Abstract
Loss of tolerance to neutrophil myeloperoxidase (MPO) underlies the development of ANCA-associated vasculitis and GN, but the mechanisms underlying this loss of tolerance are poorly understood. Here, we assessed the role of the thymus in deletion of autoreactive anti-MPO T cells and the importance of peripheral regulatory T cells in maintaining tolerance to MPO and protecting from GN. Thymic expression of MPO mRNA predominantly localized to medullary thymic epithelial cells. To assess the role of MPO in forming the T cell repertoire and the role of the autoimmune regulator Aire in thymic MPO expression, we compared the effects of immunizing Mpo−/− mice, Aire−/− mice, and control littermates with MPO. Immunized Mpo−/− and Aire−/− mice developed significantly more proinflammatory cytokine-producing anti-MPO T cells and higher ANCA titers than control mice. When we triggered GN with a subnephritogenic dose of anti-glomerular basement membrane antibody, Aire−/− mice had more severe renal disease than Aire+/+ mice, consistent with a role for Aire-dependent central deletion in establishing tolerance to MPO. Furthermore, depleting peripheral regulatory T cells in wild-type mice also led to more anti-MPO T cells, higher ANCA titers, and more severe GN after immunization with MPO. Taken together, these results suggest that Aire-dependent central deletion and regulatory T cell–mediated peripheral tolerance both play major roles in establishing and maintaining tolerance to MPO, thereby protecting against the development of anti-MPO GN.
Systemic autoimmunity to myeloperoxidase (MPO) is directly involved in causing the glomerular and vascular inflammation of ANCA-associated pauci-immune necrotizing autoimmune anti-MPO GN (AIMPOGN).1–3 ANCA induces neutrophil activation and endothelial cell adhesion, with the release of neutrophil extracellular traps containing MPO and proteases triggering endothelial injury.4,5 Experimental studies demonstrate that autoimmune anti-MPO CD4+ T cells respond to glomerular MPO deposited by degranulating neutrophils, directing injurious delayed type hypersensitivity (DTH)–mediated injury.6–8
Immunologic tolerance is maintained by central and peripheral mechanisms, allowing the immune system to discriminate between self and non-self antigens. Central tolerance involves thymic deletion of thymocytes with high-affinity interactions between the T cell receptor and self-peptide MHC complexes, preventing many potentially autoreactive T cells from entering the periphery.9 The role of central tolerance in the maintenance of tolerance to the potential kidney autoantigen, MPO, is largely unknown. The autoimmune regulator (Aire) transcription factor is important for the induction and regulation of tolerance.10–12 Aire is primarily found in lymphoid organs, particularly in the thymus where it is predominantly found in the nuclei of mature, highly MHC II–expressing13–15 medullary thymic epithelial cells (mTECs).16,17 Aire promotes the promiscuous expression of tissue-restricted antigens (TRAs) in mTECs.13,16–18 However, the mechanisms by which Aire controls the presentation of TRA expression in mTECs and its effect on tolerance and autoimmunity remain to be fully defined.
Despite central tolerance, some autoreactive cells escape the selection process, entering the periphery where they may cause autoimmunity if activated.19,20 Naturally arising CD4+CD25+Foxp3+ regulatory T cells (Tregs), mainly produced by the thymus by high-affinity interactions with thymic epithelial cells,21 are a distinct T cell population that plays a pivotal role in the maintenance of self-tolerance. Several studies demonstrate the importance of Tregs in the prevention of organ-specific autoimmunity by potently suppressing autoreactive T cells in a contact-dependent and cytokine-independent manner.22–26 Depletion of Tregs leads to the spontaneous development of some autoimmune diseases.27–29
To assess the role of central and peripheral tolerance in regulating the development of autoimmunity to MPO, we used a validated model of MPO-induced autoimmunity.6,7,30 Establishment of anti-MPO autoimmunity directs the development of focal necrotizing GN similar to that seen in human ANCA-associated GN. Our studies demonstrate the importance of both central and peripheral mechanisms in maintaining tolerance to MPO. Aire promotes thymic MPO expression and enhances central deletion of autoreactive anti-MPO T cells, whereas peripheral Tregs suppress potentially autoreactive MPO-specific CD4+ T cells. Both mechanisms limit anti-MPO GN.
Results
MPO mRNA Is Predominantly Expressed by MHC II–Expressing Medullary Thymic Epithelial Cells in an Aire-Dependent Manner
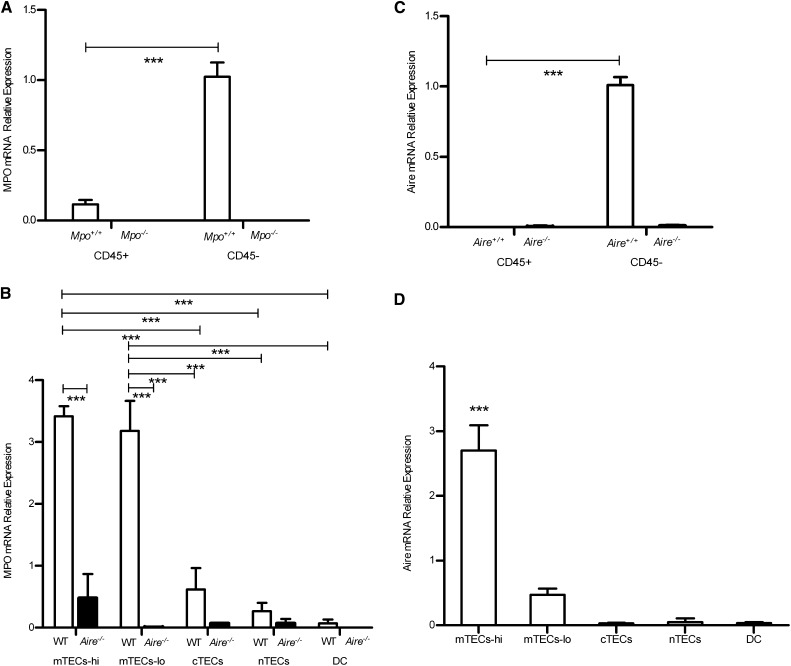
After enzymatic digestion of thymic tissue and flow cytometric sorting of thymic stromal cell (TSC) subsets, transcripts for MPO were detected in the Mpo+/+ mice, but not in Mpo−/− mice, which served as a negative control. Within the mouse thymus, MPO mRNA is highly expressed in the CD45− TSC subpopulation, but was only minimally detected in the CD45+ thymic hematopoietic subpopulation (Figure 1A). Of the CD45− population, the major cell subpopulation expressing MPO mRNA was the mTECs. MPO was expressed in both the MHC II high-expressing mTECs (mTECs-hi) and MHC II low-expressing mTECs (mTEC-lo) (Figure 1B). Expression in mTEC-hi is consistent with the known critical involvement of these cells in the development of the T cell repertoire.31 Given that Aire is expressed only on the CD45− nonhematopoietic population, and predominantly by the mature mTECs (mTECs-hi) (Figure 1, C and D), we determined whether MPO expression would be altered in Aire−/− mice. We found that MPO expression was almost absent from the mTEC-hi and mTEC-lo cells in Aire−/− mice (Figure 1B), suggesting a strong association with Aire-dependent peripheral antigen expression.
