Abstract
Objective
To determine lead level primarily in Culex quinquefasciatus (Cx. quinquefasciatus), and Culex gelidus (Cx. gelidus) larvae inhabiting lead consuming factories, and to putatively estimate eco-toxicological impact of effluents from the firms.
Methods
Third instars larvae were sampled by standard dipping method and lead concentrations in the larvae and their respective surrounding factory aquatic environments were determined through standard atomic absorption spectrophotometry (AAS).
Results
Cx. quinquefasciatus was the most abundant species followed by Cx. gelidus. The levels of lead were higher in the Cx. quinquefasciatus (1.08-47.47 µg/g), than in the wastewaters surface (0.01-0.78 µg/mL) from the factories or closer areas around factories. Other species were not reaching the criteria for lead determination.
Conclusions
The Cx. quinquefasciatus larvae can bio-accumulate the metal and can potentially serve as a biomarker of lead contamination, to complemente conventional techniques.
Keywords: Culex mosquito, Larvae, Lead, Biomarker, Eco-toxicological impact
1. Introduction
Lead is an important toxic metal contaminating sediments, soil, air, and water[1]. In Thailand, heavy metal contamination of water resources mainly originates from industrial disposal of untreated heavy metal containing effluents[2],[3]. Some aquatic invertebrate populations are very sensitive to lead, which can adversely change their community structure[1] while those inhabiting lead polluted areas may be more tolerant to the metal than those inhabiting relatively lead-free environments[4]. Assessment of lead concentrations in water was complicated by large temporal-spatial variations, and delayed response occasioned by sequestration of heavy metals from sediments[5]. These challenges are ameliorated by analysis of the metal in the aquatic organisms (e.g. mosquito larvae) instead, which can bio-accumulate the metals from surrounding aquatic environment by several thousand fold proportions[1],[6]. In this respect, aquatic organisms lower in the food chain (such as a Culex mosquito larvae) are better tools for natural bio-monitoring of the metal since they are among the first in the food chain to be exposed to pollutants[7],[8].
Heavy metals have recently been reported in mosquitoes larvae[9],[10]. Bio-monitoring of aquatic heavy metal pollution through Culex mosquito larvae bioindicators is promising, and corresponds well with requirements for their acceptance for in situ monitoring[11]. This is further underscored by the larvae's high proliferation and tolerance to pollutants in aquatic habitats[9],[10],[12]; ubiquitous presence[10],[13]; sufficient developmental time that permit significant heavy metal uptake and close interaction with ecosystem. Additionally, the larvae are relatively easy to sample and analyze for determination of heavy metal (including lead) contents, and are routinely collected in surveillance/control initiatives of Culex borne disease[14]. The samples can in turn be additionally used to monitor eco-toxicological and environmental safety from heavy metal pollutants.
This study was initiated to determine the composition, density and physiological lead levels of Culex mosquito larvae species in industrial and domestic aquatic environment, and corresponding lead levels in respective aquatic habitats. The study was also aimed at assessing the potential application of the larvae as prospective bioindicators for biological monitoring of lead environmental pollution.
2. Materials and methods
2.1. Study sites
This study was undertaken in Samut Prakan, Pathum Thani, and Bangkok provinces in central Thailand. Samut Prakan province is located at the mouth of the Chao Phraya River, 25 km south of Bangkok. Pathum Thani province is located about 60 km north of Bangkok. In Samut Prakan province there are a total of 6 987 factories of which 346 (5%) utilize lead as a source of production[2],[15]. Of the 346 factories 19 (0.2%) were estimated to be high lead consumers (categorized as battery, solder, or lead smelters/factories). Eleven of these factories (A-K) were chosen as sampling locations. The industrial district in Samut Prakan also consists of residential areas. An industrial area in Pathum Thani (L, M, N) and an urban location in Bangkok (O), both without major industrial sources of lead, were selected as reference areas representing non-specific lead sources.
