Abstract
Objective
To evaluate the anti-apoptotic and radical scavenging activities of dietary phenolics, namely ascorbic acid,α-tocopherol acetate, citric acid, salicylic acid, and estimate H2O2-induced apoptosis in renal cell carcinoma cells.
Methods
The intracellular antioxidant potency of antioxidants was investigated. H2O2-induced apoptosis in RCC-26 was assayed with the following parameters: cell viability (% apoptosis), nucleosomal damage and DNA fragmentation, bcl-2 levels and flow cytometery analysis (ROS production evaluation).
Results
The anticancer properties of antioxidants such as ascorbic acid, α-tocopherol acetate, citric acid, salicylic acid with perdurable responses were investigated. It was observed that these antioxidants had protective effect (anti-apoptotic activity) against hydrogen peroxide (H2O2) in renal cell carcinoma (RCC-26) cell line.
Conclusions
This study reveals and proves the anticancer properties. However, in cancer cell lines anti-apoptotic activity can indirectly reflect the cancer promoter activity through radicals scavenging, and significantly protect nucleus and bcl-2.
Keywords: Antioxidants, Anticancer, Apoptosis, ROS, Hydrogen peroxide, RCC-26
1. Introduction
Reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide anion radical (O2−), and the hydroxyl radical (OH−) are an entire class of highly reactive molecules derived from the metabolism of oxygen. This could be elicited by ionizing radiations and some chemical substances[1], and has been implicated in many human degenerative diseases, including aging, cancer, Huntington's disease, cardiovascular disease, mutations and neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease[2]. H2O2 belongs to the ROS, and is known to modulate diverse cellular functions in vivo by producing hydroxyl radicals through the interaction with metal ions near DNA. Also, H2O2 induces DNA damage and cell damage by lipid peroxidation[3]. Although apoptosis and necrosis have different impacts on cellular physiology, the cellular response to H2O2 is continuing from apoptosis to necrosis[4] i.e. high concentration induces necrosis converse is applicable for apoptosis[3]–[5].
Many antioxidants such as resveratrol found in grapes and other food products have anticancer or anti-carcinogenic properties along with the anti-apoptotic activity[6]. Furthermore, they can protect cells from oxidative damage and programmed cell death[7]. Antioxidants prevent carcinogenesis in murine model with anti-apoptotic activity[8].
Apoptosis, in association with oxidative stress, is an active cell death mechanism, which occurs during several pathological situations for cancer suppression[9]–[11]. It can be induced by two different pathways, the death-receptor pathway and mitochondrial pathway (mediated by caspase-8 and caspase-9, respectively). Eventually, both pathways lead to the activation of effecter caspases such as caspase-3[12]–[15]. The differential expression i.e. pro-apoptotic versus anti-apoptotic bcl-family proteins determines the inherent susceptibility of a given cell that responds to apoptotic signals. The pro-apoptotic Bax and anti-apoptotic bcl-2 are important proteins of bcl-2 family and the ratio of bcl-2/Bax play a critical role in the regulation of apoptosis[16].
Renal cell carcinoma is a common impairment, constituting 56% of all secondary malignancies. After nephrectomy, renal cell carcinoma tends to metastasize as a solitary lesion without local tumour recurrence or distant metastasis to other organs unlike other cancers[17]. Current study is designed to evaluate radical scavenging, mechanism of anticancer and the anti-apoptotic activities of antioxidants (dose dependent) with special emphasis to renal cell carcinoma (RCC-26) cell lines.
2. Materials and methods
2.1. Chemicals
Ascorbic acid (AA), α-tocopheryl acetate (α-TA), citric acid (CA), salicylic acid (SA), H2O2, RPMI-1640, were purchased from Sigma Chemicals (St.louis, MO, USA). Rabbit anti-bcl-2 and anti-rabbit-IgG-AP antibodies kit were purchased from Santa Cruz, CA, USA. Apoptotic DNA ladder kit and protease inhibitors were purchased from Roche Diagnostics, Gmbh, Germany. RCC-26 cell line was purchased from NCCS Pune, India. Dichlorofluorescin diacetate (DCFH-DA) stock solution (10 mM in methanol) was stored at -80 °C. All other chemicals were of the highest analytical grade and purchased from common sources.
2.2. Determination of H2O2 scavenging activity
The H2O2 scavenging activity of antioxidants was determined according to the method of Ruch et al with some modification, based on UV spectrophotometer. H2O2 has optimum absorbance at 230 nm, depending on its concentration. Solution of H2O2 (3.5 mM) and antioxidants were prepared in phosphate buffer (pH =7.4) followed by the determination of concentration by spectrophotometer at 230 nm[18].
Thereafter, antioxidants at different concentrations were added to H2O2 solution, separately. The absorbance of H2O2 at 230 nm was determined at appropriate time gap against a blank solution consisting of phosphate buffer as a negative control. The percentage of scavenging of H2O2 against antioxidant was calculated as per the formula:
The % of scavenged H2O2 = [( Ao-A1)/Ao ] × 100
Where Ao is the absorbance of control, A1 is the absorbance in the presence of the antioxidants.
The ability of the antioxidants to scavenge H2O2 was determined at different concentration of antioxidants against different concentration of H2O2. Here, we optimized the antioxidant property of antioxidants against 3.5 mM H2O2 at 20 and 50 mg/mL concentrations.
2.3. Cell viability assessment
Cell viability was estimated by 3-(4,5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT) method, which is based on the cleavage of a tetrazolium salt by mitochondrial dehydrogenases in viable cells[19]. Cultured RCC-26 cells (2×106) were seeded and allowed to adhere in a 96-well plate using RPMI-1640 medium. Then cells were treated with various antioxidants (20 and 50 µg/mL) followed by 2 h incubation and 3.5 mM H2O2 was added for another 3 h. After the incubation 25 µL of 5 mg/mL MTT (dissolved in PBS) was added to each well followed by incubation for 4 h at 37 °C. Thereafter, formazan crystals were dissolved in 150 µL DMSO followed by the quantitative measurement of formazan purple by the absorbance at 540 nm. Survival rate of cells (30%) treated with H2O2 (without antioxidant) was taken as control and percent viability for treated cells (with antioxidant) was then expressed with reference to % viability of control cells.