Figure 1.
Expression of MPO and Aire mRNA by thymic cell populations, including hematopoietic TECs (CD45+), nonhematopoietic TECs (CD45−), medullary TECs (mTECs), cortical TECs (cTECs), and non-TECs (nTECs) with either high (hi) or low (lo) coexpression of MHC II, and dendritic cells (DCs). (A) MPO is predominantly expressed on nonhematopoietic populations in the Mpo+/+ thymus. (B) MPO is predominantly seen in MHC II coexpressing mTECs in the WT mice compared with the Aire−/− mice. (C) Aire is expressed on the nonhematopoietic populations in the Aire+/+ thymus. (D) Within the CD45− population, Aire is predominantly expressed by the mTECs-hi cells. The asterisks denote the statistical differences between mTECs-hi with mTECs-lo, cTECs, nTECs, or DCs (***P<0.001).
MPO Protein Expression Confined to Neutrophils and Macrophages
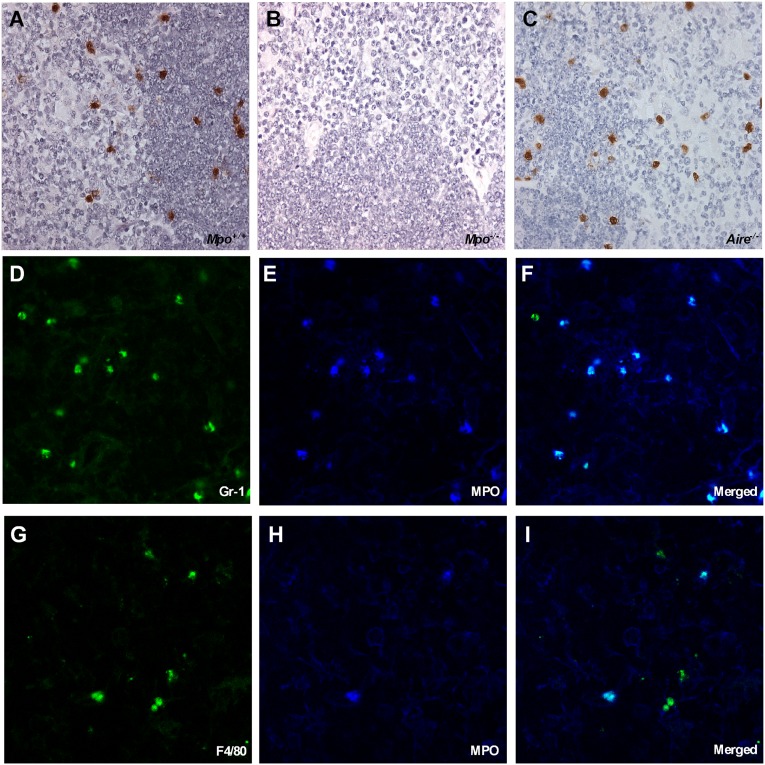
The presence of MPO protein was determined by immunohistology staining. We examined the thymi of 5-week-old Mpo+/+, Mpo−/−, and Aire−/− mice (Figure 2, A–C, respectively). MPO protein is present in Mpo+/+ and Aire−/− mice, but as expected, absent from Mpo−/− thymi (Figure 2, A–C). The MPO distribution was along the corticomedullary junction. Colocalization studies demonstrated MPO localization to neutrophils (Figure 2, D–F) and macrophages (Figure 2, G–I) in the thymic compartment of all five thymus samples. Confocal immunofluorescence microscopy revealed that all neutrophils and some macrophages are MPO positive. It was also evident that most of the MPO positive cells are neutrophils.
Figure 2.
MPO immunostaining of murine thymii demonstrated that thymic MPO expression was predominantly in neutrophils. Immunohistochemical staining for MPO protein on murine thymic tissue sections from Mpo+/+ mice (A), Mpo−/− mice (B), and Aire−/− mice (C). The immunohistochemical staining is visualized by light microscopy. The higher cell density region constitutes the cortex, whereas the lower cell density region constitutes the medulla. The MPO protein distribution is in the corticomedullary junction in the normal Mpo+/+ and Aire−/− thymi. MPO protein is not detected in Mpo−/− mice. Colocalization of thymic neutrophils (D–F) and macrophages (G–I) with MPO in Mpo+/+ mice with MPO. The immunofluorescence is visualized by laser scanning confocal microscopy. (D) Neutrophils (Gr-1 green) are almost all positive for MPO (dark blue) (E), as observed by the merged image MPO and Gr-1 (light blue) (F). Fewer macrophages are present as demonstrated by F4/80 (green) (G), but most are MPO positive (dark blue) (H) as observed by the merged image MPO and F4/80 (light blue) (I). Original magnification, ×400 in A–C; ×800 in D–I.
Thymic Aire-Dependent MPO Expression Deletes Potentially Autoreactive Anti-MPO T Cells and Protects against AIMPOGN
We assessed MPO-specific cell-mediated immune responses with regard to the Th1 and Th17 subset differentiations in lymph node cells (LNs) draining the sites of MPO immunization and serum levels of MPO-ANCA titers in groups of wild-type (WT), Mpo−/−, and Aire−/− mice. In both Mpo−/− and Aire−/− mice, the frequencies of anti-MPO autoreactive CD4+ T cells by enzyme-linked immunosorbent spot (ELISPOT) assay and the titers of circulating MPO-ANCA were increased compared with Mpo+/+ (Figure 3, A, B, F–H) and Aire+/+ littermate WT controls (Figure 3, C and I). The dermal DTH response to MPO was also increased in Aire−/− mice (Figure 3D). A control protein, ovalbumin (OVA) in Freund's complete adjuvant (FCA), did not induce anti-MPO autoimmunity in Mpo+/+ mice. Circulating anti-MPO lgG, lgG1, and lgG2c levels (Figure 3, E–G) were detected in normal Mpo+/+ mice after MPO immunization (not in OVA-immunized mice), but were significantly higher in Mpo−/− and Aire−/− mice (Figure 3H). There was no difference between Mpo+/+ and Mpo−/− mice in the frequency of Foxp3+ expression of the CD4+ T cells isolated from LNs draining the sites of injection (Figure 3, I and J). The absence of thymic MPO and Aire affected the inducible repertoire of autoreactive anti-MPO CD4+ T cells and the levels of autoantibody in a manner consistent with a role for thymic expression of MPO in deleting potentially autoreactive anti-MPO CD4+ T cells.
Figure 3.
