Abstract
Pulmonary congestion is highly prevalent and often asymptomatic among patients with ESRD treated with hemodialysis, but whether its presence predicts clinical outcomes is unknown. Here, we tested the prognostic value of extravascular lung water measured by a simple, well validated ultrasound B-lines score (BL-US) in a multicenter study that enrolled 392 hemodialysis patients. We detected moderate-to-severe lung congestion in 45% and very severe congestion in 14% of the patients. Among those patients with moderate-to-severe lung congestion, 71% were asymptomatic or presented slight symptoms of heart failure. Compared with those patients having mild or no congestion, patients with very severe congestion had a 4.2-fold risk of death (HR=4.20, 95% CI=2.45–7.23) and a 3.2-fold risk of cardiac events (HR=3.20, 95% CI=1.75–5.88) adjusted for NYHA class and other risk factors. Including the degree of pulmonary congestion in the model significantly improved the risk reclassification for cardiac events by 10% (P<0.015). In summary, lung ultrasound can detect asymptomatic pulmonary congestion in hemodialysis patients, and the resulting BL-US score is a strong, independent predictor of death and cardiac events in this population.
Volume expansion is perhaps the most insidious and common modifiable risk factor for the exceedingly high death risk of patients with kidney failure on dialysis (CKD stage 5D).1 Fluid accumulation as assessed by weight change between dialyses2 as well as total body water measured before dialysis3 or by relative plasma volume monitoring during dialysis4 predicts death and cardiovascular events independently of other risk factors in this population, and correction of volume overload is considered as a major factor underlying the beneficial effects of frequent hemodialysis schedules. Simple, noninvasive, reliable methods for measuring total body water in dialysis patients exist,5 but these estimates per se do not provide sufficient information for guiding the prescription of extracellular fluids removal during dialysis in most patients.6 In today’s dialysis population, mainly composed of elderly patients with compromised left ventricular function,7 measures of a critical component of fluid volume like extravascular lung water8 may provide useful information for risk stratification and ultrafiltration prescription in this high-risk population.
Extravascular lung water is related to the ventricular filling pressure of the left ventricle (LV; i.e., an established biomarker for risk stratification and prescribing and monitoring fluids therapy in high-risk patients).9 Lung ultrasound (US) is a novel, validated technique that has been increasingly applied to estimate lung water in patients with heart disease10 and patients with acute respiratory failure treated in intensive care units.11 The rationale of this technique is that, in the presence of lung congestion, the US beam is reflected by thickened interlobular septa, a phenomenon generating hyperechoic reverberation artifacts between edematous septa and the overlying pleura (i.e., US bundles at narrow basis going from the US transducer to the limit of the screen, the so-called lung comets,12 which can be considered as a US equivalent of B lines [BL-US] detected in chest x-rays).13 The number of these US bundles is associated with LV filling pressure and allows us to detect and quantify lung water.14 In a single-center feasibility study, we found that this technique has a high interobserver and inter-US probes reproducibility.15 Importantly, pulmonary congestion as assessed by the BL-US score is quite common among asymptomatic hemodialysis15,16 and peritoneal dialysis17 patients.
Although clinical trials remain the definitive test for the assessing the usefulness of biomarkers in clinical practice, prognostic studies are a fundamental step for testing new biomarkers. In this multicenter study, we have, therefore, investigated the prognostic value of lung US in a multicenter cohort study. The scope of this study was that of assessing whether lung US may predict death and cardiac events beyond and above classic and CKD-related risk factors and symptoms of heart failure as assessed by the New York Heart Association (NYHA) classification in the hemodialysis population.
Results
The study cohort was extracted from the whole population of 11 dialysis units, which was formed by 462 hemodialysis patients, all of whom were invited to participate into this study. Seventy patients refused. Therefore, 392 patients were enrolled into the study. The mean age was 65±15 years, 63% were males, 42% were current smokers, 9% were past smokers, and 29% were patients with diabetes (Table 1). Only 41 patients had less than or equal to five BL-US. Lung congestion was mild (BL-US ranging from 5 to 15) in 120 patients (41%), moderate to severe (BL-US=15–60) in 175 patients (45%), and very severe (BL-US>60) in the remaining 56 patients (14%).
Table 1.
