Abstract
Objective
To study the morphology, biochemistry and bioactivity of the epidermal glands of the glandular morphotype of Christella parasitica (C. parasitica) (L.) H. Lev.
Methods
Morphological studies on epidermal glands were carried out by using light microscope and scanning electron microscope. To prepare the extract, the shade-dried fronds of glandular morphotype were soaked in acetone. For antibacterial studies paper disc method was followed by using various pathogenic bacteria.
Results
Detailed micromorphological, phytochemical and bioactivity studies on a medicinal fern C. parasitica (L.) H. Lev. showed its intraspecific variation in antibacterial activity. The presence or absence of the epidermal glands was the key factor for antibacterial activity in the morphovariants of this species. The epidermal glands were orange-coloured, stalked and elongated ones of about 84.2 µm × 45 µm, and distributed on the undersurface of costa, costules and veins in croziers, young and mature leaves. Frequency of glands varied from 15/cm on costa in mature leaves to 140/cm on costules in croziers. The acetone extract of the glands showed antibacterial activities and also toxic effect against mosquito larvae and tadpoles of frog. Preliminary phytochemical analysis and HPLC studies of the gland extract showed the presence of various kinds of terpenoids, alkaloids, tannins, saponins and flavonoids in it.
Conclusions
The present study shows that epidermal glands of the glandular morphotype of C. parasitica (L.) H. Lev. have several bioactive compounds and such rare morphovariant should be conserved in nature. The next step is to isolate the pure compounds and to screen the bioactivity of individual compounds of the epidermal glands.
Keywords: Epidermal glands, Christella parasitica, Bioactivity, Phytochemical activity, Anti-bacterial activity, Morphology, Biochemistry, Glandular morphotype, Light microscope, Shade-dried frond, Bioactive compound, Morphovariant, Preliminary phytochemical analysis, Gland extract, Flavonoid
1. Introduction
Many pharmaceutical innovations are developed from a starting point of knowledge derived from the biological activities of natural organisms. In the case of the random screening of natural products, Aylward et al[1] argue that the sheer scale of the resource, in the order of 10 to 100 million species, and the continuing evolution of new screens and new disease targets imply that biodiversity will never be fully explored for its pharmaceutical potential. Although Farnsworth et al[2] agree that it is practically impossible to actually determine when a particular species has been fully investigated for its pharmaceutical properties, they made a ‘guesstimate’ that just 5 000 plant species have been exhaustively explored. Such explorations indicate that the full range of plant secondary compounds in all their complexity is only found in the angiosperms, the flowering plants and the most recently evolved major group of plants. They now, exceeding the combined diversity of algae, bryophytes, pteridophytes and gymnosperms, dominate most of the major terrestrial zones[3]. Like other groups of plants, pteridophytes also show medicinal utility and many of them like male fern or shield fern have been used medicinally from the time of Theophrastus and Dioscorides. But only limited number of pteridophytes has been subjected to experimental studies, such as screening for bioactivity and identification of bioactive compounds.
Most secondary metabolites, which are the raw materials of many commercial drugs, are restricted to single major taxa on the universal phylogenetic tree and probably only originated once, but occasionally the same compound is found in phylogenetically unrelated groups and is possibly synthesized via a different biosynthetic route. In today's higher plants, there is a clear trend that their chemical and structural complexity increases with morphological complexity. In a particular species although the bioactive compound is present in all the parts of the plant, it may be present at high concentration in a particular part like stem-bark, leaf, root, fruit and seeds. In non flowering vascular plants, searching for bioactive chemical compounds of pteridophytes is made either in aerial part, the fronds or underground part, the rhizomes. But plants in general, pteridophytes in particular possess several other microscopical structures, which may be the site of production and storage of several bioactive compounds. It has been reported that several acyl-phloroglucinols are produced and stored in internal/external glands in the rhizomes and leaves of several Dryopteris species[4]. The presence of glandular hairs or trichomes on leaf surface is of taxonomical, ecological and medicinal importance. Morphoanatomical and developmental studies on leaf secretory structures in Passiflora amethystine confirm that the leaf secretory structures are indeed extrafloral nectarines, and these findings constitute important information for studies on the taxonomy and ecology of this species[5]. Plant structures, such as trichomes, or compounds like toxins, deterrents or digestibility reducers, that directly reduce the preference or performance of an herbivore or pathogen are called direct defenses[6]. The plant Schistostephium heptalobium has long been used and the research has been conducted on the antimicrobial activity which produced positive results by Mayekiso et al[7]. The morphology of the leaf surface of this plant is characterized by oval-club shape glandular and hairyfibrous non-glandular trichomes. The scanning electron microscopy-energy dispersive spectroscopy analysis of the cuticular sac of the glandular trichome revealed the following components including Ca, K, Mg, Na and Si. However, its content was predominately composed of Ca, Mg and Na[8].