2.4. Nuclear morphology analysis
In this methodology, the observation of nuclear morphology has been measured through propidium iodide (PI) test. Cell lines were cultured and seeded on sterilized cover glasses and treated with different antioxidants, at both concentrations as mentioned earlier, in different methods for 24 h followed by the PI staining (10 µg/mL). Morphology of cell nucleus was observed by fluorescence microscope at the magnitude of 200× consisting of the sample of 2×106 cells[20]. Nucleosomal damage studies have been further carried out for more precision.
2.5. Nucleosomal damage detection
Photometric immunoassay of cytoplasmic histone associated DNA fragments was used for cell death detection of quantitative measurement of apoptosis. In brief, cells were cultured for 18 h either in presence or absence of various antioxidants (AA, CA, SA, α-TA) followed by exposure of 3.5 mM H2O2 for 3 h. Thereafter, cells were pelleted and incubated at room temperature in the lysis buffer (provided with the kit) for 30 min and centrifuged at 200× g for 10 min. 25 mL of supernatant containing mono and oligonucleosomes (released by treated cells) was used for enzyme-linked immunosorbent assay (ELISA). The results were expressed in terms of an enrichment factor, i.e., ratio of absorbance (A405 nm/A490 nm) of treated cells/absorbance (A405 nm/A490 nm) of control cells.
2.6. DNA fragmentation
DNA fragmentation was determined by using conventional agarose gel electrophoresis with the help of apoptotic DNA ladder kit, as per the manufacturer instructions. In short, RCC-26 cells (2×106) were lysed with the help of lysis buffer (6 M guanidine HCl, 100 mM Urea, and 10 mM Tris-HCl, 20% Triton-X-100, pH 4.4) and DNA was purified and separated by glass entrapment method in glass fiber fleece and eluted with the help of elution buffer (10 mM Tris, pH 8.5). DNA aliquots (1-3 µg/well) were prepared and loaded onto agarose gel (1%), containing 50 µg of ethidium bromide and were electrophoresed at 75 V for 1.5 h. DNA was visualized by placing the gel over UV-transilluminator.
2.7. Bcl-2 detection
2.7.1. Protein extraction
Firstly, cells were treated with antioxidants and then were lysed by sonication method. Cell lysate and ice-chilled methanol were mixed in the ratio of 1:4, respectively and left on ice for 1 h. Pellet of protein was collected after centrifugation at 10 000 rpm for 15 min and suspended in 0.25 M Tris-HCl buffer (pH 6.8) containing 10 µg/mL leupeptin, 1 µg/mL aprotinin and 10 µg/mL pepstatin. Total protein content was estimated by the method of Lowry et al[21].
2.7.2. Immunoblotting
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) method was used for separation of detergent soluble proteins (40 µg protein/lane) on 10% gel under reducing conditions. Protein solution was mixed with the sample buffer and protein's concentration was adjusted to 2 µg/µL. Then after proteins were transferred on nitrocellulose membrane at 100 V for 2 h. Nitrocellulose membrane was blocked overnight at 4 °C with 5% skimmed milk powder in PBS, and subsequently incubated for 1 h with rabbit-anti-bcl-2 monoclonal antibody. Membrane was washed by PBS, which contains 0.05% Tween-20 (PBS-T), and then probed with anti-rabbit-IgG conjugated with alkaline phosphates in 2.5% skimmed milk in PBS for 1 h at 37 °C. Finally, membrane was rinsed twice with PBS-T and then with PBS. Protein bands were visualized by membrane incubation with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP-NBT), a substrate for alkaline phosphatase. For comparative study, band intensity of protein was estimated by imaging densitometer software (Bio-Rad, Model GS-670) with respect to the band intensity of (H2O2-untreated) control cells.
2.8. Flow cytometry analysis
Intracellular ROS was determined by measuring changes in fluorescence resulting from intracellular probe oxidation. Renal cell carcinoma cells were cultured in RPMl-1640 medium supplemented with 10% fetal bovine serum. These viable cells (2×106/mL) were dispersed in 96-well micro plates for 24 h at 37 °C with 5% CO2. The medium was removed and cells were washed subsequently three times by PBS for the complete removal of medium, then different antioxidants were added and the microplates were incubated for 2 h. Thereafter, 20 µm 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) was loaded after another 45 min. The cells were washed with PBS for removing the DCFH-DA. 3.5 mM H2O2 was then added into the cells for another 45 min, and the change was observed by fluorescent spectrophotometer at λ ex=475 nm.
2.9. Statistical analysis
The results were expressed as mean±standard deviation. Data obtained were subjected to student's t-test for statistical analysis and statistical significance was designated as (P<0.05). Multiple comparisons were made using one way analysis of variance (ANOVA) followed by post hoc analysis using Tukey test to determine the significance of the results between different groups obtained using GraphPad Instat software.