Main demographic, somatometric, and clinical characteristics in the whole study population and patients divided according to the BL-US
| Whole Group (n=392) | US B-Lines Number | P for Linear Trend | Lung BL-US versus r (P) | |||
|---|---|---|---|---|---|---|
| <15 (n=161) | 15–60 (n=175) | >60 (n=56) | ||||
| Age (yr) | 65±15 | 63±16 | 66±13 | 68±13 | 0.01 | 0.14 (<0.001) |
| Body mass index (kg/m2) | 26±5 | 26±5 | 26±5 | 26±6 | 0.91 | −0.03 (0.60) |
| Male sex n (%) | 247 (63) | 93 (58) | 116 (66) | 38 (68) | 0.09 | 0.08 (0.09) |
| Current smokers n (%) | 164 (42) | 57 (35) | 83 (47) | 24 (43) | 0.11 | 0.03 (0.57) |
| Past smokers n (%) | 36 (9) | 14 (9) | 17 (10) | 5 (9) | 0.88 | 0.07 (0.18) |
| Patients with diabetes n (%) | 110 (29) | 40 (26) | 50 (29) | 20 (36) | 0.15 | 0.10 (0.05) |
| On anti-hypertensive treatment n (%) | 220 (56) | 89 (55) | 93 (53) | 38 (68) | 0.25 | 0.05 (0.36) |
| Dialysis vintage (mo) | 128 (65–258) | 130 (66–303) | 129 (57–253) | 112 (67–222) | 0.80 | −0.08 (0.13) |
| With cardiovascular comorbidities n (%) | 213 (55) | 77 (49) | 97 (56) | 39 (70) | 0.01 | 0.13 (0.01) |
| Systolic BP (mmHg) | 136±23 | 135±22 | 138±23 | 134±26 | 0.85 | 0.01 (0.89) |
| Diastolic BP (mmHg) | 73±12 | 73±12 | 73±12 | 69±14 | 0.10 | −0.13 (0.01) |
| Pulse pressure (mmHg) | 64±19 | 62±19 | 65±18 | 65±21 | 0.19 | 0.09 (0.07) |
| Cholesterol (mg/dl) | 151±39 | 151±41 | 150±37 | 150±45 | 0.77 | −0.05 (0.36) |
| Hemoglobin (g/dl) | 11.4±1.4 | 11.5±1.3 | 11.4±1.3 | 11.0±1.6 | 0.07 | −0.14 (0.01) |
| Albumin (g/dl) | 3.9±0.4 | 4.0±0.3 | 3.9±0.4 | 3.8±0.5 | 0.05 | −0.12 (0.02) |
| CRP (mg/L) | 4.5 (3.02–12.8) | 3.8 (3.0–7.7) | 5.0 (3.0–15.2) | 7.9 (3.6–25.5) | 0.02 | 0.17 (<0.001) |
| Calcium (mg/dl) | 8.9±0.9 | 8.9±0.8 | 8.9±0.8 | 9.11±1.3 | 0.44 | 0.02 (0.68) |
| Phosphate (mg/dl) | 4.9±1.6 | 5.0±1.3 | 4.8±1.7 | 4.9±1.7 | 0.42 | −0.06 (0.25) |
| III–IV NYHA class n (%) | 114 (30) | 37 (24) | 50 (29) | 27 (51) | 0.001 | 0.19 (<0.001) |
Data are expressed as mean ± SD, median and interquartile range, or percent frequency as appropriate.
A substantial number of patients with moderate to severe lung congestion (71%) were asymptomatic or presented slight symptoms of heart failure. Overall, 213 (55%) patients had cardiovascular (CV) comorbidities, and 114 (30%) patients were in III–IV NYHA class. Data analysis according to BL-US categories showed that patients in the top BL-US score category (>60) were significantly older, with higher C-reactive protein (CRP) and lower albumin. They were more frequently affected by CV comorbidities and more frequently presented severe heart failure (NYHA class III–IV) compared with patients in the other two BL-US categories (Table 1). Furthermore, correlation analysis by considering BL-US score as a discrete variable (Table 1, last column) identified diabetes, diastolic BP, pulse pressure (P=0.07), and hemoglobin as significant correlates of this score. In a multiple linear regression model, including all univariate predictors of BL-US (P≤0.10), only the NYHA class (β=0.13, P=0.02) and hemoglobin (β=−0.12, P=0.02) maintained an independent association with the BL-US score, whereas sex, CRP, cardiovascular comorbidities, albumin, diastolic BP, age, and diabetes were unassociated with BL-US after multivariate data adjustment (Table 2).
Table 2.
Multiple linear regression analysis of BL-US
| Standardized Regression Coefficient (β) | P | |
|---|---|---|
| Hemoglobin | −0.12 | 0.02 |
| NYHA class (III–IV) | 0.12 | 0.02 |
| Male sex | 0.09 | 0.06 |
| CRP | 0.07 | 0.21 |
| Cardiovascular comorbidities | 0.05 | 0.31 |
| Albumin | −0.05 | 0.31 |
| Pulse pressurea | 0.05 | 0.36 |
| Age | 0.05 | 0.40 |
| Diabetes | 0.03 | 0.50 |
The inclusion of diastolic BP (β=−0.10, P=0.06) instead of pulse pressure in the model did not materially affect the strength of the associations between each predictor and the BL-US (data not shown).
