Abstract
Objective
To study whether the infection of Schistosomiasis japanicum (S. japanicum) is related to enhanced proliferation and migration of cancer cells, and the molecular mechanism pertains to cancer cell metastasis in human host.
Methods
The gene of S. japanicum glutathione transferase (sjGST) cloned from S. japanicum was expressed, purified and applied in a series of assays to explore the effect of sjGST on proliferation and migration of MDA-MB-435S, and the expression of MMP2 and MMP9. Immunofluorescence assay for the binding of sjGST to MDA-MB-435S was also carried out.
Results
Results showed that sjGST enhanced proliferation and migration in human breast cancer cell MDA-MB-435S signifycantly at 50-200 nM, but did not enhance them in human lung cancer cell A549. Immunofluorescence assay for the binding of sjGST to MDA-MB-435S and A549 showed that GST was readily bound to the breast cancer cells, but showed almost no binding to human lung cancer cells. The assays for gelatinase activity showed that both MMP2 and MMP9 activities were increased significantly in the presence of sjGST (50-200 nM) in MDA-MB-435S, but they were not significant in A549.
Conclusions
Our current results show strongly that S. japanicum GST binds to MDA-MB-435S probably via its receptor, and enhances proliferation and migration of the cancer cells by up-regulatory expression of MMP2 and MMP9.
Keywords: Infection, Schistosomiasis japanicum, Glutathione s-transferase, Proliferation, Gelatinase, MMP2 and MMP9, Migration, Breast cancer
1. Introduction
Schistosomiasis japanicum (S. japanicum) is mainly present in Asia, and it threatens more than 300 millions people in south China, Japan and southeast Asian countries. Upon infection, cercariae actively penetrate into the skin of a suitable host and transform into schistosomula and during this process the worm may release substances in the circulation of the host, including glutathione transferase (GST). The enzyme presents in the worm's cells and functions as toxic substance carrier in homology dimmer, and thus neutralizes toxic effects caused by drugs, food additives, environmental chemicals and carcinogens which enter many types of cells[1]–[3]. So far it has been revealed that human host contains 4 GST genes located in chromosomes 6p encoding for α-GST, 1p for µ-GST, 11q for Pi-GST and 22q for θ-GST[4]–[7] respectively, the natural substrate of which is glutathione, a water soluble molecule widely presenting as both oxidized (GSSG) and reduced (GSHRed) forms in eukaryotic cells. Binding of GSHRed to GST causes oxidation of the substrate, and thus maintains the balance of redox states in cells[8]. Chemical studies show that internal detoxification via GST occurs in conjugation pathway (phase II) in which GST binds with substrates, forming conjugates such as GSH s-conjugate. The conjugate is then catalyzed to cysteine s-conjugate by γ-glutamyl transpeptidase and finally released in faeces and urine[9],[10]. These reactions occur in cytoplasmic space, but it is not known what it may happen when S. japanicum continuously releases S. japanicum glutathione transferase (sjGST) into the host circulation.
When the parasite moves via circulation to the portal vessels of the liver where the adult male and female worms develop and mate, they may live for 25 years during which the worm's substances may be released into the circulation. It is well-known that the worm pair produces eggs, which are responsible for major pathogenesis. The worm's antigens including sm22.6[11] can be present in patient's circulation[12],[13] and cause disorders of the host. Study showed GST could interact with nucleic acids, proteins and lipids[14] and other radicals, such as alkoxy radical and α,β-unsaturated aldehydes, which may be generated readily when lipid is attacked by hydroxyl radical and singlet oxygen, causing high oxygen pressure in cells[15]. Therefore, GST plays an important role in neutralizing these radicals, decreasing accumulation of fatty acid hydroperoxides (FA-OOH) and phospholipids hydroperoxides (PL-OOH), and preventing the production of α,β-unsaturated aldehydes in the cells[16],[17]. For instance, anti-apoptic effects of GST has been reported in leukaemia K562 cell line which expresses large amounts of α-GST. The enzyme inhibits the activation of stress-activated protein kinase/c-jun N-terminal kinase (SAPK/JNK) and caspase-3. Thus GST prevents apoptosis probably via decreasing superoxide anion under high oxygen pressure[18].