Assessment of autoimmunity in Mpo+/+, Mpo−/−, and Aire−/− mice immunized with MPO. The frequency of IFN-γ–producing LN cells (A) and IL-17A–producing LN cells (B) is enhanced in Mpo+/+ mice compared with OVA-immunized controls, but is significantly greater in Mpo−/− mice. (C and D) IFN-γ–producing LN cells as well as T cell responsiveness to MPO by DTH measurement are also enhanced in Aire−/− mice compared with Aire+/+ mice. Anti-MPO ANCA IgG titers (E), IgG1 (1:40) (F), and IgG2c (1:80) (G) are higher in MPO-immunized Mpo+/+ mice versus OVA-immunized mice, but are significantly higher inMpo−/− mice. Dotted lines represent naive Mpo+/+ mice. (H) Anti-MPO ANCA IgG titers are also enhanced in Aire−/− mice compared with Aire+/+ control littermates. (I and J) The percentage of Tregs (CD4+CD25+Foxp3+) cells within the draining LNs in both studies (Mpo+/+ versus Mpo−/− and Aire+/+ versus Aire−/−) were the same. *P<0.05; **P<0.01; ***P<0.001. Imm, immunization.
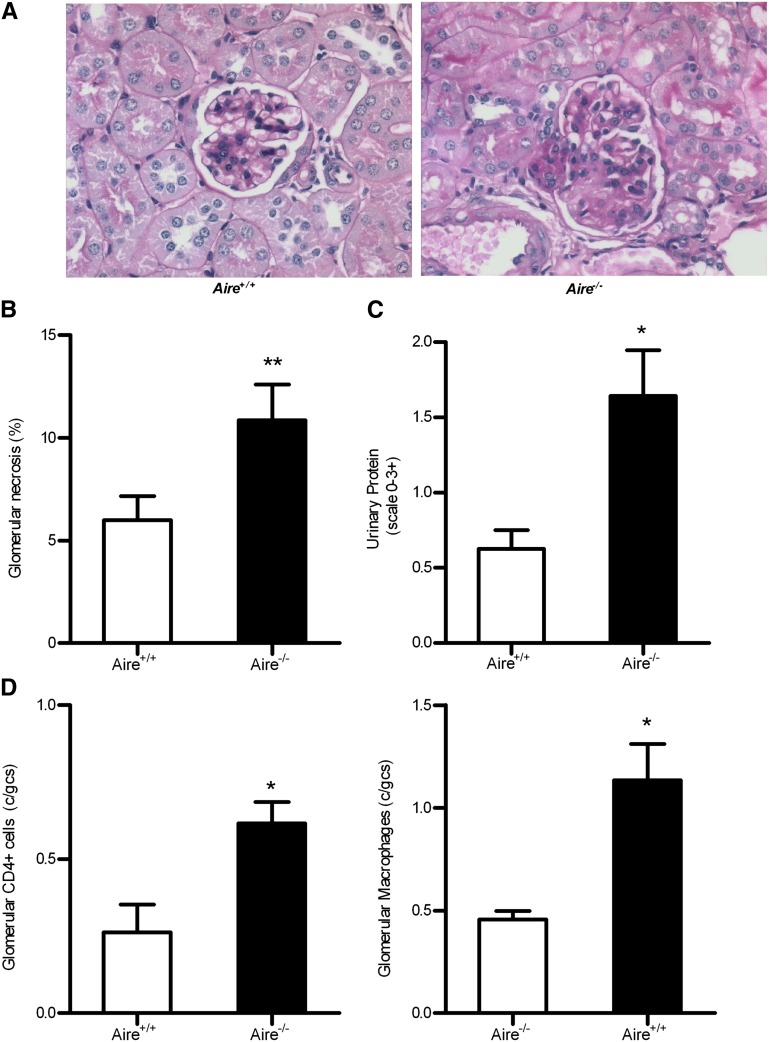
To determine the functional significance of thymic MPO expression in protecting against induced anti-MPO autoimmune focal necrotizing GN, we studied the development of glomerular injury occurring in Aire−/− versus littermate WT mice, using our previously published model.7,32 Aire−/− mice developed augmented anti-MPO GN quantified by histology (Figure 4A). Glomerular abnormalities quantified include necrosis, segmental proliferation, hypercellularity, and capillary wall thickening (Figure 4B). Exacerbation of renal injury was also demonstrated by elevated proteinuria (Figure 4C) as with glomerular accumulation of injurious CD4+ T cells and macrophages (Figure 4D).
Figure 4.
Histological injury and proteinuria were significantly increased in Aire−/− mice developing GN. Induction of GN results in enhanced focal necrotizing GN assessed by periodic acid–Schiff staining of the kidneys (A) and quantified as the percentage of glomerular segmental necrosis (B). (C) Functional renal injury quantified by urinalysis is also augmented after GN induction in the absence of Aire as well as glomerular CD4+ T cell and macrophage infiltrates (D). *P<0.05; **P<0.01. Original magnification, ×400.
Tregs Protect against the Induction of MPO Autoimmunity and AIMPOGN
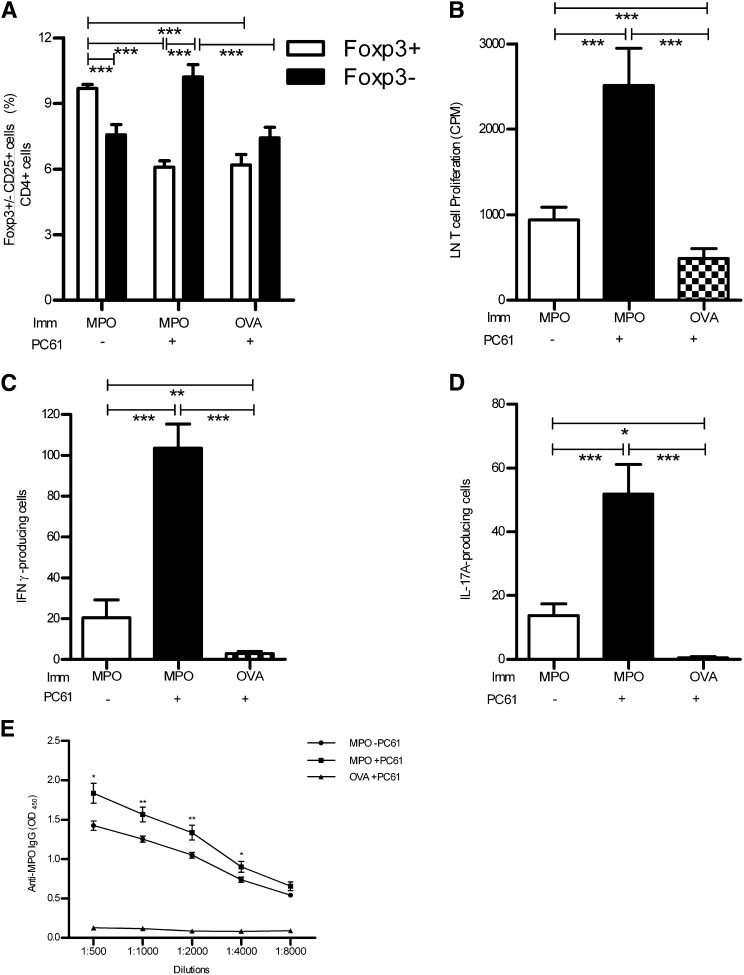
Treatment of naïve mice with the anti-CD25 mAb, PC61, decreased the Treg population significantly in MPO- and OVA-immunized mice over a 16-day period compared with mice that were not treated with the depleting antibody (Figure 5A). The CD4+CD25+Foxp3− activated T cell population was not affected by the depletion and therefore remained elevated after immunization (Figure 5A). The timing of administration of PC61 was carefully planned to allow the predominant effects to be on Tregs with minimum depletion of effector T cells after MPO immunization. It is likely that because CD25 expression is much more prominent on Tregs before immunization, these cells are more susceptible to PC61 depletion. PC61 depletion resulted in an increase in the frequency of CD4+CD25+Foxp3− activated T cell population compared with animals that were not treated with the depleting antibody (6.7±0.7×106 versus 4.5±0.7×106; P<0.05, t test).