Ultrasound Lung BL-USs, All-Cause Mortality, and Fatal and Nonfatal CV Events: Cox Regression Analyses
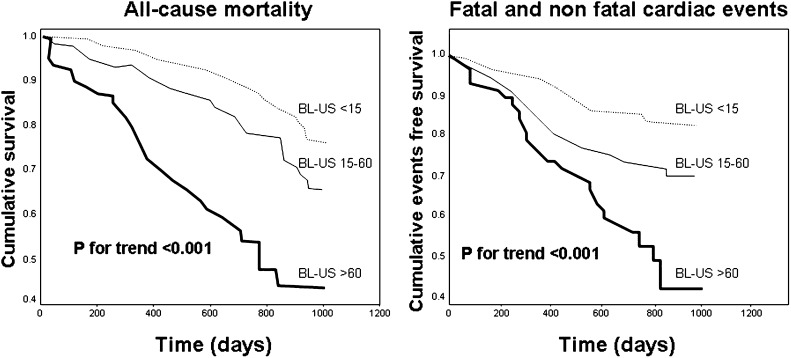
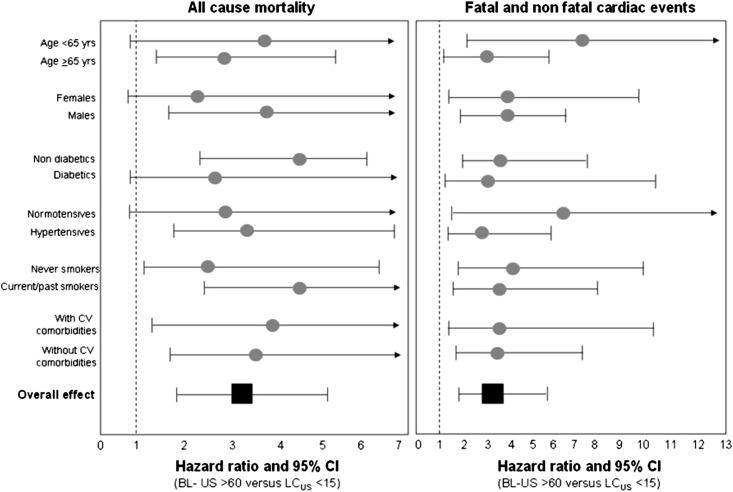
During the follow-up period (median=2.1 years, interquartile range=1.8–2.4), 96 patients died, and 90 patients had incident cardiac events that were fatal in 43 cases. In a Kaplan–Meier survival analysis, the hazard ratio of all-cause mortality increased in close parallelism with the BL-US score, and therefore, the relative risk of mortality was highest in patients with BL-US>60 (hazard ratio [HR]=4.20, 95% CI=2.40–7.20), intermediate in patients with BL-US ranging from 15 to 60 (HR=1.70, 95% CI=1.00–2.80), and lowest in patients with BL-US<15 (HR=1.00, reference group) (Figure 1 and Table 3). The same analysis carried out for cardiac events provided similar results (Figure 1 and Table 3). In multivariate Cox models, BL-US (the criteria for building these models are detailed in the Concise Methods) maintained independent predictive value mortality and cardiac events (Table 3). BL-US was superior to NYHA as a predictor of the study outcomes, because in models including both the BL-US and NYHA class, BL-US rather than NYHA was retained as significant predictor of the study outcomes (Table 3). The independent predictive power of BL-US was consistent across age, sex, diabetes, hypertension, smoking, and background cardiovascular events strata (P for interaction = NS) (Figure 2). Furthermore, no significant heterogeneity in the effect of BL-US on study outcomes was found among participating centers, and the I2 value of such an effect was not significant for both all-cause mortality and fatal and nonfatal cardiac events (P>0.80), indicating that the HR of the relationship between BL-US and study outcomes was consistent across centers. Multivariate modeling of BL-US as discrete variable provided similar results, and a 10-units increase in BL-US signaled a 6% increase for both mortality (HR=1.06, 95% CI=1.03–1.10, P<0.001) and cardiac events (HR=1.06, 95% CI=1.02–1.11, P=0.003). Furthermore, the cardiovascular hospitalization rate runs in close parallelism (P<0.001) with the BL-US<15: 19 hospitalizations/100 persons-year (95% CI=14.00–25.00); BL-US=15–60: 31 hospitalizations/100 persons-year (95% CI=25.00–38.00; BL-US>60: 50 hospitalizations/100 persons-year (95% CI=35.00–69.00).