3. Results
3.1. ROS scavenging activity of antioxidants
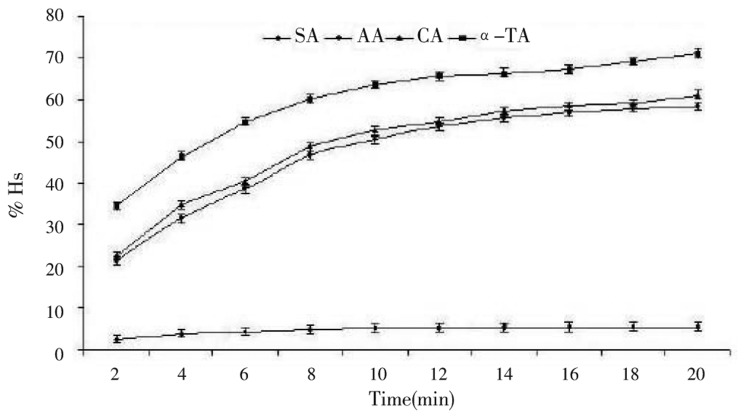
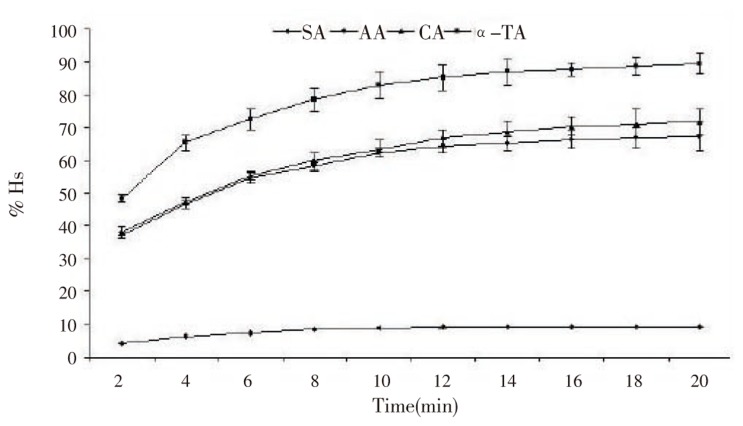
Naturally occurring antioxidants have a broad range of biochemical activities, including inhibition of ROS generation, direct or indirect scavenging of free radicals, and alteration of intracellular redox potential[2]. In this study, we have evaluated radical (generated by H2O2) scavenging activity of selected antioxidants by spectrophotometer. H2O2 scavenging (Hs) ability of the different antioxidants (at 20 and 50 µg/mL) against H2O2 was depicted in Figure 1, 2. H2O2 scavenging ability of antioxidants is concentration-dependent since it increases significantly with the increase in the concentration of antioxidants. The percentage of the H2O2 scavenging activity (%Hs) was 89.94% for α-TA, 71.78% for CA, 67.78% for AA and 9.12% for SA at 50 µg/mL. However, at 20 µg/mL concentration %Hs were 69.22, 59.05, 57.76, and 5.34 for α-TA, CA, AA, and SA, respectively. As per the results obtained, α-TA had stronger H2O2 scavenging activity among the studied antioxidants.
Figure 1. Percent H2O2 scavenging activity of different antioxidants (20 µg/mL).
Results are expressed as mean±SD, (n=5).
Figure 2. Percent H2O2 scavenging activity of different antioxidants (50 µg/mL).
Results are expressed as mean±SD, (n=5).
3.2. Cytoprotectivity (cell viability)
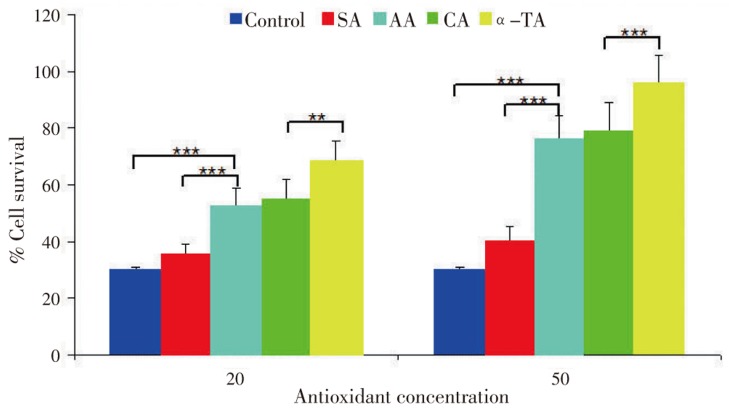
Cell viability action of antioxidants was against direct oxidative damage. It is previously accepted that high levels of free radicals or active oxygen species create oxidative stress leading to a variety of biochemical and physiological lesions which in turn result in metabolic impairment and cell death[22]. We determined cytoprotective activity of antioxidants against H2O2. It was observed that antioxidants exhibited no cytotoxic effects on RCC-26 cells instead stimulated the growth of renal cells, especially the α-TA (Figure 2). A-TA increased the cell viability and protected cells against H2O2 significantly (P<0.005) though, SA responded conversely. Moreover, AA and CA intermediated cell protection ability. These results reflected that cell survival ability was improved by antioxidants in oxidative stress conditions. Also, the enhanced cell protection ability made them plausible for functional ingredients or drugs without any significant adverse effects.
As is evident, oxidative stress is an important factor to induce cell death. In the presence of only H2O2 (control), cell death ratio reached up to 70% after 45 min of the treatment (Figure 3). As compared with the control, the reduction of cell death was observed after the treatment of each antioxidant at 20 and 50 µg/mL concentration.
Figure 3. Effect of antioxidants on cell survival after H2O2 exposure.
Results are expressed as mean±SD, (n=6).
A-TA showed the highest protective effect among the various antioxidants. The cell survival significantly increased with antioxidants treated cells at the concentration of 50 µg/mL from 20 µg/mL progressively. These results reflected the protective effect of antioxidants against intracellular oxidative damage directly induced by H2O2.
3.3. Potection of nucleosomal damage by antioxidants
H2O2 is known as a source of ROS (intra as well as extra cellular), which can cause chromosomal and nucleosomal aberrations through oxidative damage of DNA[3],[23]. We have analyzed the protective effect of antioxidants on H2O2-induced apoptosis. PI was used for staining nuclei of RCC-26 cells treated either with H2O2 alone or with both antioxidants and H2O2. Cells treated with 3.5 mM H2O2 (Figure 4) showed significant nuclear fragmentation. However, when cells were treated with antioxidants for 1 h prior to H2O2 treatment, a marked reduction in nuclear fragmentation was observed depending on type (activity or potential) of antioxidants and their concentration.