Figure 5.
Immunodepletion of Tregs (CD4+CD25+Foxp3+) is induced by mAb PC61 treatment (in control mice given rat IgG or immunized with control antigen [OVA]). Significant reduction of Tregs in the LN draining immunization sites is confirmed at the end of experiments. (A) Immunodepletion of Tregs (CD4+CD25+Foxp3+) by mAb PC61 (in control mice given isotype rat IgG1) significantly reduces the proportion of Tregs in the draining LNs of mice immunized with MPO or control antigen (OVA) compared with control-treated mice immunized with MPO. Conversely, deletion of Tregs (by PC61) results in a statistically increased percentage of effector T cells (CD4+CD25+Foxp3−) induced by MPO immunization. The proliferation of LN cells is enhanced after Treg depletion and MPO immunization (B), as is the frequency of IFN-γ–producing cells (C) and IL-17A–producing cells (D) compared with control OVA-immunized and non-Treg-depleted MPO-immunized mice. (E) Elevated levels of anti-MPO IgG titers are also observed. *P<0.05; **P<0.01; ***P<0.001. Imm, immunization.
The reduction in the Treg population after anti-CD25 treatment was associated with enhanced T cell responsiveness to MPO ex vivo. LN cells from MPO-immunized PC61-treated C57BL/6 mice proliferated more vigorously in response to MPO than mice that were given control rat IgG and those that were treated with PC61 and immunized with OVA (Figure 5B). The frequency of MPO-specific IFN-γ– and IL-17A–producing lymphocytes from the draining LNs was significantly enhanced in MPO-immunized mice compared with OVA-immunized PC61-treated animals, suggesting that Tregs modulate Th1- and Th17-mediated autoimmune responses to MPO (Figure 5, C and D).
The MPO-immunized mice treated with PC61 made enhanced autoantibody responses to MPO compared with the MPO-immunized rat IgG-treated group (Figure 5E). C57BL/6 OVA-immunized mice injected with anti-CD25 antibody do not develop significant anti-MPO autoantibody titers, showing that Treg depletion enhances autoimmune B cell responses to MPO.
Immunodepletion of Tregs Increases the Severity of Glomerular Injury
To test the importance of Tregs in the protection against ANCA-associated GN, we modified the protocol of experimental autoimmune anti-MPO GN6,7,32 to induce very modest disease in C57BL/6 mice using only a single MPO immunization. The slightly shorter 16-day experimental time course also ensured that the effects of PC61 on Tregs lasted for the full experiment. PC61-treated mice developed enhanced functional renal injury as demonstrated by an increased urinary protein/creatinine ratio compared with both control rat IgG-treated and OVA-immunized PC61-treated mice (Figure 6, A and B). The development of GN as a result of exacerbated autoimmunity to MPO resulting from Treg depletion was also demonstrated histologically. Anti-CD25 pretreatment caused the development of increased segmental glomerular necrosis (Figure 6C) in MPO-immunized mice, compared with OVA-immunized and control rat IgG-treated mice. Glomeruli of PC61-treated MPO-immunized mice showed severe segmental necrosis, protein cast formation, and glomerular cellular proliferation compared with non-Treg-depleted MPO-immunized mice and PC61-treated OVA-immunized mice, which showed only relatively mild glomerular cellular proliferation to no detectable injury (Figure 6C). These outcomes also confirmed that the glomerular injury in this model results from MPO autoimmunity with no significant contribution from the subnephritogenic dose of anti-glomerular basement membrane (GBM) antibody, which nonetheless plays a critical role of triggering disease by inducing glomerular deposition of the autoantigen, MPO.7
Figure 6.
GN is induced in mice treated with Treg-deleting mAb PC61 or control rat IgG. The protein/creatinine ratio (A) and glomerular segmental necrosis (B) are significantly enhanced in mice with Treg deletion. (C) Histologic evaluation confirms the increased incidence of severe segmental necrosis, protein cast formation, and glomerular cellular proliferation in periodic acid–Schiff-stained sections. Original magnification, ×400. ***P<0.001. Imm, immunization.
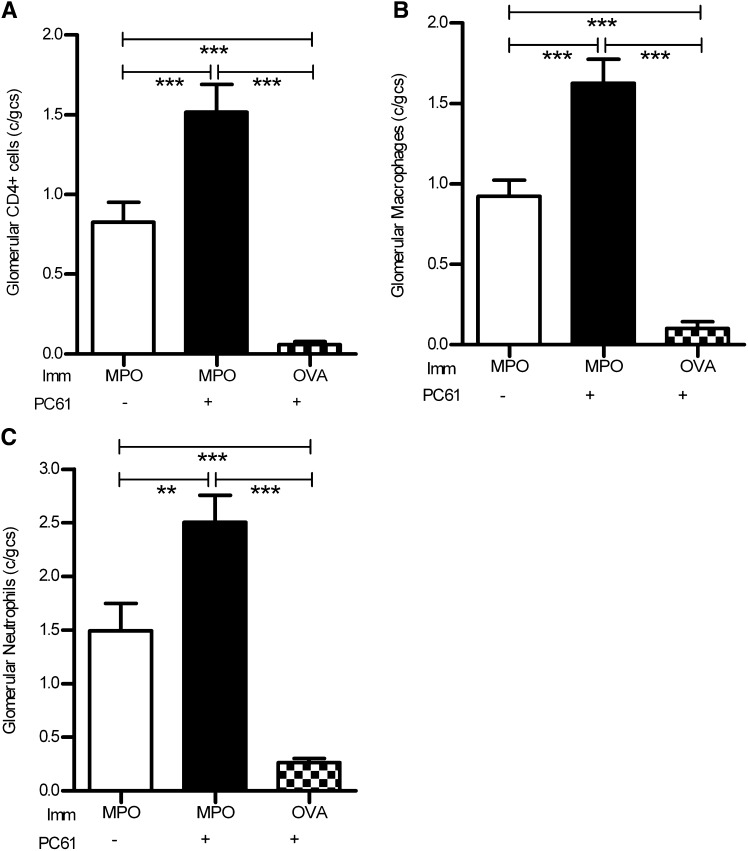
Mice with AIMPOGN augmented by anti-CD25 pretreatment developed significant glomerular influx of cellular mediators, CD4+ T cells, macrophages, and neutrophils compared with control rat IgG-treated C57BL/6 mice (Figure 7). Accumulation of CD4+ T cells, macrophages, and neutrophils was minimal in OVA-immunized PC61-treated mice, demonstrating that the influx of effectors was a result of MPO immunization and augmentation of autoimmunity from Treg depletion, and not the anti-GBM antibody alone.