Figure 1.
Kaplan–Meier survival analyses of all-cause mortality and fatal and nonfatal cardiac events according to the BL-US.
Table 3.
Crude and adjusted Cox regression analyses for all-cause mortality and fatal and nonfatal cardiac events
| Variables (Units of Increase) | Crude Analysis HR (95% CI) and P Value | Adjusted Analysis HR (95% CI) and P Value |
|---|---|---|
| All-cause mortality (n=96) | ||
| BL-US | ||
| <15 | 1a | 1a |
| 15–60 | 1.68 (1.02–2.76), P=0.04 | 1.31 (0.79–2.18), P=0.30 |
| >60 | 4.20 (2.45–7.23), P<0.001 | 3.04 (1.73–5.35), P<0.001 |
| Age (1 yr) | 1.03 (1.01–1.05), P<0.001 | |
| Current or past smoking (0=no; 1=yes) | 1.66 (1.09–2.54), P=0.02 | |
| CV comorbidities (0=no; 1=yes) | 1.26 (0.77–2.06), P=0.35 | |
| Cholesterol (1 mg/dl) | 1.00 (0.99–1.00), P=0.44 | |
| NYHA class (0=I-II; 1=III-IV) | 1.48 (0.92–2.38), P=0.10 | |
| Pulse pressure (1 mmHg)b | 1.01 (1.00–1.02), P=0.22 | |
| Albumin (1 g/dl) | 0.74 (0.45–1.21), P=0.23 | |
| Phosphate (1 mg/dl) | 0.89 (0.77–1.03), P=0.13 | |
| CRP (1 mg/L) | 1.00 (0.99–1.00), P=0.85 | |
| Fatal and nonfatal cardiac events (n=90) | ||
| BL-US | ||
| <15 | 1a | 1a |
| 15–60 | 1.88 (1.13–3.12), P=0.01 | 1.59 (0.95–2.65), P=0.08 |
| >60 | 3.75 (2.12–6.65), P<0.001 | 3.20 (1.75–5.88), P<0.001 |
| Age (1 yr) | 1.02 (1.01–1.04), P=0.01 | |
| Male sex | 1.15 (0.66–1.99), P=0.62 | |
| Current or past smoking (0=no; 1=yes) | 1.73 (1.04–2.89), P=0.03 | |
| Diabetes (0=no; 1=yes) | 0.95 (0.58–1.53), P=0.82 | |
| Dialysis vintage (10 mo) | 1.00 (0.99–1.01), P=47.00 | |
| CV comorbidities (0=no; 1=yes) | 1.92 (1.16–3.15), P=0.01 | |
| Pulse pressure (1 mmHg)b | 1.00 (0.99–1.01), P=0.41 | |
| NYHA class (0=I-II; 1=III-IV) | 0.88 (0.53–1.45), P=0.64 |
Multiple Cox regression model included all variables that were associated with study outcomes with P<0.10 at univariate analyses. The criteria for building these models are further detailed in Concise Methods. Data are expressed as HR, 95% CI, and P values.
Reference group.
The inclusion of diastolic or systolic BPs instead of pulse pressure as well as the inclusion of current smoking instead of current or past smoking into the Cox models did not materially affect the strength of the associations of each predictor and the occurrence of study outcomes (data not shown).
Figure 2.
HRs (and 95% CIs) for all-cause mortality and fatal and nonfatal cardiac events of patients with BL-US>60 versus patients with BL-US<15 (reference group) according to the presence/absence of advanced age (>65 years), sex, diabetes, hypertension, smoking (current/past versus never smokers), and background CV comorbidities. Data were derived by Cox regression analyses fitted according to the presence/absence of each risk factor.
Discrimination, Calibration, and Reclassification Abilities of BL-US
BL-US added modest discrimination ability for mortality to a prediction model based on classic risk factors (age, smoking, CV comorbidities, cholesterol, pulse pressure) and albumin, phosphate, CRP, and NYHA (model with BL-US: area under the receiver operator curve [AUC]=0.78 versus model without BL-US AUC=0.76). The same analysis carried out for fatal and nonfatal cardiac events showed similar results (model with BL-US AUC=0.73 versus model without BL-US AUC=0.69). The Hosmer–Lemeshow test indicated that the prediction models with and without BL-US were well calibrated for both all-cause mortality and fatal and nonfatal cardiac events (P>0.05). Importantly, prognostic models including BL-US increased the explained variation (i.e., an index combining discrimination and calibration) in study outcomes of models based on standard risk factors and NYHA from 24%–30% for all-cause mortality (P<0.001) and from 17%–22% for fatal and nonfatal cardiac events (P<0.001).