Some ferns belonging to the genera like Adiantum, Blechnum, Christella, Dicranopteris, etc., from the Western Ghats have been screened for their biological activities, particularly for antibacterial activity. Most of the ferns show positive results with the difference in the degree of bioactivity between species to species. But interestingly, intraspecific difference in biological activity was observed in the case of the fern Christella parasitica (C. parasitica) (L.) H. Lev., which is a highly polymorphic species[9]–[11]. Some gatherings of this species show more biological activity in contrast with some other gatherings. In order to understand such differences in biological activity, studies on morphological and phytochemical bioactivities of different gatherings were carried out based on the information from the literatures. It is known that the fern C. parasitica (L.) H. Lev. (Thelypteris parasitica (L.) Tard.) is a polymorphic species with the variation in size of the entire plant, pubescens, and depth of lobing of pinnae, etc. In the mean time there are two distinct morphotypes. They are glandular and eglandular morphotypes. The leaves of the former bear orange-coloured and elongated glands on the lower side of the costa, costules and veins, but such glands are absent in the latter. It is also important to note that the glandular morphotype is very rare in occurrence compared with the common eglandular morphotype[11]. Morphological studies on the gatherings, with or without biological activities, confirmed that the glandular morphotype has remarkable bioactivity[12]. Based on this, we studied the morphology, biochemistry and bioactivity of the epidermal glands of the glandular morphotype in details.
2. Materials and methods
C. parasitica (L.) H. Lev. was collected from Kakachi, Tirunelveli Hills (1 000 m) on the Western Ghats (Figure 1A). Voucher specimen (XCH 23365) was deposited in St. Xavier's College Herbarium, (XCH) Palayamkottai, India. Morphological studies on epidermal glands were carried out by using light microscope and scanning electron microscope. For scanning electron microscopic study, materials were coated with gold to a thickness of 100 A. Samples were observed in JEOL-JSM 5600 L. V. scanning electron microscope. For biochemical and bioactivity studies acetone extract of the glands was used. To prepare the extract, the shade-dried fronds of glandular morphotype were soaked in acetone. After soaking large amounts of materials, orange-coloured solution was obtained (Figure 1E) and was further concentrated by distillation. The dried acetone extract was used for further studies. For antibacterial studies paper disc method was followed by using various pathogenic bacteria. Toxicity study was also conducted by using mosquito larvae and frog tadpole.
Figure 1. Detailed micromorphological, phytochemical and bioactivity studies on C. parasitica.
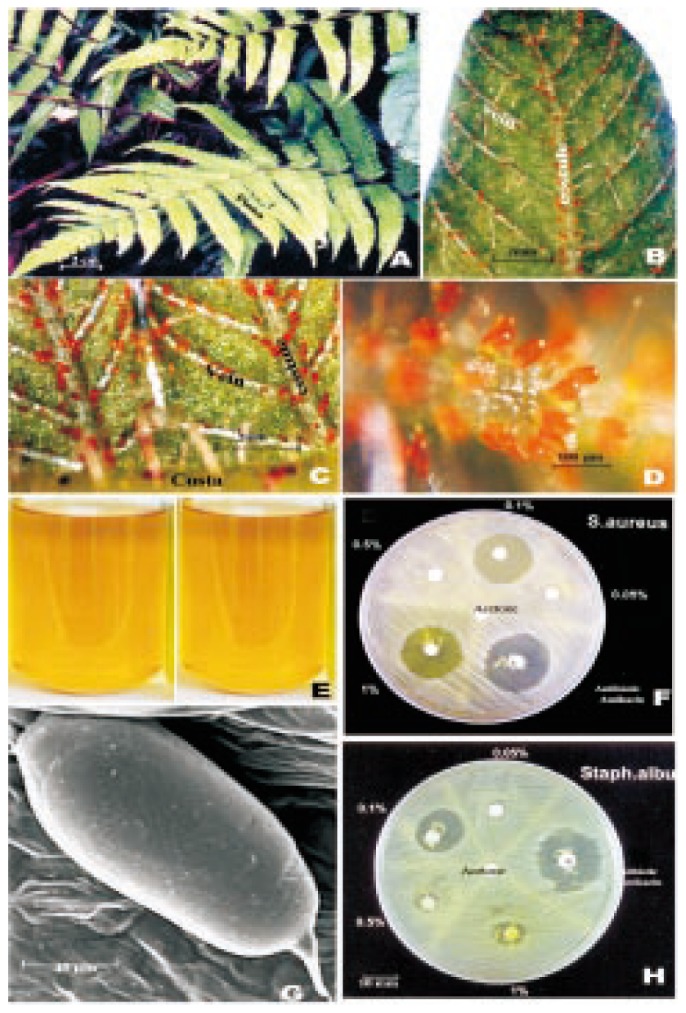
A: Leaves of C. parasitica (L.) H. Lev.; B: A pinna lobc showing orange coloured glands on the veins; C: Portion of a pinna lobe with orange coloured glands on cosutules and veins; D: Portion of a vein with cluster of orange coloured glands; E: Glandular extract in acetone; G: Scanning elcctron microscopic view of a gland; F&H: Antibacterial activity of glandular extract against S. aureus (F) and S. albus (H).