Figure 7.
Glomerular leukocytic infiltration in mice developing autoimmune anti-MPO GN. Infiltration of glomerular CD4+ T cells (A), macrophages (B), and neutrophils (C) is significantly enhanced in mice immunized with MPO (versus OVA control). However, Treg depletion with mAb PC61 is associated with significantly further augmented leukocyte infiltration of all leukocyte subpopulations. **P<0.01; ***P<0.001. Imm, immunization.
Discussion
MPO is a major autoantigen in patients with microscopic polyangiitis.33 The current studies demonstrate that in mice, MPO is expressed in the thymus in an Aire-dependent manner. MPO deletion from the thymus as seen in both Mpo−/− and Aire−/− mice increases the frequency of autoreactive anti-MPO CD4+ T cells produced in response to MPO immunization. The increased intensity of immune glomerular injury in MPO-immunized Aire−/− mice observed after the deposition of MPO in glomeruli confirms the functional importance of this increased level of induced anti-MPO autoimmunity and suggests that Aire-dependent thymic expression of MPO functionally modifies the T cell repertoire in a manner consistent with central tolerance.
To assess whether the intrathymic production of MPO transcripts is involved in the central tolerance of autoreactive anti-MPO cells, we determined the thymic cell populations in which MPO was expressed. We initially validated microarray results13,16,34 by using quantitative PCR (qPCR) to determine MPO mRNA expression in the broad hematopoietic (CD45+) and CD45− WT mice stromal cell populations as well as in the thymic epithelial cell subsets. MPO mRNA was found to be predominantly in the CD45− population and the predominant cell population expressing MPO transcripts was the mTECs. mTECs are found in the medullary region of the thymus and have an important role in self-tolerance by presenting peripheral autoantigens to developing T cells. Gene expression profiling patterns in mTECs revealed that many TRAs, including autoantigens in experimental and human autoimmune diseases, are expressed by mTECs.31,35 The mTEC population is categorized into two subpopulations (mature and immature) according to their levels of MHC II and costimulatory molecule CD80 expression,36 reflecting enhanced functional capacities of mature mTECs (mTEC-hi) to fully participate in selection and deletion of autoreactive CD4+ T cells.16,37,38 Interestingly, in this study, MPO mRNA levels were similar between the mature and immature mTEC populations. Although Aire expression has been associated with the mTEC-hi population of epithelial cells, more recent evidence demonstrate a biphasic expression before end stage terminal differentiation, where they downregulate CD80 and MHC II to intermediate levels before their cell death.39 Thus, our findings of MPO mRNA expression in both the mature and immature mTEC populations may still reflect an association with Aire. Indeed, this is supported by the loss of MPO mRNA expression in both mTEC-hi and mTEC-lo subsets in Aire−/− mice. The observation that MPO (at the mRNA level) is expressed to the same extent in mTECs-hi and mTECs-lo (WT) cells suggests that downregulation of Aire precedes downregulation of MPO.
Thymic expression of autoantigens are often measured by mRNA levels.13,16,34 However, the degree to which mRNA transcripts expressed by the mTECs are translated into whole, functional protein is unknown. We colocalized MPO protein expression within neutrophils and macrophages, but not mTECs. During the positive and negative selection processes, a large number of developing thymocytes die through apoptosis.40 Thus, the presence of neutrophils and macrophages in the thymus is likely to be associated with their involvement with clearance of apoptotic cells during thymocyte development. In the context of thymic antigen presentation, MPO peptides are likely to be expressed by mTECs as a complex with MHC II at concentrations orders of magnitude below that seen in neutrophils and macrophages and thus too low for the protein to be detected by immunohistology.
The importance of thymic MPO expression in generating tolerance to MPO was demonstrated using Mpo−/− and Aire−/− mice. Both developed significantly higher frequencies of anti-MPO CD4+ T cells after MPO immunization than was observed in WT and littermate mice. Triggering GN in Mpo−/− mice was not attempted, because the target for disease-inducing CD4+ T effectors, which is neutrophil-derived MPO, is absent, rendering these mice disease resistant. However, in Aire−/− mice, MPO expression is normal in hematopoietic cells, making these mice susceptible to anti-MPO-associated GN. The enhanced GN observed in these mice occurred as a result of the increased frequency of anti-MPO CD4+ T effectors, which developed after MPO immunization as a consequence of the lack of thymic MPO expression in Aire−/− mice. This increase in anti-MPO autoreactive CD4+ T cells was associated with the development of significantly higher titers of anti-MPO ANCA in Mpo−/− and Aire−/− mice. This was likely to be in part due to increased T cell help for B cells, but tolerance also involves the deletion of autoreactive B cells via mechanisms of B cell tolerance in the bone marrow. It is also likely that deletion of autoreactive anti-MPO B cells was similarly reduced. It is important to note that the Treg populations in both groups of mice are comparable and that the elevated autoreactivity observed in Mpo−/− mice is likely to be the result of reduced negative selection rather than decreased Tregs or that MPO-specific Tregs were not produced in the thymi of these mice.
Low levels of autoreactive CD4+ T cells not deleted by central tolerance normally persist in the periphery and are silenced by tolerogenic mechanisms such as anergy, deletion, or ignorance. However, immunologic tolerance mechanisms may pose a threat in the maintenance of tolerance because these hyporesponsive autoreactive cells can be activated upon antigen encounter under suitable conditions, such as inflammation from sepsis41 or as a result of “molecular mimicry” that might be able to mediate immune pathology and trigger autoimmunity.42–45 Recent interest has focused on the role of Tregs in peripheral tolerance in inactivating autoreactive CD4+ T cells that escaped central deletion. Administration of PC61 mAb to WT mice was associated with a significant increase in the frequency of anti-MPO CD4+ T cells after MPO immunization. This is consistent with a role for Tregs in the maintenance of tolerance to MPO in the periphery. The functional importance of peripheral tolerance mechanisms in protecting against autoimmune anti-MPO-mediated GN was demonstrated by the development of significantly enhanced necrotizing GN after triggering of disease by depositing MPO in the glomeruli of control and PC61-treated mice using anti-GBM antibody. Administration of a subnephritogenic dose of anti-GBM antibodies to immunized mice induces glomerular neutrophil recruitment and degranulation, resulting in endogenous glomerular MPO deposition. Circulating autoreactive anti-MPO CD4+ T cells direct the development of focal necrotizing GN with appearances similar to those seen in human ANCA-associated GN.6 It is important to note that the experimental model of OVA-immunized PC61-treated WT mice did not develop renal injury despite the fact that these mice could theoretically have had potentially PC61-induced augmented anti-GBM antibody-mediated injury. This control demonstrates that the subnephritogenic dose of anti-GBM antibody does not contribute to renal injury, which results from anti-MPO-specific autoimmunity found in intact mice when immunomodulated by Tregs.