In Table 4, patients who died and patients who survived were arranged according to the predicted probability of death as estimated by either a model including classic risk factors and albumin, phosphate, CRP, and NYHA (horizontal rows) or a model including the same risk factors and BL-US (vertical rows). In 6 of 96 patients who died (6%), reclassification was more accurate by using the model including BL-US to predict death. However, 7 of 96 patients who died (7%) moved to a lower risk category. Among those patients who survived, 62 of 296 (21%) patients were reclassified in a lower risk category, and 36 of 296 (12%) patients were reclassified in a higher risk category. Overall, the Net Reclassification Index (NRI) was 7.7%, a figure that just failed to reach statistical significance (P=0.10). When reclassification analysis was applied to fatal and nonfatal cardiac events (Table 4), 9 of 90 patients with cardiac events (10%) were correctly reclassified in a higher risk category for these events by the model including BL-US. However, this result was counterbalanced by an identical number of patients (n=9) who were incorrectly reclassified into a lower risk category (Table 4). Of note, among those patients without cardiac events (Table 4), 63 of 302 (21%) patients were reclassified in a lower risk category, and 33 of 302 (11%) patients were reclassified in a higher risk category, thus providing an NRI of 10%, a figure that was of statistical significance (P<0.02). Additional data analysis of prognostic models performance by the integrated discrimination improvement (IDI) showed that the inclusion of BL-US achieved a 4% IDI for all-cause mortality (P<0.001) and a 3% IDI for fatal and nonfatal cardiac events (P=0.002).
Table 4.
Reclassification ability of prediction models with and without BL-US for all-cause mortality and fatal and nonfatal cardiac events
| Model Including Standard Risk Factors Only | Model Including Standard Risk Factors and BL-US | ||
|---|---|---|---|
| <10% | 10%–20% | >20% | |
| Mortality | |||
| Patients who died (n=96) | |||
| <10% | 4 | 3 | 0 |
| 10%–20% | 3 | 6 | 3 |
| >20% | 0 | 4 | 73 |
| Patients who survived (n=296) | |||
| <10% | 71 | 11 | 2 |
| 10%–20% | 28 | 34 | 23 |
| >20% | 0 | 34 | 93 |
| Fatal and nonfatal cardiac events | |||
| Patients with fatal and nonfatal cardiac events (n=90) | |||
| <10% | 1 | 2 | 0 |
| 10%–20% | 2 | 10 | 7 |
| >20% | 0 | 7 | 61 |
| Patients without fatal and nonfatal cardiac events (n=302) | |||
| <10% | 43 | 10 | 0 |
| 10%–20% | 30 | 55 | 23 |
| >20% | 0 | 33 | 108 |
For both all-cause mortality and fatal and nonfatal cardiac outcomes, patients were divided according to the occurrence of the event of interest. Patients who died and patients who survived as well as patients with and without fatal and nonfatal cardiac events were divided in three groups according to the predicted probability of the event of interest (<10%, 20%–20%, and >20%) estimated by either a model including standard risk factors (horizontal rows) or a model including the same risk factors and BL-US (vertical rows; more details in Results).
Discussion
In this study, the degree of lung congestion measured by lung US was a better predictor of the risk of death and cardiac events than symptoms of heart failure as assessed by the NYHA score and provided additional independent information to explain variation in study outcomes over and above classic risk factors, serum albumin, phosphate, CRP, and NYHA score. Furthermore, reclassification analysis showed that the inclusion of estimates of lung congestion by US into a prediction model based on risk factors listed above significantly improves the prediction of cardiac events by the same model by 10%.
Pulmonary Water and Heart Disease in ESRD
In hemodialysis patients without apparent pulmonary disease, carbon oxide transfer is substantially compromised, denoting subclinical pulmonary edema,18 and the ventilation/perfusion ratio improves after dialysis, implying reduced extravascular lung water after fluid subtraction.19 In a study based on a double indicator dilution technique, predialysis lung water was 33% higher in asymptomatic dialysis patients without cardiac disease than in well matched healthy subjects and reverted to normal postdialysis.20 These observations were subsequently confirmed in another study based on a modified optical density dilution and ultrasound velocity technique.21 In a feasibility study in 75 patients, we showed that the measurement of pulmonary water by BL-US has good interobservers and interprobes reproducibility.15 We also found that most patients with moderate to severe lung congestion were asymptomatic,15 an observation fully confirmed in this larger cohort study. Furthermore, we reported that, both before and after dialysis, BL-US consistently associates with pulmonary pressure, left atrial volume, and particularly, ejection fraction, implicating LV dysfunction and volume overload in pulmonary congestion in dialysis patients. Thus, lung US detects congestion at a preclinical stage in a substantial proportion of patients, which could be of clinical relevance for the prevention of cardiac events in an elderly population with cardiomyopathy like the hemodialysis population.