3. Results
The light microscopic studies showed that the glands were distributed on the lower surface of the costa, costule and veins and not on the intervenal areas (Figure 1B, 1C). They occurred more densely in croziers (coiled young leaves) and uncoiled young leaves when compared with the mature leaves. The frequency of the glands in various parts and various stages was listed as follows: 15, 90 and 60/cm on costa, costule and vein, respectively in young and mature leaves; 31, 140 and 50/cm on costa, costule and vein, respectively in croziers. They were bright orange in colour (Figure 1D) and elongated with about 84.2 µm × 45 µm. Under scanning electron microscope it showed clearly that the glands were distinctly stalked with smooth surface and apiculate apex (Figure 1G). Preliminary phytochemical screening and HPLC studies (Figure 2, 3, 4) on the acetone extract of the glands showed the presence of variety of alkaloids, flavonoids, terpenoids, steroids, saponins, tannins and sugars. The acetone extract of the glands showed antibacterial activity against various bacteria like Staphylococcus albus (S. albus), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa. The concentration with maximum bactericidal activity varied from bacterium to bacterium. Thus maximum bactericidal activity, which is equivalent to 81% activity of the commercial antibiotic amikacin, was observed at 0.1% against S. albus, while both 1% and 0.1% concentrations showed maximum bactericidal activity, which is equivalent to 83% of the commercial antibiotic amikacin against S. aureus (Figure 1F, 1H). Toxicity studies, by using mosquito larvae and tadpole of frog, showed the lethal effects of the glandular extract at different concentrations. It was observed that 0.000 5% or above concentrations of glandular extract was lethal to the tadpoles. 50% mortality of mosquito larvae was seen at 0.005% and 100% mortality was found within three hours at higher concentrations(0.01%, 0.015%, and 0.02%).
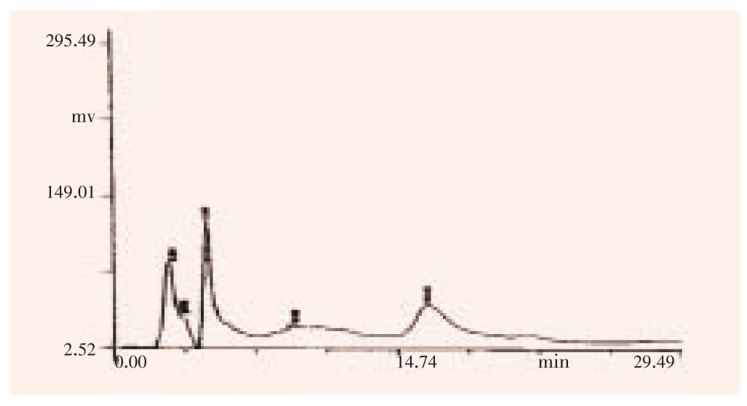
Figure 2. HPLC analysis on epidermal glands at 243 nm.
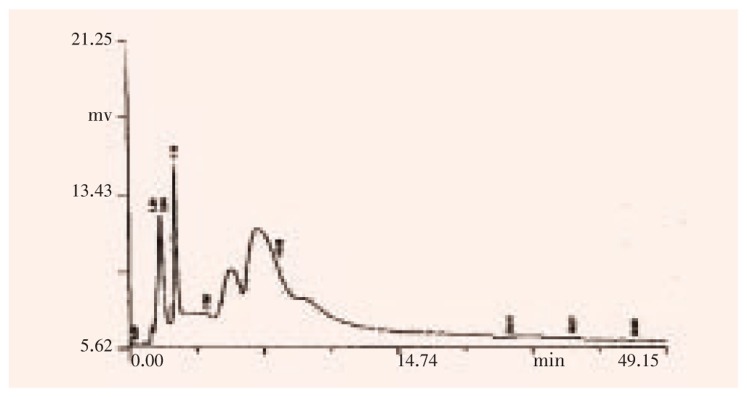
Figure 3. HPLC analysis on epidermal glands at 280 nm.
