m2)±SD | 77±19 | 65±24 | <0.001 | 77±23 | 65±20 | <0.001 |
| ACR (mg/g) median (IQR) | 8.0 (4.6–14.6) | 10.5 (5.7–23.5) | <0.001 | 8.4 (4.9–16.6) | 9.3 (5.3–20.8) | 0.22 |
| PTH (pg/ml) median (IQR) | 50 (40–65) | 56 (40–79) | <0.01 | 50 (39–65) | 56 (42–74) | <0.001 |
| Serum phosphorus (mg/dl)±SD | 3.6±0.5 | 3.8±0.6 | <0.001 | 3.8±0.5 | 3.5±0.6 | <0.001 |
| Serum calcium (mg/dl)±SD | 9.5±0.5 | 9.5±0.5 | 0.18 | 9.6±0.5 | 9.5±0.5 | 0.01 |
| Urine phosphorus (mg/dl)±SD | 47±25 | 46±25 | 0.50 | 37±21 | 56±26 | <0.001 |
| FePi (%)±SD | 16±7 | 19±10 | <0.001 | — | — | — |
| FGF23 (RU/ml) median (IQR) | — | — | — | 40 (26–60) | 47 (30–80) | <0.001 |
Table 2 shows correlations between the serum and urine markers of mineral metabolism with one another and eGFR at baseline. The strongest correlations were between eGFR and FePi and eGFR and ln(FGF23) (r=−0.40 and −0.35, respectively). The other correlations were more modest.
Table 2.
Correlations between serum and urine markers of mineral metabolism
| Mineral Marker | FePi | Ln(FGF23) | Serum Pi | Ln(PTH) | Serum Ca | eGFR |
|---|---|---|---|---|---|---|
| FePi | 1.00 | 0.21a | −0.16a | 0.25a | −0.09a | −0.40a |
| Ln(FGF23) | 1.00 | 0.22a | 0.19a | 0.05 | −0.35a | |
| Serum Pi | 1.00 | −0.16a | 0.17a | −0.08a | ||
| Ln(PTH) | 1.00 | −0.19a | −0.32a | |||
| Serum Ca | 1.00 | 0.06 | ||||
| eGFR | 1.00 |
P<0.05.
In a model adjusted for age, sex, race, and eGFR, FGF23 was strongly associated with all-cause mortality (hazard ratio [HR] per doubling=1.30, 95% confidence interval [CI]=1.19–1.43; P<0.001), whereas FePi was not (HR per doubling=0.98, 95% CI=0.83–1.16; P=0.84). Results were similar for CVD events (HR per doubling of FGF23=1.30, 95% CI=1.15–1.45; P<0.001; HR per doubling of FePi=0.94, 95% CI=0.76–1.17; P=0.58). However, we observed that the association of FGF23 with either outcome was modified by the FePi level (P interaction<0.001 for mortality and P interaction<0.05 for CVD events).
To explore the interactions, we developed four mutually exclusive categories defined by FGF23 and FePi above or below their medians and set the group with low FGF23 and low FePi as the reference category. In a model adjusted for age, sex, estrogen use (women), and race, those participants who simultaneously had high FGF23 and low FePi were at the highest risk of all-cause mortality (HR=2.21, 95% CI=1.60–3.06). The high FGF23/high FePi category was at intermediate mortality risk, whereas the low FGF23/high FePi category had risk similar to the referent category (HR=1.96, 95% CI=1.45–2.66 and HR=0.97, 95% CI=0.68–1.37, respectively) (Table 3). Additional adjustment for CVD risk factors, eGFR, ACR, and PTH did not materially change the associations.
Table 3.