Matrix metalloproteinases (MMPs) contain a family of enzymes catalyzing degradation of components of extracellular matrix, including collagen, elastin and gelatine[19]. Because of their important activity in tissue remodelling, they are recognized as pathological factors, such as rheumatoid factors and elastase, which are involved in tumour invasion and metastasis[20]. Two members of the family of enzymes have been identified to cleave denatured collagens, type IV collagen, elastin and other matrix proteins[21],[22]. These enzymes are specific for native type IV collegen chains, one being 72 kDa (MMP2), and the other 92 kDa (MMP9). Therefore the enzymic activities are taken as markers for invasion and metastasis of cancer cells.
In order to reveal the role of sjGST in circulation, we used recombinant sjGST to assay for the proliferation, migration and expression of MMP2 and MMP9 in human breast cancer cell line (MDA−MB−435S) and lung cancer cell line (A549). Our present results showed that the GST enhances the proliferation and migration by up-regulatory expression of MMP2 and MMP9 in MDA-MB-435S, which is not found in A549.
2. Materials and methods
2.1. Reagents
Recombinant Taq DNA polymerase with proofreading activity, PCR buffer, dNTP mix, and restriction enzymes were purchased from TaKaRa (Tokyo, Japan). The intermediate PCR cloning kit (pGEM-T easy vector system) was obtained from Promega (Madison, WI, USA), and expression vector (pAcGFP-N1) was purchased from Invitrogen (San Diego, California, USA). General chemicals were purchased from Sigma (Shanghai, China).
2.2. Purification of GST
The gene of sjGST was cloned in expression vector, pGEX-2T. Recombinant sjGST was purified from pGEX-2T-transformed Escherichia coli strain JM109[23]. Briefly, 100 mL of fresh LB medium containing 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl and 80 µg/mL ampicilline was inoculated with transformed BL21 (DE3) containing pGEX-2T with a cDNA fragment for sjGST and grew overnight at 37 °C with vigorous shaking. The stock cells were then transferred to 900 mL of fresh LB medium containing the same concentration of antibiotic and continued to grow for 2 h before IPTG was added to the final concentration of 0.3 mM to induce gene expression. The growth was maintained for another 4 h before harvesting the cells which were then broken in sonicator and soluble proteins in 50 mM Tris-HCl (pH 8.0) were achieved by centrifugation. Supernatant containing rGST was dialyzed against 100-fold volume of PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4), centrifuged to remove insoluble materials, filtered through 0.45 µm membrane and then loaded onto glutathione-agarose column equilibrated with PBS. The column was washed in 5-10 bed volumes of PBS or until the zero absorbance at 280 nm. The recombinant protein sjGST was eluted in elution buffer (10 mM reduced glutathione in Tris-HCl, pH 8.0). Protein concentration was estimated spectroscopically with a formula of mg/mL = 1.55A280 - 0.76A260[11], and used immediately or stored at -80 °C for use.
2.3. Assays for proliferation of MDA-MB-435S and A549
The assays were carried out similarly as we described[24]. Briefly, MDA-MB-435S or A549 cells were seeded in BD Falcon 96-well culture plates (BD Biosciences, Taipei, Taiwan) at a density of 1 500 cells per well and cultured in a complete growth medium at 37 °C in air in the presence of various concentrations of sjGST. The culture media (100 µL/well) were replaced with fresh medium containing fresh sjGST every two days. At day 2, 4, 6, and 8, cells were harvested, fixed and stained with 0.2% crystal violet for 15 min, and destained with ddH2O. The plate was air-dried, recorded (with photographs) and the dye was solubilized with 1% SDS (100 µL/well) by incubation at 37 °C for 1 h. Absorbance of the solubilized stain was measured at 570 nm using a calibrated Model 5500 Microplate Reader (Bio-Rad, Hercules, CA, USA).