Prognostic Value of Pulmonary Water Measurement by Lung US
Development of biomarkers for application in clinical practice is a complex undertaking that demands proof that the biomarker reliably reflects the targeted biologic process, evidence that the biomarker has diagnostic and/or prognostic ability over and above standard factors, and proper testing in specifically designed trials where the biomarker is face to face compared with established indicators of the same biologic process.
BL-US proved to be a strong and independent predictor of death and incident cardiovascular events22 in patients with cardiac disease, but whether the BL-US has prognostic power in other conditions is still unknown. Prognostic biomarkers should be specifically investigated in the precise population where they are proposed for clinical application. This process is particularly true in the hemodialysis population, a population with a notoriously high risk for fluid overload. We found that lung US adds significant prognostic information for death and cardiovascular events to classic risk factors, the NYHA score, and powerful risk factors in CKD patients, like hypoalbuminemia, hyperphosphatemia, and inflammation. In quantitative terms, the BL-US score significantly increased by 6% and 5% the explained variation in death rate and cardiovascular events by a model based on the above-mentioned risk factors. This result indicates that this biomarker improves the combined discrimination ability and calibration of the same model. Although the discrimination gain portended by the BL-US score was minuscule for both study outcomes, the application of this score significantly improved by 10% the reclassification of the risk for cardiac events in patients who remained free cardiovascular events. Similarly, risk reclassification in survivors improved by 7.7%, a not trivial increase, but this improvement failed to reach formal statistical significance (P=0.10). Furthermore, an analysis by the Integrated Discrimination Improvement (IDI; a cutoff-free index of performance of a prognostic model) showed that the inclusion of BL-US achieved a 4% IDI for all-cause mortality (P<0.001) and a 3% IDI for fatal and nonfatal cardiac events (P=0.002). Overall, our data show that the application of lung US may help to refine prognosis and that it adds meaningful specificity to prediction models based on classic and kidney failure-related risk factors in dialysis patients.
Our study has limitations. Although the study was multicenter and fairly large, our cohort was gathered in a limited geographical area in southern Italy. Average mortality of dialysis patients in this area is very close to the average figure in European countries.23 Furthermore, our population was composed of patients of Caucasian descent only. Therefore, the prognostic ability of lung US should be confirmed in other dialysis populations, including a larger share of other ethnicities. Finally, the usefulness of lung US remains to be tested in a formal clinical trial. A trial designed by the European Cardiovascular and Renal Medicine working group of the European Renal Association—European Dialysis Transplantation Association has recently been funded by the same society (http://www.era-edta.org/dati/pagine/allegato_201112122456.pdf; http://www.era-edta.org/privata/images/LUST_(Zoccali)_final_application.pdf).
Concise Methods
The study protocol was approved by the ethical committee at our institution, and informed consent was obtained from each participant.
Patients
From an original population of 462 hemodialysis patients, all of Caucasian descent, forming the whole population of 11 dialysis units in two regions in southern Italy (Calabria and Sicily), a cohort of 392 hemodialysis patients was available for the data analysis (Results). All patients had been on regular hemodialysis with standard bicarbonate dialysis for a median time of 126 months (interquartile range=63–256 months) and were being treated with noncellulosic membrane filters of various type; 220 patients were treated with various antihypertensive drugs (118 on monotherapy with angiotensin conversing enzyme inhibitors, calcium channel blockers, α- and β-blockers, vasodilators, diuretics, or other drugs, 71 patients on double therapy, 23 patients on triple therapy, and 8 patients on quadruple or quintuple therapy with various combinations of these drugs). All participating Renal Units adhered to recommendations by the European Renal Association—European Dialysis Transplantation Association guidelines for dialysis dose prescription and fluid volume control.24 The main demographic, somatometric, clinical, and biochemical characteristics of the study population are detailed in Table 1.
Laboratory Measurements
Blood sampling was performed after an overnight fast always during a midweek hemodialysis day (brief interval). Blood was drawn and put into tubes containing EDTA, and plasma supernatants were stored at −80°C until batch analyses. Serum cholesterol, albumin, calcium, phosphate, CRP, and hemoglobin measurements were made using standard methods in the routine clinical laboratory.
Clinical Assessment of Heart Failure
Patients were classified as symptomatic or asymptomatic on the basis of the NYHA scale,25 a scoring system that was also validated in dialysis patients.26 For the purpose of this study, patients were grouped just in two classes, the first group including NYHA I–II (70%) and the second group including NYHA III–IV (30%).