Associations of categories based on FGF23 and fractional excretion of phosphorus above versus below the median with mortality and CVD events
| FGF23/FePi Category | Participants | Events | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|---|---|
| All-cause mortalitya | ||||
| Low FGF23/low FePi | 240 | 68 | 1.00 (reference) | 1.00 (reference) |
| Low FGF23/high FePi | 197 | 62 | 0.97 (0.68, 1.37) | 0.99 (0.70, 1.42) |
| High FGF23/high FePi | 239 | 119 | 1.96 (1.45, 2.66) | 1.49 (1.07, 2.07) |
| High FGF23/low FePi | 196 | 88 | 2.21 (1.60, 3.06) | 1.98 (1.42, 2.77) |
| CVD events (MI, stroke, and CVD death)b | ||||
| Low FGF23/low FePi | 240 | 39 | 1.00 (reference) | 1.00 (reference) |
| Low FGF23/high FePi | 197 | 39 | 1.09 (0.69, 1.70) | 1.04 (0.66, 1.63) |
| High FGF23/high FePi | 239 | 66 | 1.69 (1.13, 2.53) | 1.21 (0.78, 1.86) |
| High FGF23/low FePi | 196 | 55 | 2.22 (1.46, 3.36) | 1.92 (1.25, 2.94) |
Model 1, age, sex, estrogen use (women), and race adjusted; model 2, model 1 plus diabetes, systolic BP, BMI, total cholesterol, HDL cholesterol, smoking, CRP, eGFR, ACR, and PTH.
P interaction FGF23 × FePi<0.001.
P interaction FGF23 × FePi=0.05.
Results were similar for CVD events, where the high FGF23/low FePi category was at approximately twofold risk of CVD events compared with the low FGF23/low FePi reference category in adjusted models. The high FGF23/high FePi category and low FGF23/high FePi category were at intermediate risk (HR=1.21, 95% CI=0.78–1.86 and HR=1.04, 95% CI=0.66–1.63, respectively). To examine the nature of these relationships further, we stratified participants into quartiles based on FGF23 concentration. Participants with FGF23 in the highest quartile who nonetheless had FePi below the median were at highest risk of death and CVD events (Figure 1).
Figure 1.
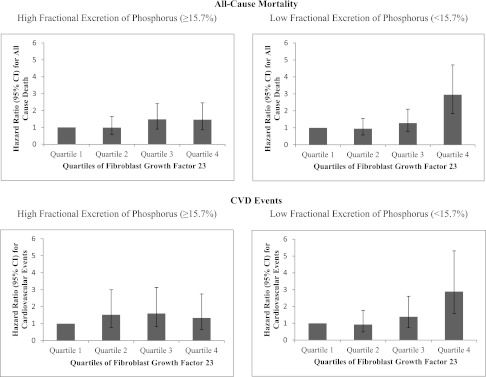
Adjusted HRs of FGF23 quartiles with mortality and CVD events stratified by urine fractional excretion of phosphorus above versus below the median. The low FGF23 quartile served as the reference category. Models were adjusted for age, sex, estrogen use (women), race, diabetes, systolic BP, BMI, total cholesterol, HDL cholesterol, smoking, CRP, eGFR, ACR, and PTH. Cut points for FGF23 quartiles were quartile 1, FGF23<27.7 RU/ml; quartile 2, FGF23=27.8–42.3 RU/ml; quartile 3, FGF23=42.3–69.5 RU/ml; quartile 4, FGF23>69.5 RU/ml.
We also explored associations when participants were stratified based on eGFR<60 ml/min per 1.73 m2 versus higher. The high FGF23/low FePi category remained the highest risk group and was associated with a two- to threefold higher risk of mortality and CVD events, regardless of CKD status (Table 4).
Table 4.
