Abstract
Objective
To investigate the ability of the methanolic extract of pineapple peel to modulate alcohol-induced lipid peroxidation, changes in catalase activities and hepatic biochemical marker levels in blood plasma.
Methods
Oxidative stress was induced by oral administration of ethanol (20% w/v) at a dosage of 5 mL/kg bw in rats. After 28 days of treatment, the rats were fasted overnight and sacrificed by cervical dislocation. Blood was collected with a 2 mL syringe by cardiac puncture and was centrifuged at 3 000 rpm for 10 min. The plasma was analyzed to evaluate malondialdehyde (MDA), catalase activity, aspartate aminotransferase (AST), alkaline phosphatase (ALP) and alanine aminotransferase (ALT) concentrations.
Results
Administration of alcohol caused a drastic increase (87.74%) in MDA level compared with the control. Pineapple peel extract significantly reduced the MDA level by 60.16% at 2.5 mL/kg bw. Rats fed alcohol only had the highest catalase activity, treatment with pineapple peel extract at 2.5 mL/kg bw however, reduced the activity. Increased AST, ALP and ALT activities were observed in rats fed alcohol only respectively, treatment with pineapple peel extract drastically reduced their activities.
Conclusions
The positive modulation of lipid peroxidation, catalase activities as well as hepatic biomarker levels of blood plasma by the methanolic extract of pineapple peels under alcohol-induced oxidative stress is an indication of its protective ability in the management of alcohol-induced toxicity.
Keywords: Blood plasma, Catalase, Lipid peroxide, Aspartate aminotransferase, Alkaline phosphatase, Alanine aminotransferase, Hepatic biomarker, Pineapple peel extract, Oxidative stress, Lipid peroxidation
1. Introduction
Oxidative stress has long been associated with chronic alcohol consumption leading to a spectrum of metabolic disorders. This is brought about by increased production of reactive oxygen species (ROS) and by impairing the protective mechanism of antioxidants[1], leading to alteration of the redox status of cells which has profound effects on cellular function and viability leading to cell death and tissue damage[2]. These free radicals cause destructive and irreversible damage to cellular components especially lipids, protein and DNA[3]. The etiology of alcohol-induced diseases has been linked to enhanced oxidative stress and decreased antioxidant status.
Various plants exhibit antioxidant activity, which protects against the damage caused by ROS[4]. The majority of the antioxidant activity has been attributed to the flavones, isoflavones, flavonoids, anthocyanin, coumarin lignans, catechins and isocatechins[5].
Pineapple (Ananas cosmosus) is a popular fruit which grows in the tropics and sub-tropics and belongs to the Bromeliaceae family. They are native to Central and Southern America and grows on the ground unlike other bromeliads that are epiphytes. The nutritional and medicinal properties of pineapples have been widely studied. They are rich in the antioxidants namely flavonoids, vitamin A and C[6]. The peels are well known ingredients in ethnomedicine. Traditionally they are used in the treatment and management of various ailments such as malaria and typhoid. Correia et al established a relationship between antioxidant activity, β-glucosidase and total phenolic content in pineapple peel/soy flour extracts[6].
This paper aims at investigating the ability of the methanolic extract of pineapple peel to modulate alcohol-induced lipid peroxidation, changes in catalase activities and hepatic biochemical levels in blood plasma of rats.
2. Materials and methods
2.2. Plant materials
Pineapple peels were collected from a local fruit seller at Oshodi, Lagos, Nigeria. They were rinsed in distilled water to remove dirt. They were air dried and grounded to fine texture with laboratory mill and were extracted with methanol for 8-10 h, distilled and concentrated using steam bath and then stored for subsequent use.
2.2. Animals
Thirty male albino rats aged 90-120 days old were used for the present investigation. They were reared at the Animal House of the Biochemistry Department of Bells University of Technology, Ota, Nigeria.
Rats were acclimatized for two weeks on normal diet of pelletized mouse chow, with water given ad libitum at room temperature under a 12-h light and dark cycle before the commencement of the experiment. They were divided into six groups as listed as follow, each consisting of five animals: Group 1: Distilled water only (5.0 mL/kg bw) (Control); Group 2: Ethanol only (Negative control); Group 3: Ethanol + Pineapple peel extract (2.5 mL/kg bw); Group 4: Ethanol + Pineapple peel extract (5.0 mL/kg bw); Group 5: Pineapple peel extract only (5.0 mL/kg bw); Group 6: Ethanol + Distilled water (5.0 mL/kg bw).