2.4. Wound-healing assays and cell counting
Migration assays were carried out according to our recent report[25] for the different concentrations of sjGST in BD Falcon 24-well culture plate (BD Biosciences). The cells were seeded in the plate wells with or without 1 mm tape stuck in the middle and grown to 100% confluent. Then the tape was taken out to make the wound region, or the cell layer was scratched in the middle of the wells using yellow tips, washed three times with serum-free medium and the cells were grown further in 1% FBS-supplemented medium containing 0.01 mg/mL human insulin (Gibco, Oxford, UK) at 37 °C. The wounded monolayer of culture was recorded under inverted microscope at 0, 6, 12, 24, 36, 48 h, and the migrated cell numbers were counted from the camera records. Cell number was counted with computerized programme image-pro-plus 6.0.
2.5. Binding of GST to MDA-MB-435S
Recombinant sjGST (100, 200 and 500 nM) was added to detached MDA-MB-435S or A549 cells, mixed and incubation at room temperature was carried out for 30 min. Then cells were washed 3 times with PBS to remove free sjGST, and then 10 nM of anti-sjGST conjugated with fluorescing substance (Santa Cruz, CA, USA) was added and incubated at room temperature for 30 min. This was followed by 3 washes in PBS, and then viewed under fluorescence microscope.
2.6. Zymographic assays for gelatinase activity
New culture supernatants centrifuged at 12 000 g for 30 min were collected and subjected to separation in non-denatured polyacrylamide gel electrophoresis (PAGE) containing 2% gelatine. After electrophoresis, gels were washed 3 times with 2.5% triton X-100, each of which lasted for 20 min. The gels were incubated in reaction buffer containing 2 M tris-HCl (pH 8.0) and 1 M calcium chloride for 16-18 h. Then the gels were stained with Commasie blue and viewed under light box. Quantification of MMP activity was measured by scanning the zymographic band with computerized programme as described above. Areas in square million meters (mm2) were taken as quantitative units in statistic calculations. We found it was inappropriate to use stored culture supernatants because the enzymes were subjected to be degraded during freeze-thaw cycle.
2.7. Statistical analysis
Significance of different proliferation (absorbance at 570 nm), migration (cell number) and gelatinase activities (relative imagine area after digestion of gelatine by gelatinase) was tested with student t-distribution using two-way formula of 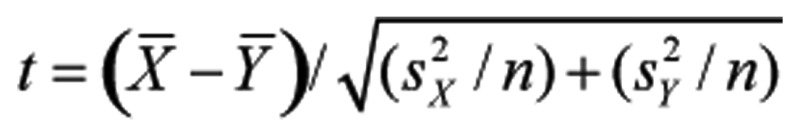 for multiple comparison. A P<0.05 was considered as statistically significant (*), P<0.01 as very significant (**) and P<0.005 as extremely significant (***).
for multiple comparison. A P<0.05 was considered as statistically significant (*), P<0.01 as very significant (**) and P<0.005 as extremely significant (***).