B-Lines Detection and BL-US Score
All measurements were performed by nephrologists of participating centers. Before the study, nephrologists had brief (2–3 hours) structured training27 on BL-US measurement at the coordinating center. After training, all of them reached an adequate competence on the application of lung US. In fact, in preliminary blind tests administered in unselected patients, the estimates of BL-US by nephrologists did not differ (±10%) from estimates by the lung US technical expert at the coordinating center (R.T.). All lung US assessments were made by these nephrologists immediately before dialysis. The method is described elsewhere,15 and more details are available in a 2-minute movie on YouTube (http://www.youtube.com/watch?v=7y_hUFBHStM). All measurements were made by an observer unaware of clinical data. BL-US has a high interobserver and interprobes reliability (concordance indexes=0.96 and 0.99, respectively).15
Follow-Up Study
After the initial assessment, patients were followed up for a median time of 2.1 years (interquartile range=1.8–2.4 years). During the follow-up period, causes of death and cardiovascular events were collected. The study end points were mortality and fatal and nonfatal cardiac events. Cardiac events were (1) myocardial infarction adjudicated on the basis of serial changes of electrocardiogram (ECG), creatine-kinase, and troponin, (2) ECG-documented angina episodes, (3) heart failure, (4) ECG-documented arrhythmia, (5) pulmonary embolism, and (6) unexpected sudden death highly suspected as of cardiac origin. Each death was independently reviewed and assigned an underlying cause by a panel of three physicians kept blind to lung US results, and discrepancies among assessors were resolved by consensus.
Statistical Analyses
Comparisons among groups were made by one-way ANOVA, Kruskal–Wallis, or chi-squared test as appropriate. Association between variables was tested by bivariate and multiple regression analysis.
The prognostic value of BL-US for predicting the study outcomes was investigated by the Kaplan–Meier analysis and Cox regression analysis. Categorization of pulmonary congestion by BL-US was done by analyzing the Martingale residuals28 in Cox’s regression analysis. By this analysis, we found that the categorization of BL-US into three categories (<15, 15–60, and >60) was the best for modeling the relationship between this variable and the incidence rate of study outcomes. In multivariate Cox models, we included all variables that correlated to the study outcomes with P≤0.10 at univariate Cox analyses. Tested covariates included traditional risk factors (age, sex, current/past smoking, diabetes, cardiovascular comorbidities, cholesterol, and arterial pressure), body mass index, antihypertensive treatment, NYHA class, risk factors related to CKD-5D (dialysis vintage, hemoglobin, albumin, calcium, and phosphate), and CRP. The additional prognostic value of BL-US for study outcomes above and beyond that provided by standard risk factors and NYHA class (basic models) was investigated by assessing the discrimination,29 calibration, and reclassification abilities (that is, by calculating the NRI and IDI) of BL-US.30,31 The explained variation in the incidence rate of study outcomes (i.e., an index combining discrimination and calibration) was calculated by −2 log likelihood statistics. The heterogeneity in the effect of BL-US for all-cause mortality and fatal and nonfatal cardiac events among centers participating into the study was investigated by a meta-analytic approach by calculating the I2 and the corresponding P value.32
Disclosures
None.
Acknowledgments
This study was supported by grants from Regione Calabria Department of Health.