Figure 4. Nuclear fragmentation due to 3.5 mM H2O2.
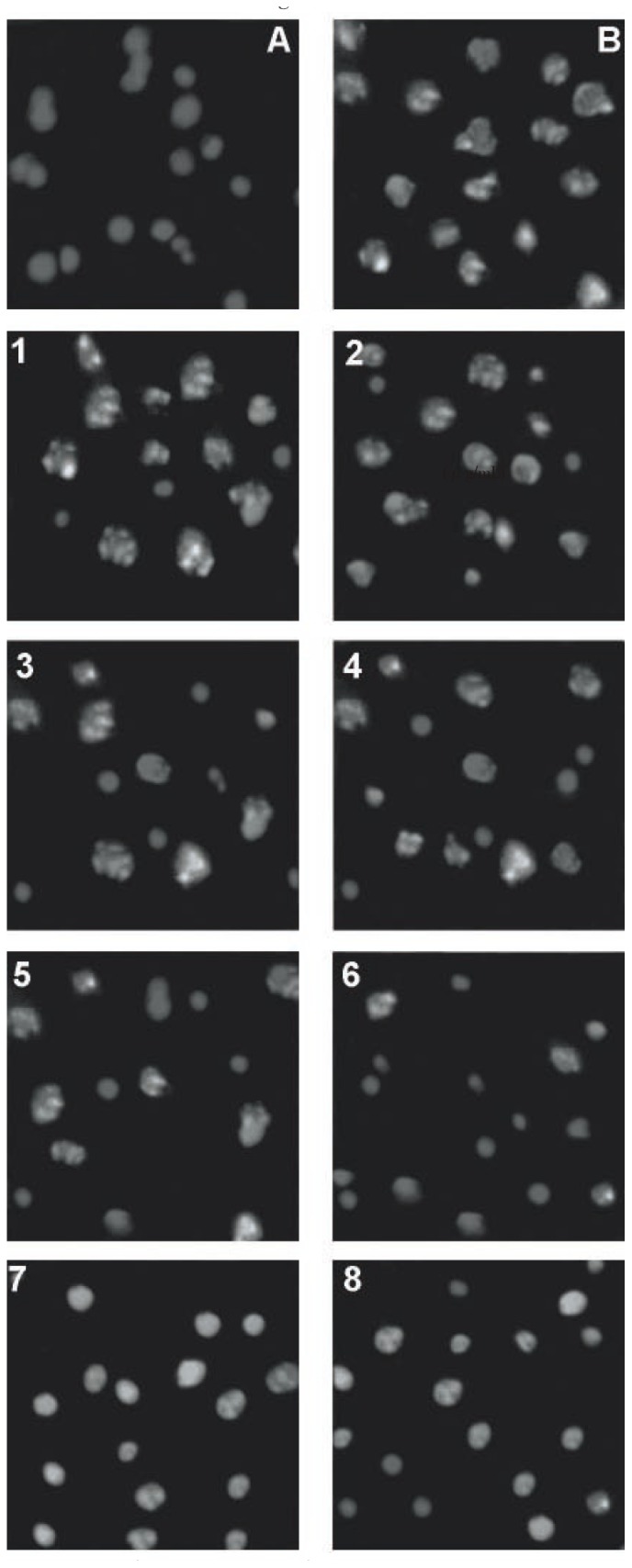
Fluorescence at the magnitude of 200×; A: Vehicle only (positive control); B: 3.5 mM H2O2 (negative control); 1, 3, 5, 7 at 20 µg/mL, 2, 4, 6, 8 at 50 µg/mL of antioxidants for 1 prior to addition of 3.5 mM H2O2 for salicylic acid, ascorbic acid, citric acid, alfa-tocopheryl acetate, respectively.
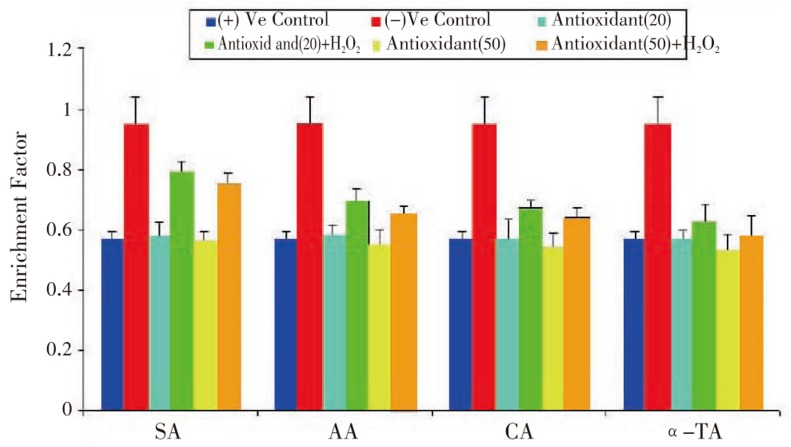
We estimated enrichment factor to detect nucleosomal damage, a quantitative measurement of apoptosis (cell death detection), which was carried out by ELISA. This method is based on the photometric immunoassay of cytoplasmic histone associated DNA fragments. Treatment of cell lines with antioxidants at two different concentrations (20 µg/mL and 50 µg/mL) with subsequent exposure to 3.5 mM H2O2, showed drastic change in enrichment factor as compared with positive and negative control. Comparing to positive (Figure 5), negative control cell showed 1.6 fold enrichment.
Figure 5. Effect of antioxidants on H2O2 induced nucleosomal damage (in terms of enrichment factor) in RCC-26.
Results are expressed as mean±SD, (n=6).