3. Results
3.1. Recombinant sjGST enhances proliferation of MDA-MB-435S
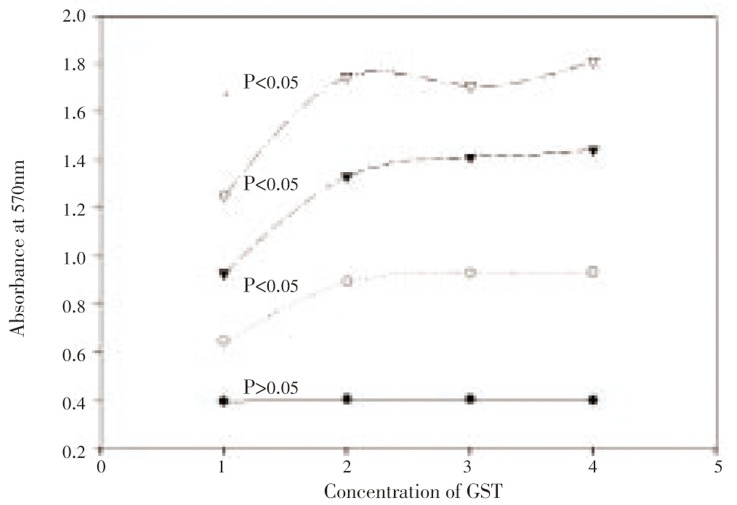
Three different concentrations of sjGST were used to incubate with human breast cancer cell line MDA-MB-435S and human lung cancer cell line A549 for assaying proliferation of the cancer cells. Assays for proliferation of MDA-MB-435S showed that sjGST significantly enhanced cell growth at the concentration of 50, 100 or 200 nM in the second day of incubation (Figure 1). Comparison of the growth of MDA-MB-435S (n=3) in the presence or absence of sjGST showed t = 8.896 4** between MDA-MB-435S alone and MDA-MB-435S incubated with 50 nM sjGST, t = 10.917 7*** between MDA-MB-435S alone and MDA-MB-435S incubated with 100 nM sjGST, and t = 9.275 8** between MDA-MB-435S alone and MDA-MB-435S incubated with 200 nM sjGST in the second day of assays. In the 4th and 6th day incubation, the different proliferations remained at extremely significant level (P<0.005). Interestingly, comparison between 50 nM and 100 nM, 50 nM and 200 nM, and 100 nM and 200 nM showed that their different proliferations did not reach to significant level in the 2nd day assay, but t = 5.585* between 50 nM and 200 nM in the 4th day assay, and t = 3.368 2* between 100 nM and 200 nM in the 6th day assay were achieved. In the incubation of A549 in the presence or absence of the same concentration of sjGST however, the different proliferations did not reach to significant level (results not shown).
Figure 1. Proliferation assays (n=3) for MDA-MB-435S in the presence or absence of sjGST.
X axis indicates treatments (MDA alone in No. 1, MDA in the presence of 50 nM sjGST in No. 2, MDA in the presence of 100 nM sjGST in No. 3, MDA in the presence of 200 nM sjGST in No. 4) and Y axis shows the absorbance at 570 nm. Statistical data showed that P>0.05 among all the treatments in the first day incubation (bottom line), P<0.05 after the 2nd day incubation (second line to the bottom), P<0.005 after the 4th day incubation (second line to the top), and P<0.005 after the 6th day incubation (top line).
3.2. Recombinant sjGST enhances migration of MDA-MB-435S
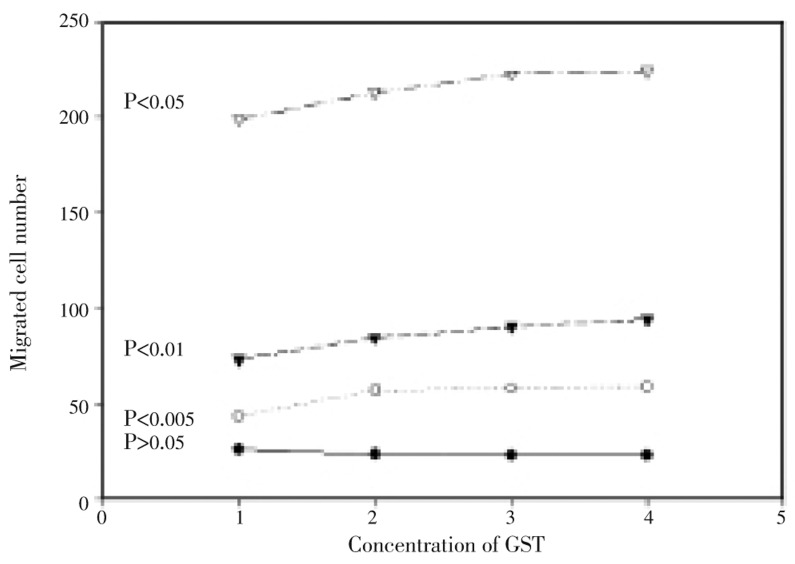
Migration rate via adhesion indicates the motorgenesis and mitogensis of majority of cancer cells, and thus it is taken as a characteristic feature of metastasis[24]. Wound healing assay is one of migration assays used frequently in cancer research. Migrated cells in wells of BD Falcon 24-well culture plate (n=5) could be fixed and stained for direct observation under reversed microscope of adhesion cells. The number of migrated MDA-MB-435S cells began to increase