These studies demonstrate that MPO is expressed in an Aire-dependent manner in the thymus and, by this mechanism, is involved in the central deletion of potentially autoreactive anti-MPO CD4+ T cells. Peripheral tolerance through natural CD4+CD25+Foxp3+ Tregs also protects from the development of induced MPO autoimmunity capable of inducing focal necrotizing GN when MPO is planted in the glomeruli.
Concise Methods
Mice
Mice were on a C57BL/6 background. C57BL/6 (Mpo+/+) mice were purchased from Monash Animal Services and housed at the Monash Medical Centre Animal Facility (Melbourne, Australia). Mpo−/− mice46 were bred at the Monash Medical Centre Animal Facility. Aire−/− mice were bred at the Department of Molecular Pathology, Centre for Cancer Biology, University of Adelaide Animal Facility. The Mpo+/+ and Mpo−/− mice were used at 7–10 weeks of age. The Aire+/+ and Aire−/− were used at 18–20 weeks of age. All experiments were approved by the Monash Medical Centre Animal Ethics Committee.
Thymus Harvesting, Single-Cell Preparation by Enzymatic Digestion, and Sorting of TSC Subsets
Thymi from adult male and female Mpo+/+, Mpo−/−, Aire+/+, and Aire−/− mice aged between 1 and 4 months were harvested. To obtain sufficient numbers of TSC for qPCR analysis, the thymi were pooled containing five mice per group. The thymi were thoroughly cleaned of fat and connective tissue, the lobes separated, and the capsule nicked with fine scissors to release thymocytes before digestion. The thymic lobes were placed in 50 ml of cold RPMI 1640 (Gibco, Grand Island, NY) in a 100-ml Schott bottle with a magnetic flea and stirred gently for 20–30 minutes at 4°C. The supernatant was then collected and the thymi transferred into fresh RPMI 1640. Any further thymocytes were removed by gentle mixing with a wide bore glass pipette until the supernatant was mostly clear of thymocytes. The thymocyte washes were discarded. For the thymus digestion, 5 ml of Liberase (0.2% w/v; Roche, Penzberg, Germany)/DNAse (0.1%; Roche) enzyme mix per thymus digest tube was added and mixed gently with a wide bore glass pipette and placed into a 37°C waterbath for digestion. The digesting thymic tissue was agitated gently every 5–10 minutes with a glass pipette. Every 15–20 minutes, the supernatant was collected and kept on ice every 15–20 minutes in 15-ml tubes containing 2 ml of FACS buffer (0.1% BSA/PBS with 0.5 mM EDTA) to neutralize enzymes. Fresh enzyme mix was then added to the digest tube. This was repeated 3–4 times until the thymi were completely digested. After digestion, all fractions were pooled and filtered using 100-μm mesh and centrifuged at 480×g for 5 minutes at 4°C to collect cells. The single-cell suspensions of adult thymus prepared by enzymatic digestion were then subjected to CD45+ bead depletion for the isolation of TSCs.
After enzymatic digestion of the thymi, cells were resuspended in FACS buffer at a concentration of 107 cells per 95 ml buffer. CD45 microbeads were added at a concentration of 5 µl/107 cells. Samples were incubated on a rotating mixer at 4°C for 20 minutes. After the incubations, any unbound beads were diluted by adding up to 10× volume of FACS buffer. Cells were centrifuged for 10 minutes at 300×g, the supernatant discarded, and pellets resuspended at a concentration of 0.5×108 cells/ml before placing on the AutoMACS Separator (Miltenyi Biotech, Bergisch Gladbach, Germany) for separation using the “Depletes” program. Fractions were eluted into separate tubes. The negative fraction contained the CD45− TSCs. The CD45+ fraction was run through a second time to obtain any remaining CD45− TSCs. Once all CD45− cells were eluted, the negative fractions were pooled and centrifuged and the cell pellets were collected for further analysis.
After CD45+ hematopoietic cell fraction had been depleted, the CD45− TSC fraction was stained and sorted into the following subsets for qPCR analysis: mTECs-hi (CD45− EpCAM+ UEA-1+ MHC IIhi); mTECs-lo (CD45− EpCAM+ UEA-1+ MHC IIlo); cortical TECs, or cTECs (CD45− EpCAM+ Ly51+ MHC II+); and non-TECs (EpCAM− MHC II−). The dendritic cell population was sorted based on CD45+ CD11c+ MHC II+. Cells were sorted using a BD Influx (BD Biosciences, Franklin Lakes, NJ).
RNA Isolation and qPCR for Murine MPO
After cell sorting, the cells were washed twice in nuclease-free PBS and cells were pelleted by centrifugation at 500×g for 5 minutes at 4°C. After the second wash, the supernatant was discarded down to the remaining 20 μl and the pellet was resuspended by flicking the Eppendorf tube gently. Lysis buffer was then added to the sample from the RNEasy Mini Kit (Qiagen, Hilden, Germany), vortexed thoroughly to lyse the cells, and snap-frozen in liquid nitrogen and stored at −80°C for later RNA extraction. RNA was extracted per the RNEasy Mini kit instructions. RNA was reverse transcribed using Superscript III (Invitrogen Life Technologies, Carlsbad, CA) and oligo-dT oligonucleotides (Invitrogen Life Technologies) according to the manufacturer’s protocol of the Superscript III first-strand synthesis system.
qPCR analysis of TSC subsets was performed on a Rotor-Gene 3000 (Qiagen) in 10-μl reactions using a Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen Life Technologies) with prevalidated qPCR primers from Qiagen. After initial holds for 2 minutes at 50°C (uracil-DNA glycosylase incubation) and then 2 minutes at 94°C (enzyme activation), PCR was performed with 40 cycles of 94°C for 15 seconds (denaturation), 60°C for 30 seconds (annealing and extension), and 1 cycle of melt analysis at 65°C–-95°C (0.5°C increments per step, 5 seconds per step). The ΔΔ threshold (CT) method was used to calculate relative levels of target mRNA. Glyceraldehyde-3-phosphate dehydrogenase was used as the housekeeper gene and the Mpo+/+ CD45− population was used as the calibrator sample. Data are presented as mean ± SEM determined from two to three groups of five thymi pooled for each population.
Expression of MPO in Mouse Thymi by Immunoperoxidase Stainings and Immunofluorescence
For the detection of MPO protein, mouse thymi were fixed in Bouin’s fixative and embedded in paraffin wax, and 3-µm-thick sections were stained using periodic acid–Schiff reagent.
For colocalization studies, mouse thymi were embedded in OCT and snap-frozen. Frozen tissues were cut at 6 µm and stained using rabbit anti-cow Cytokeratin (Dako, Glostrup, Denmark), mouse anti-mouse MPO FITC (Hycult Biotech, Uden, The Netherlands), rat anti-mouse Gr-1 647 (BD Biosciences), or rat anti-mouse F4/80 647 (BD Biosciences). Slides were examined on the same day by confocal microscopy using a Ti-E invert microscope with a C1 confocal laser point scan head attached (Nikon, Tokyo, Japan).