Adjusted associations of categories based on FGF23 and fractional excretion of phosphorus above versus below the median with mortality and CVD events stratified by CKD status
| FGF23/FePi Category | No CKD | CKD | ||||
|---|---|---|---|---|---|---|
| Participants | Events | HR (95% CI) | Participants | Events | HR (95% CI) | |
| All-cause mortality | ||||||
| Low FGF23/low FePi | 213 | 53 | 1.00 (reference) | 27 | 15 | 1.00 (reference) |
| Low FGF23/high FePi | 146 | 36 | 0.95 (0.61, 1.47) | 51 | 26 | 1.05 (0.53, 2.07) |
| High FGF23/high FePi | 109 | 32 | 1.11 (0.70, 1.76) | 130 | 87 | 1.88 (1.03, 3.43) |
| High FGF23/low FePi | 137 | 49 | 1.84 (1.22, 2.78) | 59 | 39 | 2.10 (1.08, 4.09) |
| CVD events (MI, stroke, and CVD death) | ||||||
| Low FGF23/low FePi | 213 | 33 | 1.00 (reference) | 27 | 6 | 1.00 (reference) |
| Low FGF23/high FePi | 146 | 23 | 0.90 (0.52, 1.55) | 51 | 16 | 1.53 (0.58, 4.03) |
| High FGF23/high FePi | 109 | 18 | 0.96 (0.53, 1.72) | 130 | 48 | 2.16 (0.89, 5.26) |
| High FGF23/low FePi | 137 | 32 | 1.81 (1.09, 2.99) | 59 | 23 | 2.86 (1.10, 7.41) |
Adjusted for age, sex, estrogen use (women), race, diabetes, systolic BP, BMI, total cholesterol, HDL cholesterol, smoking, CRP, eGFR, ACR, and PTH.
Discussion
In the Heart and Soul Cohort, we have previously reported that higher FGF23 concentrations are associated with all-cause mortality and CVD events.5 Similarly, in general population cohorts, higher FGF23 concentrations have been associated with mortality,9 CVD events,9 atherosclerosis,10 left ventricular hypertrophy,12 and arterial stiffness and dysfunction.11 Here, we extend these findings by evaluating the joint associations of FGF23 and renal fractional excretion of phosphorus for the first time. In this study, among 872 outpatients with a range of kidney function from normal to moderate CKD, we found that higher FGF23 concentrations are more strongly associated with mortality and CVD events when they are accompanied by lower 24-hour urine fractional excretion of phosphorus independent of eGFR, PTH concentrations, or CVD risk factors.
Klotho is a membrane-bound cofactor that is required for FGF23 binding to its receptor in the renal tubule and subsequent induction of phosphaturia.16 Kidney biopsy studies show that persons with CKD and ESRD have lower renal tubular klotho expression,17 low serum soluble klotho, and low urine klotho concentrations.18,19 Prior studies in mice show that klotho haplo insufficiency (approximately 50% reduction in klotho) is accompanied by an approximately threefold higher FGF23 concentration, suggesting that FGF23 may be increased in a compensatory fashion to induce its biologic effects when klotho is decreased.14,15 In this context, our findings could suggest that concurrent high FGF23 and low FePi may be serving as a noninvasive marker of low kidney klotho expression or activity. Future studies with kidney pathology specimens suitable for klotho assays are required to confirm this hypothesis.
Recently, other markers of renal tubular dysfunction and injury have been associated with adverse outcomes in community-living cohorts independent of eGFR and albuminuria. For example, kidney tubule and collecting duct cells are critical for acid/base regulation. Low serum bicarbonate concentrations have been identified as a marker of CKD progression independent of eGFR.20 Similarly, neutrophil gelatinase-associated lipocalin, a marker of kidney tubule cell damage originally identified in AKI studies,21 has been associated with interstitial fibrosis and tubular atrophy in renal biopsies in community-living patients with CKD.22 Moreover, higher urine neutrophil gelatinase-associated lipocalin concentrations were recently associated with incident development of CKD in community-living individuals without AKI.23 Similar findings have been reported for other markers of kidney tubule cell damage.24 Our results suggest that concurrent high FGF23 and low FePi may identify individuals at higher risk of mortality and CVD events, outcomes that are tightly linked with CKD.25 Collectively, these data suggest that noninvasive markers of kidney tubule dysfunction and injury may provide information on risk of adverse outcomes above and beyond eGFR and ACR.