significantly within 12 h of culture in the absence or presence of sjGST, but the migrated cell number was significantly larger in the presence of sjGST than that of the MDA-MB-435S control. T-values calculated from two way equation showed that t = 5.713 8*** between MDA-MB-435S alone and MDA-MB-435S in the presence of 50 nM sjGST, t = 6.085 1*** between MDA-MB-435S alone and MDA-MB-435S in the presence of 100 nM sjGST, and t = 6.184 8*** between MDA-MB-435S alone and MDA-MB-435S in the presence of 200 nM sjGST in 12 h migration assay. This different motorgenesis among the experimental treatments was remained at the best in 24 h migration assay. In the 36 h assay for cell migration however, significance between MDA-MB-435S alone and MDA-MB-435S in the presence of 50 nM sjGST decreased (t = 1.949 3), but significant difference between MDA-MB-435S alone and MDA-MB-435S in the presence of 100 nM (t = 3.070 4*), MDA-MB-435S and MDA-MB-435S with 200 nM sjGST (t = 2.631 1*) was remained (Figure 2). Similar experiments were carried out with A549, and no significant different migration was observed between A549 and A549 with sjGST.
Figure 2. Quantification of absorbance of migration assays (n=5) for motorgenesis of human breast cancer cells in the presence of sjGST.
Migrated cell numbers were accounted from the 6th (bottom line), 12th (second line to the bottom), 24th (second line to the top) and 36th (top line) hour of culture. X axis indicates different treatments (MDA alone in No. 1, MDA in the presence of 50 nM sjGST in No. 2, MDA in the presence of 100 nM sjGST in No. 3 and MDA in the presence of 200 nM sjGST in No. 4). The Y axis shows the migrated cell number. The difference among the treatments did not reach significant level (P>0.05) in the first 6 h migration, but reached extremely significant (P<0.005) after 24 h migration. The significance was remained for 36 and 48 h migration.
3.3. Recombinant sjGST binds to MDA-MB-435S
In order to study the mechanism which determines the enhanced proliferation and migration of MDA-MB-435S, sjGST was used to bind to the cancer cell. Anti-sjGST conjugated with fluorescing substance was added to bind to sjGST. Control experiment without sjGST was carried out. As expected, our results showed that anti-sjGST-fluorescin did not bind directly to MDA-MB-435S, but anti-sjGST-fluorescin bound well if sjGST was added first to the cells. Binding of sjGST to MDA-MB-435S could be observed directly under fluorescence microscope (Figure 3). Similar experiments were carried out in A549, unfortunately however, no strong enough fluorescence was observed.
Figure 3. Binding of sjGST to MDA-MB-435S.