Oxidative stress was induced by oral administration of ethanol (20% w/v) at a dose of 5.0 mL/kg bw. The treatment lasted for 28 days. The dose of ethanol, pineapple peel extract and the period of treatment were selected on the basis of previous studies by Faremi et al[8].
At the end of the treatment, the rats were fasted overnight and sacrificed by cervical dislocation.
The animals used in the present study were maintained in accordance with the rules guiding the use of animals in experimental studies by Bells University of Technology, Ota, Nigeria.
2.3. Plasma preparation
Blood was collected with a 2 mL syringe and needle by cardiac puncture and was centrifuged at 3 000 rpm for 10 min and the plasma was analyzed to evaluate some biochemical parameters.
2.4. Parameter assays
Enzyme activities were measured by enzymatic colorimetric method using Randox kits which covers alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) according to the method of Amanvermez et al[9]. Catalase activity was evaluated according to the method of Chance and Maehly[10].
The extent of lipid peroxidation fractions was determined by measuring the level of malondialdehyde (MDA) formed according to the method of Chowdhury et al[11].
2.5. Statistical analysis
Statistical significance of the difference between experimental groups was calculated using the student's t-test. A P<0.05 was considered significant. One-way ANOVA was also performed.
3. Results
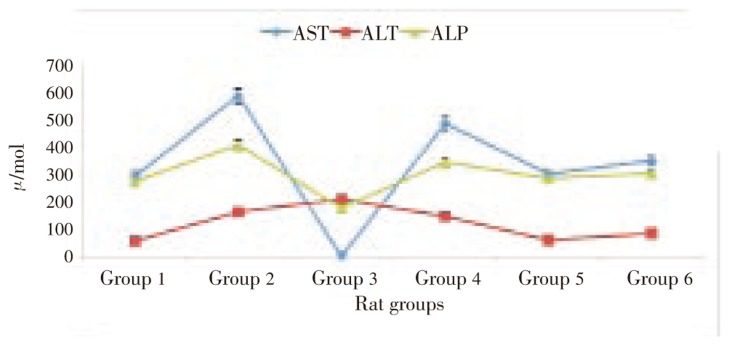
Results of the effect of pineapple peel extract on plasma hepatic biomarker levels were presented in Figure 1. Feeding of alcohol caused an elevated level in all the studied hepatic biomarkers. Treatment with pineapple peel extract at 2.5 mL/kg bw was observed to cause significant decrease in AST (99.8%) and ALP (56.53%) levels. ALT level was observed to increase on treatment with pineapple peel extract at 2.5 mL/kg bw. However, treatment with a double dose (5.0 mL/kg bw) caused a 10.06% decrease.
Figure 1. Effect of pineapple peel extract on plasma hepatic biomarkers of alcohol-induced oxidative stressed rats.
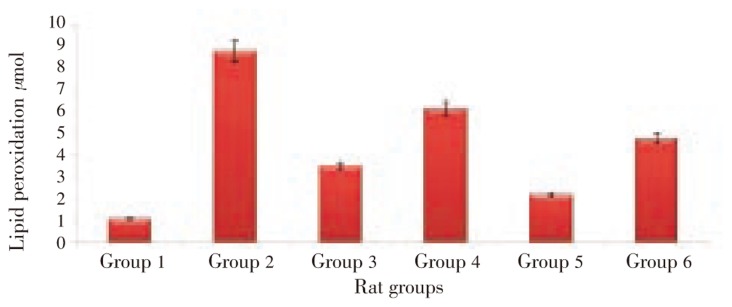
Figure 2 showed the effect of pineapple peel extract on lipid peroxidation in plasma of alcohol-fed rats. Feeding on alcohol was observed to cause an elevation of MDA level. Treatment with pineapple peel extract at 2.5 mL/kg bw caused a 60.16% reduction in the MDA level. This was observed to be more effective than treatment with double dose (5.0 mL/kg bw). Treatment with distilled water was observed to reduce the MDA level by 45.63%.