Induction of MPO Autoimmunity in AIMPOGN in Aire−/− and Aire+/+ Mice
Aire−/− (n=7) and control Aire+/+ littermates (n=4) were immunized with 20 μg of native mouse MPO (nmMPO)47 in FCA subcutaneously at the base of the tail at day 0. At day 7, the mice were immunized again with 20 μg of nmMPO in Freund's incomplete adjuvant subcutaneously at the neck. Nine days after immunization, GN was induced in all mice planting endogenous MPO in their glomeruli using an established technique32 of administering 1.5 mg of sheep anti-mouse GBM globulin intravenously on 2 consecutive days to assess the development of renal injury. Administration of a subnephritogenic dose of anti-GBM antibody recruits and degranulates neutrophils, depositing MPO in the target organ in the same manner likely to occur in the human disease through neutrophil degranulation. Mice were humanly culled at day 20. The time course of this model was previously used and published by our group.7
Induction of MPO Autoimmunity and Immunodepletion by PC61 in the Model of AIMPOGN
Systemic autoimmunity to recombinant mouse MPO (rmMPO), which induces autoreactivity to nmMPO,47 was assessed by immunizing Mpo+/+ or Mpo−/− mice with 10 µg of rmMPO in FCA subcutaneously at the base of the tail. Control Mpo+/+ mice were immunized with 10 µg of OVA in FCA. Mice were humanely culled 10 days postimmunization when T cell autoreactivity to MPO was established.8
The assessment of the role of Tregs in the model of experimental autoimmune anti-MPO GN was performed at day −2 by CD4+CD25+Foxp3+ Tregs depletion in naïve Mpo+/+ mice (n=8, each group) by a single intraperitoneal injection of 1 mg of a well characterized anti-IL-2 receptor–specific Treg-deleting monoclonal rat anti-mouse CD25 mAb, PC61,48 whereas control mice were administered 1 mg of isotype rat IgG1. At day 0, experimental autoimmune anti-MPO GN was induced by immunizing 8-week-old male mice (n=8, each group) with 40 μg of nmMPO in FCA (450 μl subcutaneously at the base of the tail and the neck; Sigma, St. Louis, MO) to assess the role of Tregs in maintaining tolerance to MPO. PC61-treated control mice (n=8) were immunized with OVA (Sigma) in FCA. Nine days after immunization, GN was induced in all mice by planting endogenous MPO in their glomeruli using a well established technique6,7,30 of administering 1.5 mg of sheep anti-mouse GBM globulin intravenously on 2 consecutive days to assess the functional capacity of Tregs to immunomodulate induced ANCA-associated vasculitis. Mice were humanely culled 4 days (day 14) after the second intravenous injection. The single immunization with nmMPO and the shorter overall time course of this experiment were to induce very mild disease in control antibody-treated mice and allow PC61 to have its effects on Tregs throughout the experiment.
Assessment of Systemic Autoimmune Responses to MPO
IFN-γ and IL-17A production was assessed by ELISPOT (BD Biosciences and eBiosciences, respectively) with harvested draining LN cells. Both IFN-γ and IL-17A ELISPOT assays were performed according to the manufacturer’s instructions. Briefly, 5×105 cells/well were incubated with or without 5 μg/ml of antigen (OVA or rmMPO) for 18 hours at 37°C in 5% CO2 on ELISPOT plates precoated with purified anti-mouse IFN-γ the previous night. The next day, IFN-γ–secreting cells were detected using biotinylated anti-mouse IFN-γ, streptavidin-horseradish peroxidase, and 3-amino-9-ethyl-carbazole. The number of IL-17A–secreting cells was determined by first incubating 5×105 cells/well with or without 5 μg/ml of antigen for 18 hours at 37°C in 5% CO2 on ELISPOT plates precoated with purified anti-mouse IL-17A the night before. The next day, IL-17A–secreting cells were detected using biotinylated anti-mouse IL-17A, avidin-horseradish peroxidase, and 3-amino-9-ethyl-carbazole. Spots were enumerated with an automated ELISPOT reader system.
For the T cell proliferation assay, a single-cell suspension from the LNs was prepared aseptically. We incubated 5×105 cells/well in triplicate in the presence or absence of 5 μg/ml of antigen (OVA or rmMPO) at 37°C in 5% CO2 for 72 hours. Cells were pulsed with 0.5 μCi 3H-thymidine/well for the final 18 hours, and were then harvested onto glass fiber filters using an automated cell harvester.
Circulating mouse anti-MPO antibody titers in sera were measured by ELISA. The sera were prepared from cardiac puncture blood after mice were sacrificed. One hundred microliters of rmMPO or 5 μg/ml of nmMPO in 0.05 M carbonate/bicarbonate buffer (pH 9.6) was coated onto 96-well polystyrene microtiter plates (Nunc; Thermo Fisher Scientific, Waltham, MA) for 18 hours at 4°C. The assay was performed at room temperature. The absorbance values were read at 450 nm using a Bio-Rad 550 microplate reader (Bio-Rad, Hercules, CA) and analyzed using Microplate Manager software (version 5.2; Bio-Rad).
Flow Cytometry
Draining LNs were harvested, meshed, and pushed through cell strainers to create single-cell suspensions. Cells (5×105) were stained for CD4, CD25, and Foxp3 expressions. Cells were first labeled with CD4-APC/CY7 (GK1.5) and CD25-FITC or CD25-APC (7D4) in 1% BSA/PBS for 30 minutes at 4°C, and then fixed and permeabilized with 1× Fix/Perm solution before labeling with Foxp3-PE mAb (FJK-16s) in 1% BSA/PBS for 30 minutes at 4°C. After incubation, cells were washed twice in 1% BSA/PBS and analyzed on a BD FACSCANTO II flow cytometer. The data were analyzed with FlowJo software.
Assessment of Renal Injury
Mpo+/+, Mpo−/−, and C57BL/6 mice were housed individually in cages for urine collection over the final 24 hours of experiments and proteinuria (mg/24 h) was measured using a modified Bradford’s assay method49,50 to determine functional renal injury. Urinary creatinine concentrations were measured by enzymatic assays and protein/creatinine ratios were determined. The urine samples from Aire+/+ and Aire −/− mice were tested by dipstick (Combur 10 Test; Roche) for proteinuria and scored on a 0–3+ scale.
To assess histologic renal injury, kidney tissue was fixed in formalin and embedded in paraffin, and 4-µm tissue sections were cut and stained with periodic acid–Schiff using coded slides. Because focal/segmental necrosis of glomeruli is a major characteristic of human anti-MPO GN, this abnormality was used to quantify glomerular injury in mice in these studies. Segmental necrosis on glomerulus was diagnosed when >25% of the glomerular area was necrotized. The percentage of glomeruli with segmental necrosis was used as a quantitative indicator of the severity of histologic glomerular injury. The proportion of glomerular necrosis was determined by evaluating a minimum of 50 glomerular cross-sections per mouse.