A recent study reported that FGF23 directly induced cardiac myocyte hypertrophy in mice through pathways independent of klotho.14 The idea that FGF23 may be directly toxic does not necessarily exclude the concept that higher levels may mark renal tubule dysfunction. Kidney tubule resistance to FGF23 actions or klotho deficiency may secondarily lead to higher FGF23 concentrations,14 and higher FGF23 concentrations, in turn, may have adverse consequences on cardiac cells.
Strengths of this study include simultaneous availability of serum FGF23 concentrations, 24-hour urine phosphorus, multiple potential confounding variables, and long-term follow-up for adjudicated CVD events and mortality. The study also has important limitations. Despite the unique availability of 24-hour urine measurements in a relatively large cohort, urine measurements were made at a single point in time, and the effect of variability in these measurements over time cannot be assessed. Use of vitamin D supplements, serum vitamin D concentrations, and klotho measurements were not available. We used the C-terminal FGF23 assay; whether the results would be similar using the full-length assay is uncertain. Participants were predominantly male; all had prevalent CVD at baseline, and few had advanced CKD. Whether results generalize to individuals without CVD or with more advanced CKD requires future study.
In conclusion, in community-living individuals with stable CVD and a spectrum of kidney function ranging from normal to moderate CKD, higher FGF23 concentrations are more strongly associated with mortality and CVD events when simultaneously accompanied by lower urine fractional excretion of phosphorus. Concurrent measurement of FGF23 and urine FePi may provide a noninvasive index of kidney tubule resistance to FGF23 action. The association of FGF23 with adverse clinical outcomes reported here and elsewhere may, in part, reflect kidney tubule dysfunction that is not captured by measurement of eGFR and albuminuria alone.
Concise Methods
Participants
The Heart and Soul Study is an observational study designed to investigate the influence of psychosocial factors on the progression of CVD. Study design and recruitment techniques have been described elsewhere.26 Briefly, persons with prevalent coronary artery disease in San Francisco Bay area outpatient clinics were invited to participate if they met one of the following inclusion criteria: a history of myocardial infarction (MI), angiographic evidence of >50% stenosis in more than or equal to one coronary artery, evidence of exercise-induced ischemia using treadmill or nuclear testing, history of coronary artery revascularization, or documented diagnosis of coronary artery disease by an internist or cardiologist. Exclusion criteria included inability to walk 1 block, MI within the previous 6 months, or plans to leave the area within 3 years. The Institutional Review Boards of the participating centers approved the study protocol, and all participants provided written informed consent.
From September of 2000 to December of 2002, a total of 1,024 participants enrolled and had a baseline study visit, during which time they provided medical history, underwent a physical examination, and answered a comprehensive health status questionnaire. Participants also provided fasting (12-hour) morning venous blood samples and 24-hour urine collections.5 For the present analysis, we excluded participants with insufficient blood for FGF23 measurement (n=36) or missing serum phosphorus (n=5), urine phosphorus (n=92), urine creatinine (n=1), or any covariate data (n=18), resulting in a final sample of 872 participants.
Measurements
FGF23
Plasma FGF23 concentrations were measured two times in each individual in the same baseline blood specimen using a C-terminal human ELISA (Immutopics; www.immutopicsint.com), and results for each individual were averaged.27 The intra-assay coefficient of variation (CV) was <5.1%, and interassay CVs were 9.9% at a concentration of 36.4 RU/ml and 12.6% at a concentration of 379 RU/ml.
Urinary Fractional Excretion of Phosphorus
Serum and urine creatinine were measured using the rate Jaffé method. Serum phosphorus was measured on fasting morning blood specimens using a Vitros 950IRC analyzer (Ortho Clinical Diagnostics; www.orthomedical.com). The range was 0.3–13 mg/dl with a CV <3.5%. Urine phosphorus was measured in 24-hour urine specimens using the Cobas 6000 analyzer (Roche Diagnostics; www.roche.com) with lower limit of detection 3.4 of mg/dl and CV=1.4%–1.7%.