In addition, we have evaluated that antioxidants protect DNA against H2O2 by conventional agarose gel electrophoresis. DNA gel electrophoresis (Figure 6) pattern obtained after the treatment of different antioxidants with cell lines. Oxidative stress in cell lines by H2O2 for 2 h was able to produce DNA fragments (lane III) which were absent in control (lane II) and significantly visualized in cells treated with the antioxidants (lane IV,V, VI, VII at 50 µg/mL and lane VIII, IX, X, XI at 20 µg/mL). Pre-treatment of cells with antioxidants for 18 h prior to H2O2 exposure inhibited the fragmentation of DNA. However, protection of DNA against H2O2 depends upon the concentration and potential of antioxidants. Antioxidants at a concentration of 50 µg/mL exhibited good DNA protective activity (Figure 6), and α-TA mainly showed the best DNA protective activity amongst all antioxidants. Other antioxidants AA and CA can intermediate, and SA had very low DNA protective activity against H2O2. The same order was followed at 20 µg/mL, however, protection was directly correlated with the concentration of antioxidants.
Figure 6. Agarose gel electrophoresis for DNA fragmentation.
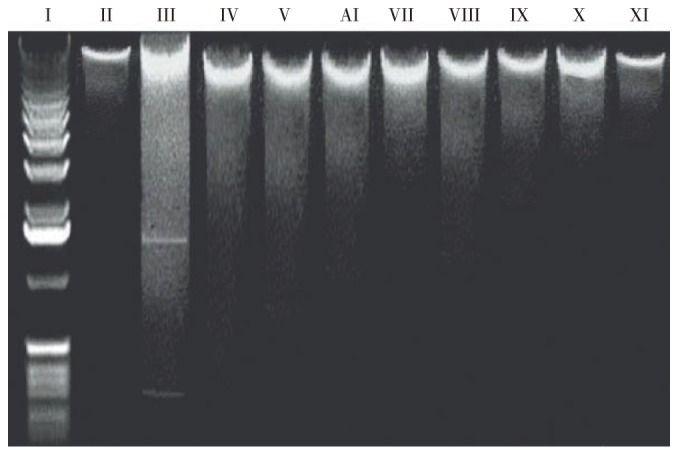
Lane I: Marker; Lane II: Normal RCC-26 (positive control); Lane III: H2O2 (negative control); Lane IV, V, VI, VII: for 20 µg/mL and Lane VIII, IX, X, XI for 50 µg/mL of antioxidant prior to addition of H2O2, salicylic acid, ascorbic acid, citric acid, alfa-tocopheryl acetate, respectively.
3.4. Effect of antioxidants on bcl-2 level
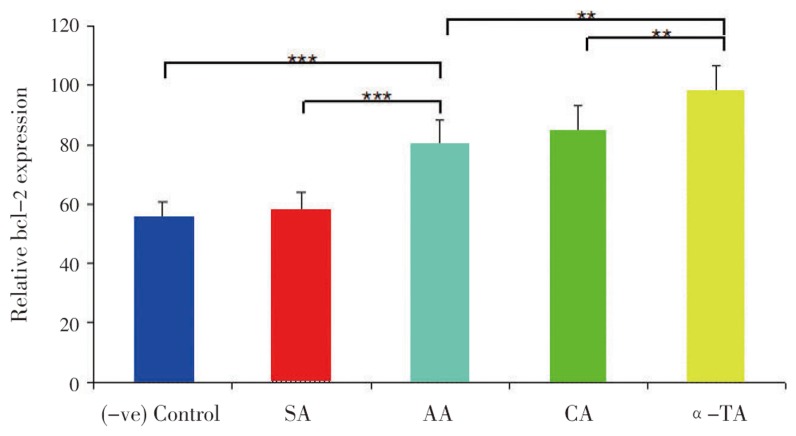
Antioxidants raised bcl-2 level against the pro-oxidant in the cell mediated reaction. Thereafter, cells become resistant to oxidant and oxidant-induced apoptosis[24]. Results (Figure 7, 8) indicated that antioxidants (SA, AA, CA and α-TA) treated at both concentrations 20 and 50 µg/mL to cell lines for 18 h significantly protected the bcl-2 levels. Additionally, only H2O2 treatment (vegative control) for 2 h significantly reduced the bcl-2 levels as compared with positive control RCC-26 cell lines. The protection of bcl-2 by antioxidants was in the following order α-TA > CA > AA » SA at both (20 and 50 µg/mL) concentration.
Figure 7. Effect of antioxidants on bcl-2 level (20 µg/mL).
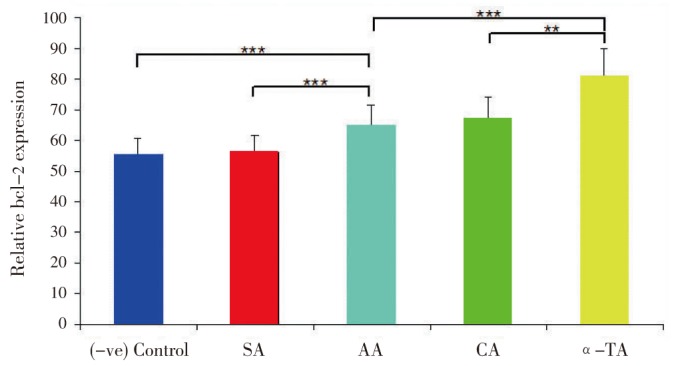
Relative bcl-2 levels in which cell without treatment with H2O2 (positive control) was taken as 100%, where antioxidants were taken at 20 µg/mL. Results are expressed as mean±SD, (n=6).
Figure 8. Effect of antioxidants on bcl-2 level (50 µg/mL).
Relative bcl-2 levels in which cell without treatment with H2O2 (positive control) was taken as 100%, where antioxidants were taken at 50 µg/mL. Results are expressed as mean±SD, (n=6).