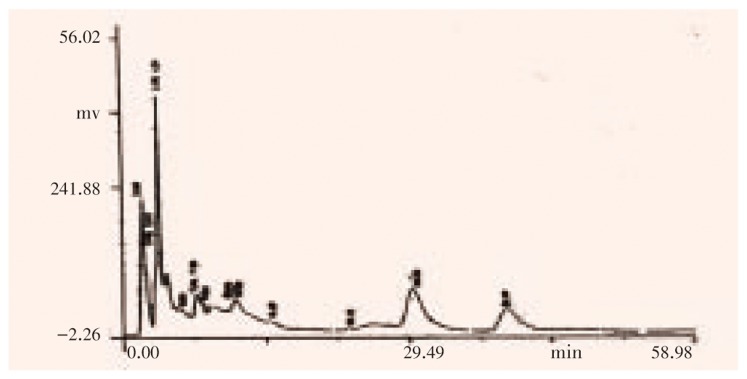
Figure 4. HPLC analysis on epidermal glands at 215 nm.
4. Discussion
C. parasitica is a large species complex with many morphotypes and cytotypes[13]–[15],[17]. Both glandular and eglandular morphotypes, diploid, triploid and tetraploid cytotypes, have been reported from South India. The diploid plants are eglandular while the tetraploid plants are either glandular or eglandular. Presence of glands in C. parasitica is the dominant character[16]. In Christella cylindrothrix (Rosenst.) Holttum the underside of the lamina is more or less downy-pubescent, and invariably provided with capitate, long-stalked and bright orange coloured glands on the veins as well as the general surface[18]. Cylindrical, unicellular, orange coloured glands with abbreviated stalks are present on the lower surface of the frond in Christella arida (D. Don) Holttum. Indian specimens of Christella papilio (Hope) Holttum are devoid of glandular hairs, which are abundant in specimens from Thailand and Malaya[16].
Preliminary phytochemical screening showed the difference in the presence or absence of triterpenoid in glandular and eglandular morphotypes of C. parasitica (L.) Lev.[17]. Various types of flavonoids such as astragarin, kaempferol glucoside and kaempferol rutinoside have been reported in C. parasitica[18]. But it is not known whether the material is glandular or eglandular morphotype. It is reported that several acyl-phloroglucinols are produced and stored in internal / external glands in the rhizomes and leaves of several Dryopteris species[4].
In order to confirm the parts with bioactive compounds, antibacterial activity was studied by using the leaves without glands, which had been removed by soaking the leaves in acetone. Minimum bactericidal activity was observed in the extract of empty leaves, while medium and maximum degrees of activities were observed in the extract of leaves with glands and the extracts of pure glands, respectively.
The present study confirmed the trend that, in today's higher plants, chemical and structural complexity increases with morphological complexity. Chemical and chemotaxonomical studies on Dicranopteris species from the Western Ghats also showed the intraspecific variation in the presence / absence of secondary metabolites[19]. The ferns and isolated flavonoids are presented as follows: Dicranopteris linearis (D. linearis) (Burm. f.) Underwood var. tenuis Manickam & Irud: quercitrin, iso-quercitrin; D. linearis var. brevis Manickam & Irud: afzelin, quercitrin; D. linearis var. sebastiana Panigr & Dixit: astragarin, isoquercitrin, rutin, kaempferol. Thus morphological, chemical and physiological diversities are interrelated and all are ultimately controlled by genetical diversity. The tropical flora with high degree of biodiversity should be exhaustively explored to find out the useful morphotype, chemotype and genotype.
In conclusion, the present study confirms that the epidermal glands of the glandular morphotype of C. parasitica (L.) H. Lev. have several bioactive compounds and such rare morphovariant should be conserved in nature. The next step is to isolate the pure compounds and to screen the bioactivity of individual compound of the epidermal glands.