2.2. Larval and water collections
Mosquito larvae were collected in the 11 locations in Samut Prakan. Larvae were collected in larval habitats with water originating from either industrial or domestic wastewater. Industrial wastewater was water discharged directly from factories (4 locations: A, B, C, and D). Domestic wastewater originated from various domestic uses accumulating in sewage ponds, leaky or broken sewers and septic tanks, or in pools under or nearby houses (8 locations: A, E, F, G, H, I, J, and K). In location A, larvae were collected from both industrial and domestic wastewater habitats. There was no visible physical connection between industrial and domestic wastewater, except in location H, where wastewater released from the factory mixed with domestic wastewater. Mosquito larvae were also collected in Pathum Thani in ponds from factory effluents before (location L), after (location N), and in a sewage treatment pond (location M); and in Bangkok in a sewage pond inside the campus of Mahidol University (location O).
Three dips were taken to estimate larval densities using a 1 000 mL dipper. In habitats with less than ten larvae in three dips additional two dips were taken. Dipper contents were taken to the laboratory and transferred to white plastic trays. The number of larvae were counted and reported as the average number of larvae per dip per habitat. Twenty to thirty fourth-instar larvae were randomly selected for mounting and species identification, while the remaining larvae were bred through to the adult stage for validation of their species status. Mosquito larvae and adults were identified under a stereo-microscope using keys developed by Rattanarithikul and Panthusiri[16]. Additional 3-10 dips were taken from each habitat for determination of physiological levels of lead in the larvae. Two water samples were taken from each mosquito habitat (100 mL for each sample; 2 mL of nitric acid was added for preservation). Water samples were refrigerated (4 °C) until analyzed. Temperature and pH of water in each wastewater habitat were recorded at the time of collection. The water temperature ranged from 22-27 °C with a pH of 6-8.
2.3. Chemical analysis
A sample of 80 fourth-instar (about 100-120 mg) Culex quinquefasciatus (Cx. quinquefasciatus) larvae per habitat were pooled and analyzed for lead content. Two samples were analyzed where larval numbers collected exceeded 160 larvae. Since densities of Culex gelidus (Cx. gelidus) larvae were lower than 80, the available fourth-instar larvae were treated as one sample and analyzed. One hundred mL of water sample collected from the larval habitats were placed in a 120 mL bottle. Larvae were rinsed three times with distilled water and weighed. Both larvae and water were digested by nitric acid following the standard digestion method of International Atomic Energy Agency (IAEA)[17]. Atomic absorption spectrometry (GFAAS; Hitachi Z-8200, Zeeman background correction) was used to detect lead concentrations. Prior to analysis, the hollow cathode lamps for lead metal was aligned, and calibration tests were conducted for lead metal. Sensitivity checks were determined by testing a standard solution of metal at specific concentrations. The spectrophotometer was further calibrated by analyzing standard solutions for lead metal. Standard solutions of 0.0, 0.1, 0.2, 0.5 and 1.0 ppm were prepared from stock solutions of 1 000 ppm. Blank solutions were tested to recalibrate the spectrophotometer. If the spectrophotometer failed to give a reading of 0.0 ppm during recalibration, the samples that were analyzed just before recalibration were re-tested. Additionally, analytical blanks were prepared to determine the detection limit. The estimated lead detection limit for this spectrometer was 1 µg/L. Lead concentrations in mosquito larvae were recorded in µg/g wet weight and in water in µg/mL (ppm).
2.4. Data analysis
Mean larval densities were compared between lead (Samut Prakan) and non-lead (Pathum Thani and Bangkok) locations; and between industrial and domestic wastewater (in Samut Prakan) using the Kruskal-Wallis test. Mean lead concentrations were compared between non-lead industrial reference areas (Pathum Thani and Bangkok) and lead industrial areas (Samut Prakan), between larval species; and between larvae from industrial wastewater, domestic wastewater, and water from reference areas using non-parametric Kruskal-Wallis test. Statistical analysis were performed using SPSS statistical software (SPSS Corporation, Chicago, Illinois Statistical Package version 11.0). Biological concentration factor (BCF) was calculated and expressed as the proportion of lead uptake in fourth stage larvae and water habitat (BCF=lead concentration in larvae/lead concentration in water).