The fractional excretion of phosphorus (FePi [%]) was calculated as [urine phosphorus (mg/dl)/serum phosphorus (mg/dl)]×[serum creatinine (mg/dl)/urine creatinine (mg/dl)]×100.
All-Cause Mortality and Cardiovascular Events
Participants or their proxies were contacted annually from the time of enrollment through May 2, 2012, and they were asked about hospitalizations, cardiac procedures, and death. Two independent and blinded adjudicators classified events using medical records, electrocardiograms, death certificates, and coroners’ reports. In the event of disagreements, the adjudicators conferred, reconsidered their decision, and requested a third blinded adjudicator when necessary.
All-cause mortality was determined by review of death certificates. CVD event was defined as MI, stroke, transient ischemic attack, or CVD death. MI was defined by cardiac biomarkers, electrocardiography results, and cardiac symptoms or signs according to the American Heart Association Criteria.28 Stroke was defined as a new neurologic deficit not secondary to brain trauma, tumor, infection, or other cause. Transient ischemic attack was defined as a focal neurologic deficit (in the absence of head trauma) lasting more than 30 seconds and no longer than 24 hours, with rapid symptom evolution to the maximal level of deficit in less than 5 minutes with subsequent complete resolution. CVD death was defined as (1) death during the same hospitalization in which an acute MI was documented or (2) death not explained by other causes that occurred within 1 hour of the onset of terminal symptoms.
Other Measurements
Age, sex, race/ethnicity, and smoking status were determined by questionnaire. Participants brought all medication bottles to the baseline visit, and this information was recorded by study personnel. BP was measured with a calibrated sphygmomanometer in the supine position after 5 minutes of rest. Weight and height were measured in light clothes and without shoes. BMI was calculated as weight (in kilograms) divided by height (in meters) squared. Diabetes was defined as self-reported history of diabetes or use of oral glucose-lowering medications or insulin. Urine albumin was measured using nephelometry and indexed to urine creatinine to yield ACR in milligrams per gram.29 Cystatin C was measured using a BNII nephelometer (Siemens; www.medical.siemens.com) that used a particle-enhanced immunonephelometric assay (N Latex Cystatin C).30 The assay range is 0.195–7.330 mg/L, with intra-assay CV ranging from 2% to 2.8% and interassay CV ranging from 2.3% to 3.1%. The GFR was estimated using the equation eGFR=76.7×(serum cystatin C)−1.19.31 Plasma PTH levels were measured using the Roche immunoassay on an Elecsys E170 automated analyzer. The lower limit of detection was 6 pg/ml. The CV of the assay is 1.8% at a concentration of 167 pg/ml and 3.0% at 20 pg/ml. Serum calcium was measured using a Vitros 950IRC analyzer (Ortho Clinical Diagnostics; www.orthomedical.com). The assay range was 1–14 mg/dl with a CV<2%. High-sensitivity CRP concentrations were measured with the Roche assay (Roche Diagnostics; www.roche.com) and the Beckman Extended Range assay (Galway, Ireland). The interassay CV was 6.7%, and the intra-assay CV was 6.2%. Total cholesterol and HDL levels were measured using standard clinical analyzers.
Statistical Analyses
Participants were divided into two categories based on FGF23 concentrations above or below the median. Participants’ baseline characteristics were compared between groups using the t test for continuous variables, the Mann–Whitney U test for skewed variables, and the chi-squared test for categorical variables. This analysis was repeated categorizing participants into two groups based on urine FePi levels above or below the median. We used Pearson correlations to examine the cross-sectional relationship of FePi, natural log-transformed FGF23, serum phosphorus, natural log-transformed PTH, serum calcium, and eGFR.