3.5. Protective action of antioxidants against direct oxidative stress
It is documented that antioxidants have been used to inhibit apoptosis since apoptosis is initially thought to be oxidative stress mediated phenomenon[25]. We attempted to observe the changes in DCF fluorescence with fluorescence microscopy (Figure 9). ROS and oxidative stress agents (H2O2) cause cell damage (Figure 9B). Enzymes were released from cell membrane like esterase cleaved ester bond of DCF-DA and DCF was produced which reflects fluorescence. Photomicrograph is a typical microscopic presentation of the DCF fluorescence in antioxidants treated RCC-26. Cells treated with 3.5 mM H2O2 for 45 min showed elevated levels of fluorescence as compared with the positive control samples (Figure 9A) clearly indicating that antioxidants at concentrations of 20 and 50 µg/mL for 2 h significantly inhibited the oxidation of DCFH into DCF. The intensity of fluorescence and number of fluorescecent cells was showed in microphotograph for antioxidants in following order SA »AA> CA> α-TA indicating the protective activity of cell against ROS (H2O2 as source) in following order α-TA >CA>AA»SA.
Figure 9. Fluorocytometric assay (ROS production evaluation with DCFH-DA assay/DCF fluorescence in RCC-26 cells.
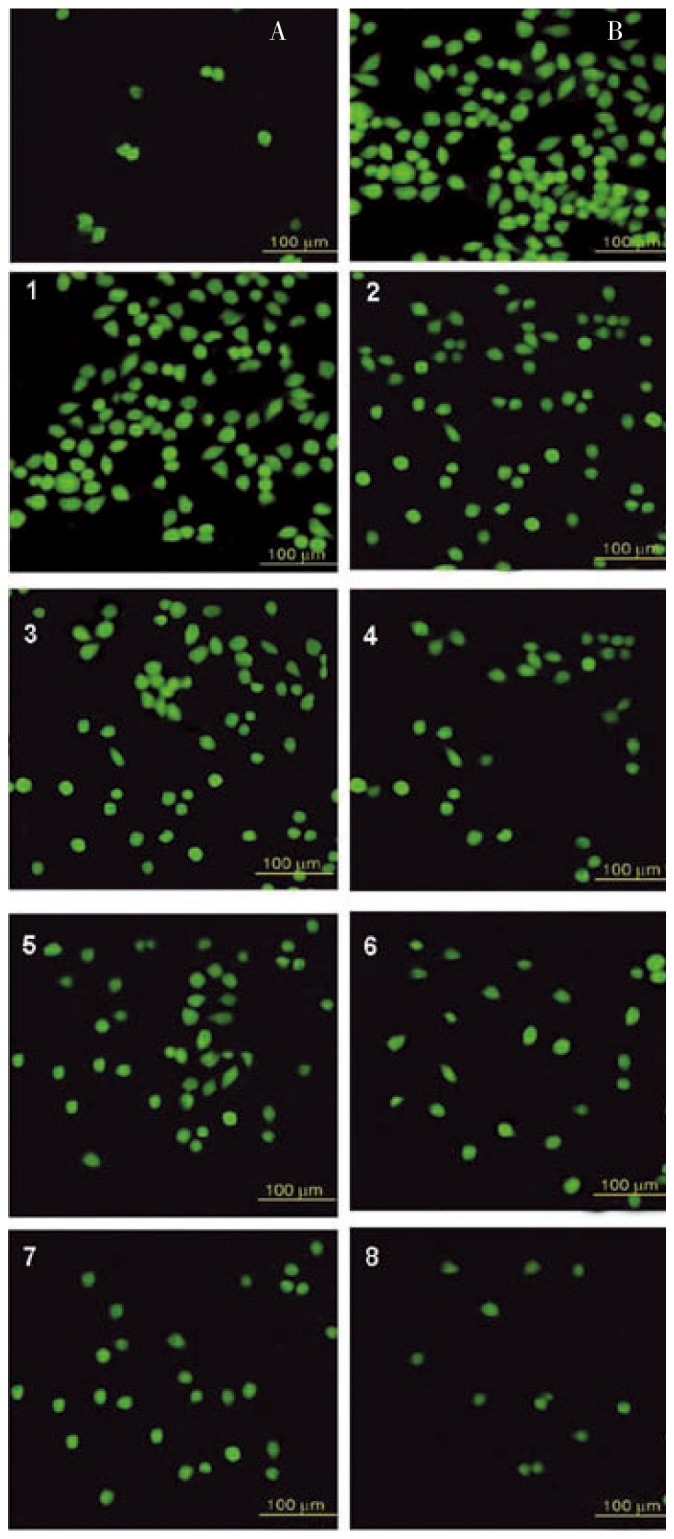
A: Vehicle only (positive control); B: 3.5 mM H2O2 only (negative control); 1, 3, 5, 7 at 20 µg/mL; 2, 4, 6, 8 at 50 µg/mL, of antioxidants for 1 h prior to addition of 3.5 mM H2O2 for salicylic acid, ascorbic acid, citric acid, alfa-tocopherol acetate, respectively.
4. Discussion
There are sufficient evidences revealing the vital range of biological activities of antioxidants such as direct or indirect scavenging of free radicals, anticancer, antimutagenesis[2] and anti-apoptotic activity[25]. Although the role of apoptosis in pathological situation for cancer suppression has been already evaluated[9]–[11]. This study demonstrates that antioxidants have the potential scavenging effect on intracellular oxidative damage directly induced by H2O2 which, in turn, lead to an improvement in the percentage cell viability. Also, the anti-apoptotic effect of antioxidants on cancerous cells (RCC-26) is quite evident. The protective effect of antioxidants over cells against ROS has been studied. Antioxidants prevent radical (especially hydroxyl radical generated by H2O2) mediated cell damage and indirectly suppress apoptosis. Thus, poly-phenolic antioxidants show significant cell protecting activity and inhibit apoptosis. Again, our findings are in agreement with those obtained for H2O2 scavenging activity, indicating major structural features responsible for the anti-apoptotic activity against reactive radicals.