3. Results
3.1. Culex species diversity and density
Cx. quinquefasciatus, Cx. gelidus, Culex sitiens (Cx. sitiens), Culex vishnui (Cx. vishnui), and Culex pseudovishnui (Cx. pseudovishnui) were detected in the sampling sites among which Cx. quinquefasciatus was the most abundatnt (Table 1). Species diversity was higher in the non-lead industrial area of Pathum Thani (5 species) than in the lead industrial area of Samut Prakan (3 species). In Samut Prakan, three species were found in domestic wastewater (Cx. quinquefasciatus, Cx. gelidus, and Cx. sitiens) and two species in industrial wastewater (Cx. quinquefasciatus, and Cx. gelidus). Apart from Pathum Thani there were no locations where species co-existed. Cx. quinquefasciatus was the only species found in Bangkok. The density of Cx. quinquefasciatus was significantly higher in domestic wastewater (156 larvae/dip/location) than in industrial wastewater (57 larvae/dip/location) (χ2=6.49, df=1, P=0.01). The density of Cx. gelidus in domestic wastewater was 59 larvae/dip/location and in factory wastewater 25 larvae/dip/location (χ2=0.0, df=1, P=1.0).
Table 1. Characteristics of breeding places and mosquito larvae densities collected in various breeding habitats.
| Province | Locations | Type of factory | Larval habitat | Mean density of larvae/dip |
||||
| Cx. quinquefasciatus | Cx. gelidus | Cx. sitiens | Cx. vishnui | Cx. pseudovishnui | ||||
| Samut Prakan | A | Battery producer | Industrial | 23 | - | - | - | - |
| B | Battery producer | Industrial | 88 | - | - | - | - | |
| C | Lead smelter | Industrial | - | 64 | - | - | - | |
| D | Lead smelter | Industrial | - | 8 | - | - | - | |
| A | Battery producer | Domestic | 182 | - | - | - | - | |
| E | Battery producer | Domestic | - | 22 | - | - | - | |
| F | Battery producer | Domestic | 172 | - | - | - | - | |
| G | Battery producer | Domestic | - | 32 | - | - | - | |
| H | Battery producer | Domestic | - | - | 106 | - | - | |
| I | Lead smelter | Domestic | 152 | - | - | - | - | |
| J | Lead smelter | Domestic | - | 22 | - | - | - | |
| K | Lead smelter | Domestic | 170 | - | - | - | - | |
| Pathum Thani | L | Non-lead industry | Pond before M | 7 | 5 | 2 | 2 | 2 |
| M | Non-lead industry | Sewage treatment pond | - | - | - | - | - | |
| N | Non-lead industry | Pond after M | 13 | 5 | 3 | 1 | 0 | |
| Bangkok | O | Non-industry | Sewage pond | 118 | - | - | - | - |
3.2. Lead concentrations in mosquito larvae and water
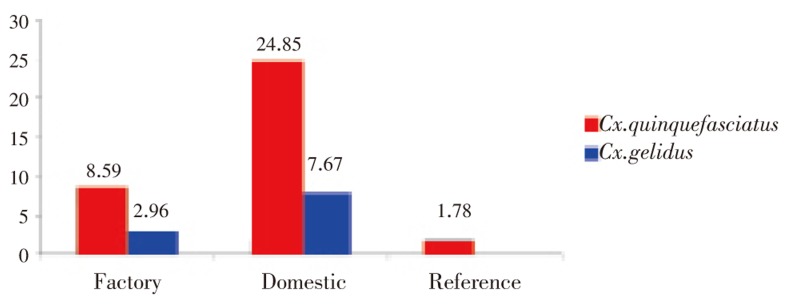
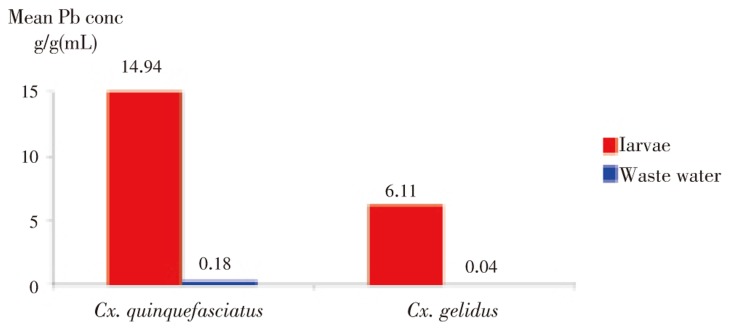
Lead was detected in Cx. quinquefasciatus and Cx. gelidus larvae in all locations. There was no significant difference in lead concentrations between Cx. quinquefasciatus and Cx. gelidus mosquito species (χ2=0.39, df=1, P=0.53). Mean lead concentration in Culex spp. larvae from lead industrial areas (Samut Prakan; larvae from domestic wastewater: 17.48 µg/g wet weight and larvae from industrial wastewater: 5.78 µg/g wet weight) were significantly higher (χ2=7.53, df=2, P=0.02) than in non-lead industrial reference areas (Pathum Thani and Bangkok; 1.78 µg/g wet weight) as shown in Figure 1. Mean lead concentration in Culex larvae was higher (11.76 µg/g, n=25) than in wastewater (0.1 µg/mL, n=25) (Figure 2). The BCF for these two species ranged between 12 and 2 262 times (Table 2). Lead contamination was very high in both industrial and domestic wastewater at location A and in domestic wastewater in location H, all in Samut Prakan province (Table 2).