Next, we created four mutually exclusive groups based on FGF23 and FePi levels above versus below the median. We used Cox proportional hazards regression to evaluate the associations of FGF23/FePi categories with all-cause mortality and CVD events using the low FGF23/low FePi group as the referent. The first model was adjusted for age, sex, race/ethnicity, and estrogen use (in women). The second model was additionally adjusted for CVD risk factors (diabetes, systolic BP, BMI, total cholesterol, HDL cholesterol, smoking, and CRP), eGFR, ACR, and PTH. We tested nested models with and without an FGF23×FePi interaction term, and statistical significance was determined using the likelihood ratio test. Last, we evaluated whether associations seemed similar in persons with or without CKD. We stratified participants based on presence or absence of CKD (eGFR<60 ml/min per 1.73 m2) and evaluated the four mutually exclusive FGF23/FePi categories within each strata.
Analyses were conducted using Stata SE version 11.0 (StataCorp, College Station, TX), and P values<0.05 were considered statistically significant for all analyses, including interaction terms.
Disclosures
None.
Acknowledgments
J.R.D. was supported by National Heart, Lung, and Blood Institute Training Grant T32 HL007261. J.H.I. was supported by National Heart, Lung, and Blood Institute Grant R01HL096851. The Heart and Soul Study was supported by the Department of Veterans Epidemiology Merit Review Program, the Department of Veterans Affairs Health Services Research and Development Service, National Heart, Lung, and Blood Institute Grant R01 HL079235, the American Federation for Aging Research (Paul Beeson Scholars Program), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), and the Ischemia Research and Education Foundation. This ancillary study was supported by the American Heart Association and the National Heart, Lung, and Blood Institute. Fibroblast growth factor-23 and urine measurements were supported by grants from the American Heart Association and the Sandra A. Daugherty Foundation. This material is the result of work supported with resources of the Veterans Affairs San Diego Healthcare System.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Fibroblast Growth Factor-23 and Outcomes: New Answers, New Questions,” on pages 523–525.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012090894/-/DCSupplemental.
References
- 1.Liu S, Quarles LD: How fibroblast growth factor 23 works. J Am Soc Nephrol 18: 1637–1647, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Zoppellaro G, Faggin E, Puato M, Pauletto P, Rattazzi M: Fibroblast growth factor 23 and the bone-vascular axis: Lessons learned from animal studies. Am J Kidney Dis 59: 135–144, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH: FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant 25: 3983–3989, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ix JH, Shlipak MG, Wassel CL, Whooley MA: Fibroblast growth factor-23 and early decrements in kidney function: The Heart and Soul Study. Nephrol Dial Transplant 25: 993–997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG: Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza MA, Hansen T, Johansson L, Ahlström H, Larsson A, Lind L, Larsson TE: Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant 24: 3125–3131, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Mirza MA, Larsson A, Lind L, Larsson TE: Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 205: 385–390, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE: Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207: 546–551, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA: Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: The Heart and Soul Study. Am J Kidney Dis 58: 737–745, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE: Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 23: 1641–1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuro-o M: Klotho in health and disease. Curr Opin Nephrol Hypertens 21: 362–368, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Nickolas TL, Forster CS, Sise ME, Barasch N, Valle DS, Viltard M, Buchen C, Kupferman S, Carnevali ML, Bennett M, Mattei S, Bovino A, Argentiero L, Magnano A, Devarajan P, Mori K, Erdjument-Bromage H, Tempst P, Allegri L, Barasch J: NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int 82: 718–722, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhavsar NA, Köttgen A, Coresh J, Astor BC: Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 233–240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS: Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 300: 2379–2388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H, AHA Council on Epidemiology and Prevention. AHA Statistics Committee. World Heart Federation Council on Epidemiology and Prevention. European Society of Cardiology Working Group on Epidemiology and Prevention. Centers for Disease Control and Prevention. National Heart, Lung, and Blood Institute : Case definitions for acute coronary heart disease in epidemiology and clinical research studies: A statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 108: 2543–2549, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Erlandsen EJ, Randers E, Kristensen JH: Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest 59: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