During various physiological and pathological processes, ROS (like OH−, O2− etc.) is generated from H2O2 by mitochondria at both extra and intracellular levels[26]. This induces oxidative chromosomal damage as well as direct breakage of DNA[23]. H2O2 itself is not reactive enough, however, in association with super oxide anions can damage many cellular components[27]. Thus, these antioxidants which possess the property of scavenging of free radicals or ROS can protect oxidative damage followed by the decrease in the level of pro-oxidants such as H2O2[28]. Therefore, H2O2 (ROS generating agent) has been used to evaluate the radical scavenging activity of different antioxidants. In parallel, we have studied radical scavenging activity of different oxidants at different concentration. Our results, in agreement with earlier reports, have reflected the inhibition of carcinogenesis and mutagenesis by antioxidants via the formation of adducts (with DNA), masking the binding sites which are occupied by mutagens or carcinogens[23]. Furthermore, antioxidants have large number of hydrogen atoms showing the importance to be available for abstraction and subsequent reduction and neutralization of the oxidative species. Moreover, they remain stable through electron delocalization across both side chains and conjugated ring. Therefore, antioxidants could reduce the bioavailability and the exposure of noxious radicals towards DNA. The antioxidants (novel radical scavengers) could scavenge ROS generated by electron leakage and protect cells against apoptosis induced by ROS.
As is cited in the literature, H2O2 induces oxidative nucleosomal and chromosomal damage through ROS[3],[23], and these ROS are scavenged by polyphenolic antioxidants[2]. In the current study the protective effect of different antioxidants against H2O2 mediated nucleosomal damage in cancer cell lines especially RCC-26 was investigated. Here, we observed that these antioxidants protect nucleosome against ROS produced by H2O2. However, we hypothesized the dose dependent protection of nucleosome by antioxidants and their anti-apoptotic activity[25]. This dose dependency could be explained in terms of the ROS level and extent of oxidative stress.
Anti-apoptotic property of bcl-2 is quite evident either by controlling the membrane potential of mitochondria and/or inhibiting calcium ion depletion of endoplasmic reticulum[29],[30]. The mechanism of anti-apoptosis through bcl-2 is explained by its pro-oxidant, though indirect, nature. The rise in the level of pro-oxidant is directly related with the increase in intrinsic cellular anti-oxidant. Here the increase of bcl-2 expression is in coherence with above mentioned obervation and could be correlated like, bcl-2 mediated reaction leads the cells to make them resistant to oxidant and oxidant-induced apoptosis[24]. The anti-apoptotic function and its efficiency are controlled by the bcl-2 family members, which function partly by interacting physically with one another at mitochondrial level[31]. Here, our findings have shown the importance and role of bcl-2 over the H2O2-induced apoptosis and its prevention by antioxidants in renal cell carcinoma cells. Interestingly, antioxidants used in the present study significantly reduced the H2O2 mediated bcl-2 degradation and/or reduction in the expression. Moreover, cell survival is prolonged by the bcl-2 gene product by inhibiting apoptosis induced by H2O2 mediated stimuli. Here, we observed significant changes in bcl-2 protein levels in RCC-26 cells treated with antioxidants. Presumably, there are some unknown mechanisms accounting for preventing RCC-26 from H2O2-induced apoptosis. However, this change in bcl-2 level is dose dependent and also depends on antioxidant potency of the compounds.
The bond dissociation enthalpy (BDE) plays an important role in molecular properties of the compounds which make the OH− weaker and its reaction with the free radicals become faster. As per the hypothesis already proposed, more the exothermic reactions of antioxidant with free radicals, lesser is the barrier which in turn leads the antioxidants to react faster with the radicals thereby preventing its reaction with the biological substrate[32]. We argue that α-TA is an effective antioxidant due to its larger BDE. Also, the physical and chemical environment of antioxidants seems to be an important factor for its reactivity. The position, number of hydroxyl groups, BDE, IP, and presence of bulky groups near OH-group have considerable effect on antioxidant activity of these phenolics[33]. Apart from that, enhanced anti- apoptotic effect of α-TA can be partly attributed to its role in activation of ROS metabolizing enzymes like SOD[34].
ROS are mainly generated at the complex III site (bcl complex) in mitochondria. During respiratory chain the complex III, shuttles transfer electrons from Q10 to cytochrome c and release two protons to their inter-membrane space which in turn reduces cytochrome c. The occurred electron leakage and the these electron transports are associated with a redox shift which creates interference in the mitochondria and build up of ROS playing a major role in apoptotic cascade[35]–[38]. Considering these findings, we evaluated the protective role of antioxidants in apoptotic cell death induced by H2O2, which is a source of ROS (complex III inhibitor).
In conclusion, current study shows that the antioxidants, preferentialy α-TA, significantly inhibit cell damage and exhibit DNA protective effect in RCC-26 cell lines against H2O2 induced oxidative stress. The anti-apoptotic activity of the antioxidants is mediated through scavenging ROS and can be partly attributed to bcl-2 recruitment. The anti-apoptotic activity of bcl-2 dependent mechanism is also an important finding giving insight into themolecular mechanism of reported antioxidant. Present findings bring new insight in the direction of therapeutic intervention for cancer.
Acknowledgments
The research work is supported by the All India Council for Technical Education (AICTE) for funding, New Delhi, India. We are also thankful to the analytical technical facilities of All India Institute of Medical Sciences (AIIMS), New Delhi, India. Authors also acknowledge the kind support of Dr. Gaurav Kaushik, PGIMER, Chandigarh for providing chemicals and his valuable guidance. No writing assistance was utilized in the production of the manuscript.