Acknowledgments
Mr. K Paul Raj is grateful to the University Grant Commission, SERO, Hyderabad, India, for the award of Teacher Fellowship under tenth plan. We thank Dr. Peter Kosh, Regional Research Laboratory, Council of Scientific and Industrial Research, Thiruvananthapuram, Kerala, India, for his help in scanning electron microscopic studies. We also give our gratitude to Dr. S Daniel Wesley, SPIC Research Foundation, Tuticorin, India, for his help in HPLC studies.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Aylward BA, Barbier EB. What is biodiversity worth to a developing country? Capturing the pharmaceutical value of species information. London: London Environmental Economics Centre; 1992. [Google Scholar]
- 2.Farnsworth NR, Morris RW. Higher plants-the sleeping giant of drug development. Am J Pharm Sci Support Public Health. 1976;148:46–52. [PubMed] [Google Scholar]
- 3.Friis EM, Chaloner WG, Crane PR. The origin of angiosperms and their biological consequences. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 4.Wollenweber E, Stevens JF, Ivanic M, Deinzer ML. Acylphloroglucinols and flavonoid aglycones produced by external glands on the leaves of two Dryopteris ferns and Currania robertiana. Phytochemistry. 1998;48(6):931–939. [Google Scholar]
- 5.Rocha DI, da Silva LC, Vaente VMM, Francino DMRT, Meira RMSA. Morphoanatomy and development of leaf secretory structures in Passiflora amethystina Mikan (Passifloraceae) Aust J Bot. 2009;57(7):619–626. [Google Scholar]
- 6.Bezemer TM, Dam NMV. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol. 2005;20(11):617–624. doi: 10.1016/j.tree.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Mayekiso B, Madlokazi N, Magwa ML. Antibacterial activity of Schistostephium heptalobium leaf extracts. Med Aromat Plant Sci Biotechnol. 2008;2(1):40–42. [Google Scholar]
- 8.Buyisile M. Morphological and chemical composition of the essential oil of the leaf of Schistostephium heptalobium. Afr J Biotechnol. 2009;81509(8):-1519. [Google Scholar]
- 9.Manickam VS, Irudayaraj V. Cytology of ferns of the Western Ghats, South India. New Delhi: Today & Tomorrows Printers and Publishers; 1988. [Google Scholar]
- 10.Manickam VS, Irudayaraj V. Thelypteridaceae of the Western Ghats, South India. Indian Fern J. 1990;7:100–117. [Google Scholar]
- 11.Manickam VS, Irudayaraj V. Pteridophyte flora of the Western Ghats, South India. New Delhi: BI Publications; 1992. [Google Scholar]
- 12.Manickam VS, Benniamin A, Irudayaraj V. Antibacterial activity of leaf glands of Christella parasitica (L.) Lev. Indian Fern J. 2005;22:87–88. [Google Scholar]
- 13.Irudayaraj V, Dominic RS, Manickam VS. Studies on intraspecific variation in south Indian ferns: rediscovery of the rare diploid cytotype of Christella parasitica (Thelypteridaceae: Pteridophyta) 1995;15(2):41–50. [Google Scholar]
- 14.Panigrahi G, Manton I. Cytological and taxonomical observations on some members of the Cyclosorus parasiticus complex. Bot J Linn Soc. 1958;55(363):729–743. [Google Scholar]
- 15.Loyal DS. Cytomorphological studies in the eastern Himalayan Thelypteridaceae. In: Bhardwaj TN, Gena CB, editors. Aspects of plant sciences: perspectives in pteridology-present and future. Jaipur: University of Jaipur; 1991. pp. 171–248. [Google Scholar]
- 16.Holttum RE. Studies in the family Thelypteridaceae XI. The genus Christella leville, sect. Christella. Kew Bull. 1976;31(2):293–339. [Google Scholar]
- 17.Irudayaraj V. Studies on intraspecific variation in South Indian ferns VI: preliminary phytochemical analysis of epidermal glands in Christella parasitica (L.) Lev. Flora Fauna. 1996;2(2):155–157. [Google Scholar]
- 18.John De Britto A, Manickam VS, Tanaka N, Gopalakrishnan G. Flavonoid glycosides of Christella parasitica (L.) Lev. of Western Ghats of South India. J Indian Chem Soc. 1996;73:285–286. [Google Scholar]
- 19.Patric RD, Manickam VS, John De Britto A, Gopalakrishnan G, Ushioda T, Satoh M, et al. et al. Chemical and chemotaxonomical studies on Dicranopteris species. Chem Pharm Bull. 1995;43(10):1800–1803. doi: 10.1248/cpb.43.1800. [DOI] [PubMed] [Google Scholar]