For assessment of glomerular macrophages, neutrophils, and CD4+ T cell infiltrations, frozen periodate lysine paraformaldehyde-fixed kidneys were cut at 6 μm and stained using a three-layer immunoperoxidase technique, including FA/11 (anti-CD68) for macrophages, RB6–8C5 (anti-Gr-1; DNAX, Palo Alto, CA) for neutrophils, and GK1.5 (anti-CD4+; American Type Culture Collection, Manassas, VA) for CD4+ T cells. A minimum of 40 glomeruli per mouse were assessed using coded slides, and results are expressed as cells per glomerular cross-section (c/gcs).
Statistical Analyses
All data are presented as the mean ± SEM. An unpaired t test was used for statistical analysis, and one-way ANOVA and Tukey’s post hoc test were used for multiple group comparisons.
Disclosures
None.
Acknowledgments
This study is supported by grants from the National Health and Medical Research Council of Australia (606684 to S.R.H and 1023059 to H.S.S.), the Swedish Society of Medicine (SLS-96661 to B.A.L), and the Swedish Research Council (524-2010-6723 to B.A.L.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318: 1651–1657, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Xiao H, Falk RJ: Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 17: 1235–1242, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Morgan MD, Harper L, Williams J, Savage C: Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol 17: 1224–1234, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE: Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ: Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 584: 3193–3197, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR: Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 17: 1940–1949, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ooi JD, Chang J, Hickey MJ, Bogdan-Borza D, Fugger L, Holdsworth SR, Kitching AR: The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A 109: E2615–E2624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein L, Hinterberger M, Wirnsberger G, Kyewski B: Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol 9: 833–844, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Aaltonen J, Björses P, Perheentupa J, Horelli-Kuitunen N, Palotie A, Peltonen L, Lee YS, Francis F, Henning S, Thiel C, Leharach H, Yaspo ML, Finnish-German APECED Consortium : An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17: 399–403, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N: Positional cloning of the APECED gene. Nat Genet 17: 393–398, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Mathis D, Benoist C: Aire. Annu Rev Immunol 27: 287–312, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D: Projection of an immunological self shadow within the thymus by the aire protein. Science 298: 1395–1401, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hubert FX, Kinkel SA, Crewther PE, Cannon PZ, Webster KE, Link M, Uibo R, O’Bryan MK, Meager A, Forehan SP, Smyth GK, Mittaz L, Antonarakis SE, Peterson P, Heath WR, Scott HS: Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol 182: 3902–3918, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hubert FX, Kinkel SA, Webster KE, Cannon P, Crewther PE, Proeitto AI, Wu L, Heath WR, Scott HS: A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol 180: 3824–3832, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Derbinski J, Gäbler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B: Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 202: 33–45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, Ikawa T, Satoh R, Kawamoto H, Mouri Y, Matsumoto M: Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med 205: 2827–2838, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D: The cellular mechanism of Aire control of T cell tolerance. Immunity 23: 227–239, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Salama AD, Chaudhry AN, Ryan JJ, Eren E, Levy JB, Pusey CD, Lightstone L, Lechler RI: In Goodpasture’s disease, CD4(+) T cells escape thymic deletion and are reactive with the autoantigen alpha3(IV)NC1. J Am Soc Nephrol 12: 1908–1915, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Goverman JM: Immune tolerance in multiple sclerosis. Immunol Rev 241: 228–240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ: Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2: 301–306, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM: CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol 160: 1212–1218, 1998 [PubMed] [Google Scholar]
- 23.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S: Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: Induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 10: 1969–1980, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S: Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol 12: 1145–1155, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM: Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 164: 183–190, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Shevach EM: CD4+ CD25+ suppressor T cells: More questions than answers. Nat Rev Immunol 2: 389–400, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155: 1151–1164, 1995 [PubMed] [Google Scholar]
- 28.Mottet C, Uhlig HH, Powrie F: Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170: 3939–3943, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Morris GP, Brown NK, Kong YC: Naturally-existing CD4(+)CD25(+)Foxp3(+) regulatory T cells are required for tolerance to experimental autoimmune thyroiditis induced by either exogenous or endogenous autoantigen. J Autoimmun 33: 68–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summers SA, Steinmetz OM, Gan PY, Ooi JD, Odobasic D, Kitching AR, Holdsworth SR: Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum 63: 1124–1135, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Derbinski J, Schulte A, Kyewski B, Klein L: Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2: 1032–1039, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Gan PY, Summers SA, Ooi JD, O’Sullivan KM, Tan DS, Muljadi RC, Odobasic D, Kitching AR, Holdsworth SR: Mast cells contribute to peripheral tolerance and attenuate autoimmune vasculitis. J Am Soc Nephrol 23: 1955–1966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Paassen P, Tervaert JW, Heeringa P: Mechanisms of vasculitis: How pauci-immune is ANCA-associated renal vasculitis? Nephron, Exp Nephrol 105: e10–e16, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Guerau-de-Arellano M, Mathis D, Benoist C: Transcriptional impact of Aire varies with cell type. Proc Natl Acad Sci U S A 105: 14011–14016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotter J, Kyewski B: Regulating self-tolerance by deregulating gene expression. Curr Opin Immunol 16: 741–745, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nelson AJ, Hosier S, Brady W, Linsley PS, Farr AG: Medullary thymic epithelium expresses a ligand for CTLA4 in situ and in vitro. J Immunol 151: 2453–2461, 1993 [PubMed] [Google Scholar]
- 37.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Holländer GA, Reith W: Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29: 451–463, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L: Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol 11: 512–519, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa Y, Hirota F, Yano M, Kitajima H, Miyazaki J, Kawamoto H, Mouri Y, Matsumoto M: Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med 207: 963–971, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surh CD, Sprent J: T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature 372: 100–103, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H: Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65: 305–317, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Gran B, Hemmer B, Vergelli M, McFarland HF, Martin R: Molecular mimicry and multiple sclerosis: Degenerate T-cell recognition and the induction of autoimmunity. Ann Neurol 45: 559–567, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin MC, Lee SM, Kalume F, Morcos Y, Dohan FC, Jr, Hasty KA, Callaway JC, Zunt J, Desiderio D, Stuart JM: Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med 8: 509–513, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D: Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 14: 1088–1096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honeyman MC, Stone NL, Falk BA, Nepom G, Harrison LC: Evidence for molecular mimicry between human T cell epitopes in rotavirus and pancreatic islet autoantigens. J Immunol 184: 2204–2210, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ: Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest 107: 419–430, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apostolopoulos J, Ooi JD, Odobasic D, Holdsworth SR, Kitching AR: The isolation and purification of biologically active recombinant and native autoantigens for the study of autoimmune disease. J Immunol Methods 308: 167–178, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Setiady YY, Coccia JA, Park PU: In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol 40: 780–786, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 50.Huang XR, Tipping PG, Apostolopoulos J, Oettinger C, D’Souza M, Milton G, Holdsworth SR: Mechanisms of T cell-induced glomerular injury in anti-glomerular basement membrane (GBM) glomerulonephritis in rats. Clin Exp Immunol 109: 134–142, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]