Figure 1. Lead concentration among wastewater from factory, domestic area close to factory and reference area.
Figure 2. Lead concentrations in mosquito larvae and industrial and domestic wastewater in Samut Prakan province, Thailand.
Table 2. BCF, mean lead concentrations of mosquito larvae and wastewater in a lead industrial area of Samut Prakan province, Thailand.
| Locations | Cx. quinquefasciatus (µg/g wet weight) | Cx. gelidus (µg/g wet weight) | Cx. sitiens (µg/g wet weight) | BCF | Wastewater (µg/mL) |
| A | 9.5 | - | - | 12 | 0.780* |
| B | 7.67 | - | - | 219 | 0.035 |
| C | - | 1.83 | - | 122 | 0.015 |
| D | - | 4.09 | - | 273 | 0.015 |
| A | 31.71 | - | - | 117 | 0.270* |
| E | - | 16.59 | - | 133 | 0.125 |
| F | 21.16 | - | - | 705 | 0.030 |
| G | - | 3.02 | - | 302 | 0.010 |
| H | - | - | 3.23 | 7 | 0.440* |
| I | 45.24 | - | - | 2 262 | 0.020 |
| J | - | 3.95 | - | 198 | 0.020 |
| K | 1.28 | - | - | 64 | 0.020 |
| L | Low Larvae | - | - | - | 0.010 |
| M | No larvae | - | - | - | 0.030 |
| N | 1.73 | - | - | 288 | 0.006 |
| O | 1.21 | - | - | 121 | 0.010 |
* Exceeding national allowed limits from industrial effluents of 0.2 mg/L (Pollution Control Department, 2003).
4. Discussion
Lead was present in water and mosquito larvae in all study areas. Lead concentrations in wastewater from domestic and industrial sources in two of the study sites in Samut Prakan province (locations A and H) exceeded the national lead limits (0.2 mg/L) allowed in industrial effluents[15]. This confirms lead contamination problem in Thailand, particularly in residential areas close to lead-consuming factories. Uptake of lead in food crops grown in such areas could cause a potential human health problem. The widely consumed water spinach (Ipomoea aquatica) is commonly grown in water originating from domestic wastewater. Although, Gothberg et al[18] claimed that lead accumulation in Ipomoea aquatica in the Bangkok region did not cause a direct threat to human health, lead accumulation in food crops grown in risk areas, such as the ones studied here, should be closely monitored.
We found significantly higher concentrations of lead in Culex larvae collected around lead-consuming factories compared with reference areas, indicating the potential function of these species as rough indicators of lead pollution. Particularly high concentrations of lead were found in Cx. quinquefasciatus collected from domestic wastewater sources. Concentrations of almost 50 µg/g wet weight were similar to lead concentrations found in caddisfly larvae in a contaminated stream in Japan[19].
Cx. quinquefasciatus could be a good candidate for lead monitoring because it is ubiquitous, abundant and has been shown to have an increased lead uptake in relation to increased lead exposure in laboratory experiments[9]. Our data showed large variations in lead concentration in Cx. quinquefasciatus (1.0-50.0 µg/g wet weight), in wastewater (0.01-0.78 µg/mL), and the BCF (12 and 2 262). The very high BCFs in some locations indicate that lead uptake in Culex larvae could accumulate in the biotic food chain, since mosquito larvae often serve as food for both vertebrate and invertebrate aquatic predators. Although our study did not show any clear relationship between lead concentration in mosquito larvae and wastewater, our data still suggest that Cx. quinquefasciatus larvae could be a valuable indicator species for detecting lead contamination. A larger and more comprehensive study is necessary to clarify such relationships.