Footnotes
Foundation Project: Supported by All India Council for Technical Education.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48(5):825–834. [PubMed] [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Ueda N, Shah SV. Endonuclease-induced DNA damage and cell death in oxidant injury to renal tubular epithelial cells. J Clin Invest. 1992;90(6):2593–2597. doi: 10.1172/JCI116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, et al. et al. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22(1–2):73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 5.Lennon SV, Martin SJ, Cotter TG. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson IT, Williamson G, Musk SR. Anticarcinogenic factors in plant foods: a new class of nutrients? Nutr Res Rev. 1994;7(1):175–204. doi: 10.1079/NRR19940011. [DOI] [PubMed] [Google Scholar]
- 7.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. et al. Cancer chemo preventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 8.Clement MV, Hirpara JL, Chawdhury S, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92(3):996–1002. [PubMed] [Google Scholar]
- 9.Iwashita K, Kobori M, Yamaki K, Tsushida T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 2000;64(9):1813–1820. doi: 10.1271/bbb.64.1813. [DOI] [PubMed] [Google Scholar]
- 10.Kobori M, Iwashita K, Shinmoto H, Tsushida T. Phloretin-induced apoptosis in B16 melanoma 4A5 and HL60 human leukemia cells. Biosci Biotechnol Biochem. 1999;63(4):719–725. doi: 10.1271/bbb.63.719. [DOI] [PubMed] [Google Scholar]
- 11.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukemia HL-60 cells. Eur J Cancer. 1999;35(10):1517–1525. [PubMed] [Google Scholar]
- 12.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29(3–4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92(1):57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 14.Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie. 2002;84(2–3):203–214. doi: 10.1016/s0300-9084(02)01376-7. [DOI] [PubMed] [Google Scholar]
- 15.Mathiasen IS, Jaattela M. Triggering caspase-independent cell death to combat cancer. Trends Mol Med. 2002;8:212–220. doi: 10.1016/s1471-4914(02)02328-6. [DOI] [PubMed] [Google Scholar]
- 16.Tjalma WA, Weyler JJ, Bogers JJ, van Dam PA, van Marck EA, Buytaert PM, et al. et al. The importance of biological factors (bcl-2, bax, p53, PCNA, MI, HPV and angiogenesis) in invasive cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2001;97(2):223–230. doi: 10.1016/s0301-2115(00)00541-8. [DOI] [PubMed] [Google Scholar]
- 17.Heffess CS, Wenig BM, Thompson LD. Metastatic renal cell carcinoma to the thyroid gland: a clinicopathologic study of 36 cases. Cancer. 2002;95(9):1869–1878. doi: 10.1002/cncr.10901. [DOI] [PubMed] [Google Scholar]
- 18.Ruch RJ, Cheng SJ, Klauning JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Shin HT, Hwang HJ, Kim JH. Antioxidant activity of extract from Alpinia katsumadai seed. Phytother Res. 2003;17(9):1041–1047. doi: 10.1002/ptr.1291. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 22.Ames B. Micronutrients prevent cancer and delay aging. Toxicol Lett. 1998;102:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman HM. The bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem. 1995;270(8):3487–3490. [PubMed] [Google Scholar]
- 25.Hockenhery DM, Oltavai ZN, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 26.Davies KJ, Hochstein P. Ubisemiquinone radicals in liver: implications for a mitochondrial Q cycle in vivo. Biochem Biophys Res Commun. 1982;107(4):1292–1299. doi: 10.1016/s0006-291x(82)80138-1. [DOI] [PubMed] [Google Scholar]
- 27.Kaur H, Perkins J. The free radical chemistry of food additives. In: Aruoma OI, Halliwell B, editors. Free radicals and food additives. London: Taylor and Francis Limited; 1991. pp. 17–35. [Google Scholar]
- 28.Pazdzioch-Czochra M, Widenska A. Spectrofluorimetric determination of hydrogen peroxide scavenging activity. Anal Chim Acta. 2002;452:177–184. [Google Scholar]
- 29.Distelhorst CW, Lam M, McCormick TS. Bcl-2 inhibits hydrogen peroxide-induced ER Ca2+ pool depletion. Oncogene. 1996;12(10):2051–2055. [PubMed] [Google Scholar]
- 30.Shimizu S, Eguchi Y, Kamiike W, Lacronique V, Matsuda H, Tsujimoto Y, et al. et al. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci USA. 1998;95(4):1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinkel S, Gross A. Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13(8):1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 32.Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 2003;41(6):753–758. doi: 10.1016/s0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 33.Chen ZY, Chan PT, Ho KY, Fung KP, Wang J. The antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem Phys Lipids. 1996;79(2):157–163. doi: 10.1016/0009-3084(96)02523-6. [DOI] [PubMed] [Google Scholar]
- 34.Xiao DZ, Tian XW, Li SC, Yong FZ. Effects of α-tocopheryl acetate supplementation in pre-slaughter diet on antioxidant enzyme activities and fillet quality of commercial-size Sparus macrocephalus. J Zhejiang Univ Sci B. 2007;8(9):680–685. doi: 10.1631/jzus.2007.B0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adachi S, Cross AH, Babior BM, Gottlieb RA. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272(35):21878–21882. doi: 10.1074/jbc.272.35.21878. [DOI] [PubMed] [Google Scholar]
- 36.Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17(1):37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Silva JP, Gustafsson DM, Rustin P, Larsson NG. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci USA. 2001;98(7):4038–4043. doi: 10.1073/pnas.061038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Graaf AO, Meijerink JP, van den Heuvel LP, DeAbreu RA, de Witte T, Jansen JH, et al. et al. Bcl-2 protects against apoptosis induced by antimycin A and bongkrekic acid without restoring cellular ATP levels. BBA. 2002;1554(1–2):57–65. doi: 10.1016/s0005-2728(02)00213-x. [DOI] [PubMed] [Google Scholar]