Persons named in the acknowledgements declare that no conflict of interest is related to this manuscript. No funding was received by private industries. On behalf of all coauthors and persons named in the acknowledgements, I certify that no conflict of interest is related to this manuscript. I received permission from all persons named in the acknowledgements. The paper was not published before in any language, and it is not under consideration for publication elsewhere. It was not presented before in abstract form. Hereby, I certify that I had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Lung US in CKD Working Group: Giovanni Alati (Servizio Dialisi Ospedale di Tropea), Rosalia Boito and Rosita Lucà, (Divisione di Nefrologia e Dialisi Ospedale “S. Giovanni di Dio,” Crotone), Graziella Bonanno (“Azzurra” Ambulatorio Medico Nefrologico e Tecniche Dialitiche, Catania), Simonetta Cassani (Divisione di Nefrologia e Dialisi CAL Ospedale Mariano Santo Cosenza), Antonio Chippari and Teresa Cicchetti (Divisione di Nefrologia e Dialisi Ospedale “Giannettasio” Rossano), Anna Clementi and Maurizio Garozzo (U.O. di Nefrologia e Dialisi Ospedale S. Marta e S. Venera Acireale), Domenico Lo gozzo (Servizio Dialisi di Acri), Domenico Mancuso and Francesco Mollica (U.O. di Nefrologia, Dialisi e Trapianto, Ospedale Annunziata, Cosenza), Giuseppe Natale (Servizio Nefrologia e Dialisi Ospedale “Jazzolino” Vibo Valentia), Vincenzo Panuccio (UO di Nefrologia e Dialisi di Reggio Calabria), and Arcangelo Sellaro (Centro Dialisi ASL 5 Mesoraca).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Mees EJ: Volaemia and blood pressure in renal failure: Have old truths been forgotten? Nephrol Dial Transplant 10: 1297–1298, 1995 [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC: Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R: Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 56: 512–517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921–933, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Charra B: Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int 11: 21–31, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 8.Staub NC: Pulmonary edema. Physiol Rev 54: 678–811, 1974 [DOI] [PubMed] [Google Scholar]
- 9.McGee WT, Mailloux P, Jodka P, Thomas J: The pulmonary artery catheter in critical care. Semin Dial 19: 480–491, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Picano E, Gargani L, Gheorghiade M: Why, when, and how to assess pulmonary congestion in heart failure: Pathophysiological, clinical, and methodological implications. Heart Fail Rev 15: 63–72, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein DA, Mezière GA: Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 134: 117–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G: Ultrasound lung comets: A clinically useful sign of extravascular lung water. J Am Soc Echocardiogr 19: 356–363, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T, International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) : International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38: 577–591, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E: “Ultrasound comet-tail images”: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest 127: 1690–1695, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, Picano E, Zoccali C: Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 3: 586–594, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A: Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 135: 1433–1439, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Panuccio V, Enia G, Tripepi R, Torino C, Garozzo M, Battaglia GG, Marcantoni C, Infantone L, Giordano G, De Giorgi ML, Lupia M, Bruzzese V, Zoccali C: Chest ultrasound and hidden lung congestion in peritoneal dialysis patients. Nephrol Dial Transplant 27: 3601–3605, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Bedetti G, Gargani L, Sicari R, Gianfaldoni ML, Molinaro S, Picano E: Comparison of prognostic value of echographic [corrected] risk score with the Thrombolysis in Myocardial Infarction (TIMI) and Global Registry in Acute Coronary Events (GRACE) risk scores in acute coronary syndrome. Am J Cardiol 106: 1709–1716, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Romaldini H, Rodriguez-Roisin R, Lopez FA, Ziegler TW, Bencowitz HZ, Wagner PD: The mechanisms of arterial hypoxemia during hemodialysis. Am Rev Respir Dis 129: 780–784, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Wallin CJ, Jacobson SH, Leksell LG: Subclinical pulmonary oedema and intermittent haemodialysis. Nephrol Dial Transplant 11: 2269–2275, 1996 [DOI] [PubMed] [Google Scholar]
- 21.MacRae JM, Joseph G, Kislukhin V, Krivitski NM, Heidenheim AP, Lindsay RM: Determining lung water volume in stable hemodialysis patients. ASAIO J 52: 430–437, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E: Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 13: 830–835, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Registry ERA-EDTA: 2009 Report, Italy, Calabria, 2009. Available at: http://www.rc.ibim.cnr.it/Registro/survival%20report%202009%20Calabria.pdf Accessed August 14, 2012
- 24.Tattersall J, Martin-Malo A, Pedrini L, Basci A, Canaud B, Fouque D, Haage P, Konner K, Kooman J, Pizzarelli F, Tordoir J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG guideline on dialysis strategies. Nephrol Dial Transplant 22[Suppl 2]: ii5–ii21, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hurst JW, Morris DC, Alexander RW: The use of the New York Heart Association’s classification of cardiovascular disease as part of the patient’s complete Problem List. Clin Cardiol 22: 385–390, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postorino M, Marino C, Tripepi G, Zoccali C, Calabrian Registry of Dialysis and Transplantation : Prognostic value of the New York Heart Association classification in end-stage renal disease. Nephrol Dial Transplant 22: 1377–1382, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Mottola G: Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc Ultrasound 4: 34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grambsch PM, Therneau TM, Fleming TR: Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics 51: 1469–1482, 1995 [PubMed] [Google Scholar]
- 29.Tripepi G, Jager KJ, Dekker FW, Zoccali C: Statistical methods for the assessment of prognostic biomarkers (Part I): Discrimination. Nephrol Dial Transplant 25: 1399–1401, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Tripepi G, Jager KJ, Dekker FW, Zoccali C: Statistical methods for the assessment of prognostic biomarkers(part II): Calibration and re-classification. Nephrol Dial Transplant 25: 1402–1405, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JPT, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558, 2002 [DOI] [PubMed] [Google Scholar]