It is also found a significantly higher density of Cx. quinquefasciatus larvae in the more lead-polluted domestic water than in industrial water. The abundance of organisms often reflects the presence of pollutants in an ecosystem. A decrease in species diversity and population density is usually an indication of various stress factors, such as pollution[20]. However, it has been shown that Cx. quinquefasciatus larval and pupal abundances in organically polluted water increase as the amount of organic waste increase[21], but only up to a certain pollution level, when water becomes too polluted to sustain further mosquito development[22].
We also found high concentrations of lead in larvae in both domestic and industrial wastewater in the lead-industrial area in Samut Prakan. Interestingly, lead concentrations were in general higher in domestic wastewater than in industrial wastewater. We do not know the reason for this, but it could be that domestic wastewater is contaminated from both domestic and industrial sources, and from lead deposits in soils originating from lead-consuming factories. A relatively limited flow and distribution of domestic wastewater would also tend to concentrate lead pollution. Industrial wastewater sometimes flow to larger areas and consequently become diluted. More detailed studies are needed to understand the dynamics of heavy metal contamination in these areas.
Three species of Culex larvae were found around battery and lead factories in Samut Prakan province. Cx. quinquefasciatus is the most dominant species followed by Cx. gelidus. Cx. sitiens is only found in one location. Lead is detected in all three species, indicating that all species have the potential to function as bioindicator species. However, further studies are needed to clarify the potential of each species to accumulate heavy metals and their potential suitability as bioindicators.
An interesting result from our field studies is an apparently higher tolerance to lead contamination than found in laboratory studies. In experiments by Kitvatanachai et al[9], there was almost 100% mortality of Cx. quinquefasciatus larvae when exposed to lead concentrations of 0.25 µg/mL. However, in two of the locations (A and H), the lead contamination in wastewater exceeded 0.25 µg/mL. It might be possible that larvae in this highly lead polluted wastewater had a low lead uptake and a high excretion rate of toxic substances from their bodies for survival. It could also be possible that there are large variations in wastewater lead content within one breeding habitat and that larval development can continue in pockets with low lead concentrations.
In conclusion, mosquitoes of the Culex genus, particularly, Cx. quinquefasciatus, are potential bioindicators of lead for early warning of contamination of heavy metals in aquatic ecosystems. In our study we showed high uptake (high BCF) of lead in mosquito larvae breeding in lead contaminated sites close to lead-consuming factories in industrial areas of central Thailand. The higher lead concentrations in mosquito larvae from lead contaminated areas further support these findings. However, more research is needed to expand the knowledge of the roles of mosquito larvae for bioindication, and contamination of other heavy metals needs to be studied.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.WHO . Lead environment aspects: environmental health criteria. Geneva: WHO; 1989. [Google Scholar]
- 2.Kerdpratum S, Walansathira S. Lead and cadmium in Bangplee, Samut Prakan province's water resource. J Huachiew Chalermprakiet University. 2001;5(9):27–37. [Google Scholar]
- 3.Suwanna P, Sirikul K, Phacharakon K, Wansuk S, Nongnud T, Acacia A. Heavy metals in wild banana prawn (Fenneropenaeus merguiensis De Man, 1888) from Chantaburi and Trat provinces, Thailand.(Report) J Shellfish Res. 2007;26(4):1193–202. [Google Scholar]
- 4.Blanck H, Wangberg SA, Molander S. Pollution-induced community tolerance-a new ecotoxicological tool. In: Cairns J, Pratt JR, editors. Functional testing of aquatic biota for estimating hazards of chemicals. Philadephia: American Society for Testing and Materials; 1988. pp. 219–230. [Google Scholar]