Figure 2. Effect of pineapple peel extract on lipid peroxidation in blood plasma of alcohol-induced oxidative stressed rats.
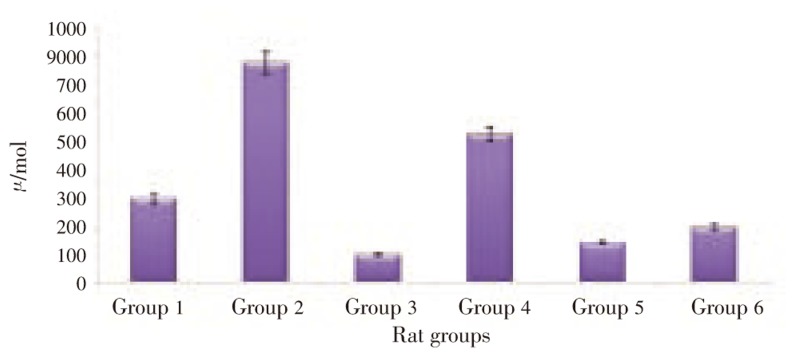
Results on the effect of pineapple peel extract on catalase activities in plasma of alcohol-fed rats were presented in Figure 3. An elevated level in catalase activity was observed in alcohol-fed rats. Treatment with pineapple peel extract at 2.5 mL/kg bw reduced the activity by 87.46%, while treatment with a double dose (5.0 mL/kg bw) was observed to reduce the activity by 32.67%. 74.94% decrease in the catalase activity was observed when treated with distilled water.
Figure 3. Effect of pineapple peel extract on catalase activity of alcohol-induced oxidative stressed rats.
4. Discussion
Alcohol is generally accepted to be a toxic compound as they induce oxidative stress leading to significant increase in lipid peroxidation and protein oxidation which damage lipids and proteins as well as altering the endogenous antioxidant capacity[12]. In this present study, the protective activity of pineapple peels against alcohol-induced lipid peroxidation, changes in catalase activities and hepatic biochemical levels in blood plasma was reported.
The present ethanol toxicity study recorded significant increase in plasma hepatic ALP, AST, and ALT levels. These increased levels were in accordance with previous reports[13],[14]. The toxic actions of ethanol on proteins and on hepatocytes cellular organelles could be responsible for these increased enzyme levels[15]. Increased plasma hepatic biomarkers have been attributed to the leakage of these enzymes from the liver cytosol into the blood stream and/or change in the permeability of liver cell membrane indicating hepatic damage[16]. Treatment with pineapple peel extract for 28 days was observed to significantly decrease the enzyme levels, signifying the modulatory effect of the extract on the hepatic biomarkers and its hepatoprotective potentials.
Lipid peroxidation has been reported to be a marker of oxidative stress[17]. Free radicals induce lipid peroxidation, resulting in reactive molecules such as MDA and 4-hydroxy-2-nonenal (HNE). Both of these can react with proteins to form MDA-protein and HNE-protein adducts which have adverse effect on cell functions and biochemistry[18]. Ethanol can generate the ROS that caused lipid peroxidation. Treatment with pineapple peel extract for 28 days was observed to significantly reduce plasma MDA level indicating a protective role of the extract against alcohol-induced toxicity. This may be attributed to the presence of phytochemicals such as phenol and flavonoids. This is further supported by evidences indicating the use of natural extracts from plant source in reducing the risk of oxidative stress due to their rich source of phytochemicals[3].
The increased activities of catalase on feeding with alcohol can be attributed to increased synthesis due to induction. These enzymes are induced due to oxidative stress brought about by ethanol metabolism as catalase play a major role in alcohol degradation[19]. These enzymes have been reported to play an important role in maintaining physiological levels of oxygen and hydrogen peroxide by catalysing the dismutation of oxygen radicals and hydrogen peroxides generated from alcohol breakdown. Treatment with pineapple peel extract for 28 days was observed to significantly reduce the catalase activities, signifying its protective and/or modulatory potentials against alcohol-induced oxidative stress.