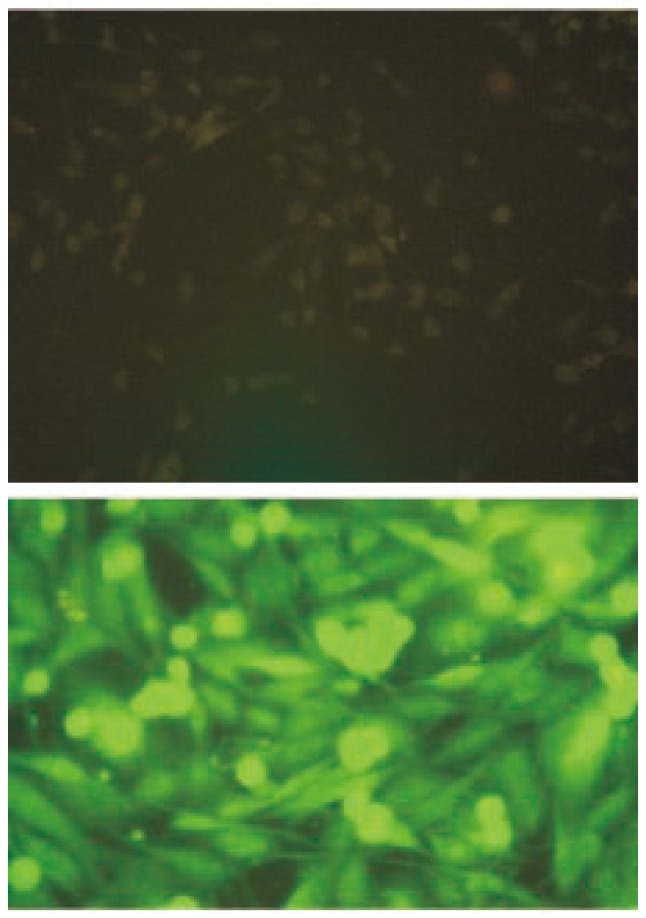
The upper figure (×50) shows that MDA-MB-435S cells were directly bound with anti-sjGST-fluorescin, washed, and then observed under fluorescence microscope. No fluorescence was emitted from the cells. The bottom figure (×200) shows that when MDA-MB-435S cells were incubated with sjGST followed by anti-sjGST-fluorecin, strong fluorescence could be observed.
3.4. Gelatinase activity is enhanced under the stimulation of sjGST
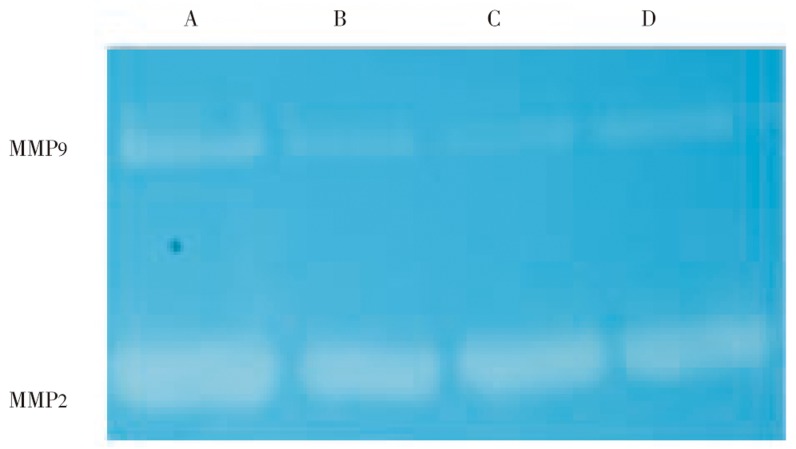
Culture supernatants (20 µL) were loaded onto polyacrylamide gel containing 2% of gelatine and separation was carried out with constant 100 mV until bromophenol blue reached to the bottom of the gel. The gel was washed and incubated to reveal the gelatinase activity (Figure 4). Different expression of MMP9 and MMP2 was clearly shown with zymographs. Again, when the same experiment was carried out in A549, the different expression of MMP2 and MMP9 was not clearly shown.
Figure 4. Zymographic study of gelatinase activity.
New culture supernatants were separated in polyacrylamide gel containing 2% gelatine. MMP9 (92 kDa) and MMP2 (72 kDa) are present in the culture supernatants and contain gelatinase activity.