- 5.Miesbauer H, Kock G, Füreder L. Determination of trace elements in macrozoobenthos samples by total-reflection X-ray fluorescence analysis. Spectrochim Acta Part B At Spectrosc. 2001;56:2203–7. [Google Scholar]
- 6.Wilson JG, Elkaim B. The toxicity of freshwater: estuarine bioindicators. In: Jeffrey DW, Madden B, editors. Bioindicators and environmental management. London: Academic Press Ltd; 1991. pp. 311–322. [Google Scholar]
- 7.Lu PY, Metcalf RL, Vogel FR, Hassett J. Model ecosystem studiesof lead and cadmium and of urban sewage sludge containing these elements. J Environ Qual. 1975;4(4):505–509. [Google Scholar]
- 8.Anderson RL, Walbridge CT, Fiandt JT. Survival and growth of Tanytarsus dissimilis (Chironomidae) exposed to copper, cadmium, zinc and lead. Arch Environ Contam Toxicol. 1980;9:329–335. doi: 10.1007/BF01057412. [DOI] [PubMed] [Google Scholar]
- 9.Kitvatanachai S, Apiwathnasorn C, Leemingsawat S, Wongwit W, Tornee S. Determination of lead toxicity to Culex quinquefasciatus mosquitoes in the laboratory. Southeast Asian J Trop Med Public Health. 2005;36(4):862–874. [PubMed] [Google Scholar]
- 10.Mireji P, OKeating J, Hassanali A, Mbogo CM, Nyambaka H, Kahindi S, et al. et al. Heavy metals in mosquito larval habitats in urban Kisumu and Malindi, Kenya, and their impact. Ecotoxicol Environ Saf. 2008;70(1):147–153. doi: 10.1016/j.ecoenv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkin SP. In situ biological monitoring of pollution in terrestrial and aquatic ecosystems. In: Calow P, editor. Handbook of ecotoxicology: volume 1. Oxford: Blackwell Scientific; 1993. pp. 397–427. [Google Scholar]
- 12.Whelan P, Hayes G, Carter J, Wilson A, Haigh B. Detection of the exotic mosquito Culex gelidus in the Northern territory. Commun Dis Intell. 2000;24:74–75. doi: 10.33321/cdi.2000.24.11. [DOI] [PubMed] [Google Scholar]
- 13.Mulla MS, Thavara U, Tawatsin A, Kong-Ngamsuk W, Chompoosri J. Mosquito burden and impact on the poor: measures and costs for personal protection in some communities in Thailand. J Am Mosq Control Assoc. 2001;17:153–159. [PubMed] [Google Scholar]
- 14.White GB. Geographical distribution of arthropod-borne diseases and their principal vectors. Geneva: World Health Organization, WHO Vector Biology Division; 1989. [Google Scholar]
- 15.Pollution Control Department, Ministry of Natural Resources and Environment . Water quality standards. Bankok: Pollution Control Department; 2003. [Google Scholar]
- 16.Rattanarithikul R, Panthusiri P. Illustrated keys to the medically important mosquitoes of Thailand. Southeast Asian J Trop Med Public Health. 1994;25(suppl 1):31–66. [PubMed] [Google Scholar]
- 17.IAEA . Laboratory procedure book: training work shop on the analysis of trace metals in biological and sediment samples. Monaco: International Atomic Energy Agency; 2009. [Google Scholar]
- 18.Gothberg A, Greger M, Bengtsson BE. Accumulation of heavy metals in water spinach (Ipomoea aquatica) cultivated in the Bangkok region, Thailand. Environ Toxicol Chem. 2002;21:1934–1939. [PubMed] [Google Scholar]
- 19.Boudou A, Ribeyre F. Fundamental concepts in aquatic ecotoxicology. In: Boudou A, Fibeyre F, editors. Aquatic ecoloticology: fundamental concepts and methodologies. Florida, Boca Raton: CRC Press; 1989. p. 3575. [Google Scholar]
- 20.Tochimoto H, Maki T, Afzal M, Tanabe S. Accumulation of trace metals in aquatic insect Stenopsyche marmorata Navas transferred in streams. Ecotoxicol Environ Saf. 2003;56:256–264. doi: 10.1016/s0147-6513(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 21.Sinha VP. Further observations on the physio-chemical factors of the breeding places of the Cx. quinquefasciatus Say=fatigans Wied. Mosq News. 1976;6:358–360. [Google Scholar]
- 22.Rutz DA, Axtell RC, Edwards TD. Effect of organic pollution levels on aquatic insect abundance in field pilot-scale anaerobic animal waste lagoons. Mosq News. 1980;40(3):403–409. [Google Scholar]