Pineapple peel extract may carry out these antioxidant activities by one or the following mechanisms: scavenging free radicals; interaction with oxidative cascade and preventing its outcome; singlet oxygen quenching; inhibition of oxidative enzymes and activation of antioxidant enzymes; and metal chelating[14],[20].
The positive modulation of lipid peroxidation, catalase activities as well as hepatic biomarker levels of blood plasma by the methanolic extract of pineapple peels under alcohol-induced oxidative stress is an indication of its protective ability in the management of alcohol-induced toxicity.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 2.Kaur J, Bansal MP. Effect of vitamin E on alcohol-induced changes in oxidation stress and expression of transcription factors NFkB and AP-1 in mice brain cerebral hemispheres. Indian J Exp Biol. 2008;46:562–567. [PubMed] [Google Scholar]
- 3.Reddy DV, Padmavathi P, Paramahamsa M, Varadacharyulu NC. Amelioration of alcohol-induced oxidative stress by Emblica officinalis (Alma) in rats. Indian J Biochem Biophys. 2010;47:20–25. [PubMed] [Google Scholar]
- 4.Erukainure OL, Oke OV, Owolabi FO, Umanhonlen EE. Investigation into the chemical properties and antioxidant activities of Myristica fragrans. Biosci Res Commun. 2010;22(2):109–114. [Google Scholar]
- 5.Aqil F, Ahmed I, Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk J Biol. 2006;30:177–183. [Google Scholar]
- 6.Wood R. The whole foods encyclopedia. New York: Prentice-Hall Press; 1988. [Google Scholar]
- 7.Correia RTP, Mccue P, Vattem DA, Magalhães MMA, Macêdo GR, Shetty K. Amylase and helicobacter pylori inhibition by phenolic extracts of pineapple wastes bioprocessed by rhizopus oligosporus. J Food Biochem. 2004;28(5):419–434. [Google Scholar]
- 8.Faremi TY, Suru SM, Fafunso MA, Obioha UE. Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol. 2008;46(8):2658–2664. doi: 10.1016/j.fct.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Amanvermez R, Ankaralı S, Tunçel OK, Tomak L, Alvur M. Effect of chronic high dose-alcohol consumption on the general biochemical parameters. Turk J Biochem. 2009;34(3):113–120. [Google Scholar]
- 10.Chance B, Maehly AC. Assay of catalase and peroxidase. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- 11.Chowdhury P, Soulsby M. Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Ann Clin Lab Sci. 2002;32(2):188–192. [PubMed] [Google Scholar]
- 12.Kannan M, Wang L, Kang YJ. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med. 2004;229:553–559. doi: 10.1177/153537020422900614. [DOI] [PubMed] [Google Scholar]
- 13.Bain PJ. In: Duncan and Prasses vetinary laboratory medicine pathology. 4th ed. Latimer KS, Mahaffey EA, Prasse K, editors. Ames: Iowa State University Press; 2003. [Google Scholar]
- 14.Abovwe JA, Erukainure OL, Oke OV, Okafor OY, Ajiboye AJ. Modulatory effects of Globimetula braunii on lipid peroxidation and antioxidant status in hypercholesteremic rats. Invent Rapid Ethnopharm. 2010;1:1. [Google Scholar]
- 15.Soliman KM, Hamed MA, Ali SA. Hepatoprotective effect of carnosine on liver biochemical parameters in chronic ethanol intoxicated rat. Med J Islam World Acad Sci. 2006;16(2):77–86. [Google Scholar]
- 16.Naik SR, Panda VS. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007;27:393–399. doi: 10.1111/j.1478-3231.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 17.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2005;43:20–24. [PubMed] [Google Scholar]
- 18.Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown-focus on adducts. Alcohol Res Health. 2003;27(4):285–290. [PMC free article] [PubMed] [Google Scholar]
- 19.Koolman J, Roehm K. Color atlas of biochemistry. Stuttgart: Georg Thieme Verlag; 2005. p. 320. [Google Scholar]
- 20.Rukkumani R, Aruna K, Varma PS, Rajasekeran KN, Menon VP. Comparative effects of curcumin and an analog curcumin onn alcohol and PUFA induced oxidative stress. J Pharm Pharm Sci. 2004;7(2):274–283. [PubMed] [Google Scholar]