A: Supernatant of MDA-MB-435S in the presence of 200 nM sjGST; B: Supernatant of MDA-MB-435S in the presence of 100 nM sjGST; C: Supernatant of MDA-MB-435S in the presence of 50 nM sjGST; D: Supernatant of MDA-MB-435S in the absence of sjGST. The bands were quantified with imagin-pro-plus 6.0.
3.5. Quantification of expression of gelatinase activity
Gelatinase activity (n=5) was quantified with computerized programme image-pro-plus 6.0 in which the zymographic bands were scanned, calculated and expressed as square million meters. Statistical data showed that the different expression of MMP9 and MMP2 in the presence or absence of sjGST in MDA-MB-435S cells was significant (P<0.05) or very significant (P<0.01).
4. Discussion
Internal cytoplasmic GST has been reported to occur at phase II in detoxicification pathway[8],[9], protecting cells from damage by free radicals. Detoxification occurred in cytoplasmic space can be divided into two phases. In phase I, toxic substances may be bound with P450 and turned into intermediates. In phase II, GST binds and turns the intermediates into non-toxic substances which are then released through drainage systems. But nothing is known if S. japanicum continuously releases sjGST into circulation of the host, such as sm22.6 antigen in patient sera[11]–[13]. Therefore, we design and carry out the present study to examine the mechanism of action of sjGST in cancer cells.
Firstly, we used a range of sjGST concentration (50-200 nM) in cellular assays for proliferation of human breast cancer cells (MDA-MB-435S) and found that 50-100 nM of sjGST significantly stimulated cell proliferation. The significant difference in proliferation in the presence or absence of sjGST could be detected from the 2nd day of incubation and remained in the 6th day experiment, suggesting that sjGST significantly shortened the time of cell cycle in MDA-MB-435S. Interestingly, when the same experiment was carried out in human lung cancer cell line A549, the stimulation was not significant, suggesting differentially “acceptance” of sjGST could be possible.
Secondly, migration experiment was carried out to study the difference among different treatments in the presence or absence of sjGST. Migrated cell number was counted. Statistical data showed that motorgenesis under different treatments was significantly different from 12 h migration, reached at maximum and remained in the 36 h migration. These results strongly suggest that sjGST not only shortens the cell cycle and promotes mitosis, but also stimulates motorgenesis of MDA-MB-435S.
The sjGST is a 26 kDa protein, and therefore it seems likely that it acts on the cell via certain substance, e.g. sjGST receptor on MDA-MB-435S cells. If sjGST binds well to the surface of MDA-MB-435S, it can be revealed in situ experiment by immune fluorescence. We designed another experiment that allowed sjGST to bind to the surface substance on MDA-MB-435S cells, then anti-sjGST-fluorescin was used to reveal the site of sjGST. Observation with fluorescence microscope showed it was indeed bound well to the surface of MDA-MB-435S. We believe there is likely a sjGST receptor on the surface of MDA-MB-435S cells.
In order to reveal the mechanism in which sjGST stimulates proliferation and migration of MDA-MB-435S, zymographic experiment was carried out to show gelatinase activity, especially MMP2 and MMP9. Studies have shown that gelatinase activities are responsible for migration and metastasis of cancer cells[19]–[22], especially secreted proteins MMP2 and MMP9. Our present study showed the expression and quantification of the two enzymes are significantly enhanced in the presence of sjGST, suggesting that molecular is related to proliferation and migration.
The results of our present work indicates that the infection of S. japanicum and the release of sjGST in circulation of human host cause proliferation and migration of certain cancer cells, such as MDA-MB-435S, by up-regulatory expression of MMP2 and MMP9. Recombinant sjGST may work via its receptor on the surface of MDA-MB-435S, and further studies should be conducted to uncover its signal pathways.
Acknowledgments
This work was supported by grants from National Science Council (NSC 98-2314-B-110-001-MY3), the Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan (93A10503) and China Medical University (CMU97-118) Taiwan, ROC. RR was supported by NSYSU international PhD training programme.
Footnotes
Foundation Project: Supported by grants from National Science Council (NSC 98-2314-B-110-001-MY3).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mannervik B, Danielson UH. Glutathione transferases: structure and catalytic activity. CRC Crit Rev Biochem. 1988;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- 2.Andujar-Sanchez M, Smith AW, Clemente-Jimenez JM, Rodriguez-Vico F, Las Heras-Vazquez FJ, Jara-Perez V, et al. et al. Crystallographic and thermodynamic analysis of the binding of S-octylglutathione to the Tyr 7 to Phe mutant of glutathione S-transferase from Schistosoma japonicum. Biochemistry. 2005;44:1174–1183. doi: 10.1021/bi0483110. [DOI] [PubMed] [Google Scholar]
- 3.McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, et al. et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 5.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function, and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 7.Nebert DW, Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Hum Genomics. 2004;1:460–464. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liska DJ. The detoxification enzyme systems. Altern Med Rev. 1998;3:187–198. [PubMed] [Google Scholar]
- 9.Lash LH. Role of renal metabolism in risk to toxic chemicals. Environ Health Perspect. 1994;102:75–79. doi: 10.1289/ehp.94102s1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kursula I, Heape AM, Kursula P. Crystal structure of non-fused glutathione S-transferase from Schistosoma japonicum in complex with glutathione. Protein Pept Lett. 2005;12:709–712. doi: 10.2174/0929866054696154. [DOI] [PubMed] [Google Scholar]
- 11.Lin YL, He S. Sm22.6 antigen is an inhibitor to human thrombin. Mole Biochem Parasitol. 2006;147:95–100. doi: 10.1016/j.molbiopara.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Webster M, Roberts M, Fulford AJ, Marguerite M, Gallisot MC, Diagne M, et al. et al. Human IgE responses to rSm22.6 are associated with infection intensity rather than age per se, in a recently established focus of Schistosomiasis mansoni. Trop Med Int Health. 1998;3:318–326. doi: 10.1046/j.1365-3156.1998.00234.x. [DOI] [PubMed] [Google Scholar]
- 13.Webster M, Correa-Oliveria R, Gazzinelli G, Viana IR, Fraga LA, Silveira AM, et al. et al. Factors affecting high and low human IgE responses to schistosome worm antigen in an area of Brazil endemic for Schistosomiasis mansoni and hookworm. Am J Trop Med Hyg. 1997;57:487–494. doi: 10.4269/ajtmh.1997.57.487. [DOI] [PubMed] [Google Scholar]
- 14.Kidd P. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;2:155–176. [Google Scholar]
- 15.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehyes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S. Role of glutathione S-transferases in protection against lipid peroxidation. Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- 19.Liotta AL, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 20.Crawford HC, Matrisian LM. Tumor and stromal expression of matrix metalloproteinases and their role in tumor progression. Invasion Metastasis. 1995;14:234–245. [PubMed] [Google Scholar]
- 21.Murphy G, Crabbe T. Gelatinases A and B. Methods Enzymol. 1995;248:470–484. doi: 10.1016/0076-6879(95)48030-7. [DOI] [PubMed] [Google Scholar]
- 22.Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 23.He S, Lin YL. In vitro stimulation of C1s proteolytic activities by C1s-presenting autoantibodies from patients with system lupus erythematosus. J Immunol. 1998;160:4641–4647. [PubMed] [Google Scholar]
- 24.Lin YL, Chen HL, Kuo HM, He S. NK3 and NK4 of HGF enhance filamin production via STAT pathway, but not NK1 and NK2 in human breast cancer cells. Acta Pharmacol Sin. 2008;29:728–735. doi: 10.1111/j.1745-7254.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- 25.He S, Liao TT, Chen YT, LIN YL. GST enhances proliferation-migration and protects against shikonin-induced cell death in MDA-MB-435S. Kao Hsiung J Med Sci. :2011. doi: 10.1016/j.kjms.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]