Abstract
Objective
To explore various unexplored locations where Penicillium spp. would be available and study the production of penicillin from the isolated Penicillium spp. in different media with altered carbohydrate source.
Methods
The collected soil samples were screened for the isolation of Penicillium chrysogenum (P. chrysogenum) by soil dilution plate. The isolated Penicillium species were further grown in different production media with changes in the carbohydrate source. The extracted penicillin from various isolates was analyzed by HPLC for the efficacy of the product. Further the products were screened with various bacterial species including methicillin resistant Staphylococcus aureus (MRSA). And the work was extended to find the possible action on MRSA, along with characterization using other pathogens.
Results
From the various soil and citrus samples used for analysis, only the soil sample from Government General Hospital of Bangalore, India, and Sanjay Gandhi Hospital, Bangalore, India, showed some potential growth of the desired fungi P. chrysogenum. Different production media showed varied range of growth of Penicillium. Optimum production of penicillin was obtained in maltose which proved maximum zone of inhibition during assay. Characterization of penicillin on pathogens, like wild Escherichia coli strain, Klebsiella spp., and MRSA, gave quite interesting results such as no activity on the later strain as it is resistant. HPLC data provided the analytical and confirmation details of the penicillin produced. Accordingly, the penicillin produced from the soil sample of Government General Hospital had the high milli absorbance unit of 441.5 mAu compared with that of the penicillin produced from Sanjay Gandhi Hospital sample, 85.52 mAu. Therefore, there was a considerable change in quantity of the penicillin produced from both the samples.
Conclusions
The Penicillium spp. could be possibly rich in hospital contaminants and its environments. This research focuses on various unexplored sources of medical ailments, and also shows that the growth of penicillin is high in maltose rich media that could possibly enhance the growth.
Keywords: Penicillium chrysogenum, Soil, Citrus, Penicillin, Bioefficacy, Antibiotic activity, Penicillium spp., Production, Soil dilution plate, Staphylococcus aureus, Characterization, Production media, Zone of inhibition, Carbohydrate source, Isolation, Efficacy, Pathogen
1. Introduction
Fungi are an important component of the soil microbiota typically constituting more of the soil biomass than bacteria, depending on soil depth and nutrient conditions[1]. The role of fungi in the soil is an extremely complex and is fundamental to the soil ecosystem. They perform ecological services that strongly impact the quality of human life and have enormous potential for providing economic benefits, e.g., the isolation and identification of the soil fungus Penicillium leading to a large pharmaceutical industry of antibiotics[2]. It is estimated that there are 1.5 million fungal species on earth, of which only about 70 000 have been described till recently[3].
According to the recent research done by “marketresearch.com”, China's demand for penicillin has grown at a fast pace in the past decade. In the next five years, both production and demand will continue to grow. Accordingly, new studies that examine China's economic trends, investment environment, industry development, supply and demand, industry capacity, industry structure, marketing channels and major industry participants regarding historical data[3] and long-term forecasts through 2012 and 2017 are presented.
This present investigation was aimed to explore the mycoflora diversity of antibiotics producing Penicillium chrysogenum (P. chrysogenum) from various soil and citrus sources. The expected outcome of this work is to explore antibiotics for the upcoming threat by the mutant pathogens. Results of this study suggest that soil and citrus fruit samples seem to be good source of Penicillium species.
2. Materials and methods
2.1. Sample collection
The soil samples were collected from four different hospitals of Bangalore city, i.e., Sanjay Gandhi Hospital Bangalore (SGH), Government General Hospital Bangalore (GGH), Victoria Hospital Bangalore, and Vanivilas Women's and Children's Hospital Bangalore. The deep wet soil samples were collected as the Penicillium species, usually habitats in wet and moist location. The samples were stored in small plastic bags until transported to the laboratory, and then stored at 4 °C until process. Similarly, the rotten citrus fruit samples, such as rotten orange, Citrus limetta, and lemon were also collected from fruit vendor's wastes and market wastes. They were also stored in polythene bags and after reaching laboratory kept in cool open air away from sunlight promoting fungal growth.
2.2. Isolation of Penicillium from samples
The soil samples were processed further using the soil dilution plate[4] in potato dextrose agar.
2.3. Screening and identification of P. chrysogenum
The Penicillium spp. was identified from the colonies in the petriplates by its shiny blue green mold surrounded by white mycelium. Such colonies were visualised under light microscope using phenol blue to confirm the mycelium and conodia of the fungi. The species identification was done based on the method of Raper et al[5].
2.4. Production of penicillin
Method of production of penicillin in the three different production media was done in laboratory scale. Hence three shaking flasks were taken in which the production media was prepared. The flasks along with the media were sterilized at 15 psi, 121 °C for 20-45 min. The sterilized media were cooled to room temperature before inoculation. The flasks with production media were transferred to the clean air laminar flow chamber and 2 mL of the 4-5 days seed culture was added to it and shaken well. After inoculation the flasks with culture were maintained in a shaking incubator for 3-5 days at 25-28 °C and 50 rpm speed until harvest (approximately 7-10 days).
2.5. Antibiotic assay
The routine antibiotic assay of the production was carried out every three days. This routine antibiotic sensitivity assay showed the production of penicillin in the media. The assay procedure for penicillin was followed as described by Raahave[6] with some modifications.
2.6. Sugar utilization analysis
The sugar utilization analysis provided the data of how the P. chrysogenum utilized the carbon source by the quantification of the sugar content. DNS method is simple, sensitive and adoptable during handling of a large number of samples at a time for reducing sugar analysis.
2.7. Purification and identification of penicillin
The purification of penicillin from the production media began with filtration of the broth. In the first stage, large solids and microbial cells were separated by filtration, as filtration is the most versatile method for removing the insoluble from the broth. Penicillin rich aqueous broth was treated with activated charcoal to remove pigments and impurities. After filtration and carbon treatment, penicillin recovery was done by liquid-liquid extraction (solvent extraction). Penicillin was extracted from an aqueous phase into the solvent butyl acetate. Solute recovery was carried out by evaporation of the extracted sample. The identification of purified penicillin was done by thin layer chromatography (TLC) with benzene: ethyl acetate: acetic acid (40:40:20) as solvent and visualized in UV illuminator.
2.8. Characterization of purified penicillin
Characterization of purified extract and crude extract of penicillin was finally analyzed for its activity on three different pathogenic organisms, i.e., Klebsella spp., wild strain of Escherichia coli (E. coli), and methycillin resistant Staphylococcus aureus (MRSA). A bacterial lawn of the foresaid bacterial species was spread on nutrient agar plate and a well was bored on the bacterial agar plates. And randomly selected samples which had a high rate of inhibition during routine assay were used for characterization. 100 µL of the samples from crude and purified extract were loaded in two different wells bored in a single plate. The plates were kept for inhibition at 37 °C for 16 to 24 h, and the results were noted.
2.9. HPLC analysis of penicillin
HPLC analysis of penicillin was carried out in a SCIGENICS, cyber lab manufactured device, with UV detector set at 254 nm. The column used for analysis is C-18. The mobile phase consisted of methanol: phosphate buffer (85:15, v/v) at flow rate 1 mL/min. Standard used for comparison is Pencom®13 (commercially available penicillin injection).
3. Results
After exploring the mycoflora of Penicillium species and inoculating them in production media with different carbohydrate sources, the optimal penicillin production was determined and results were briefed.
3.1. Isolation and screening of P. chrysogenum
From the various soil and citrus samples used for analysis, only the soil sample from GGH (Figure 1), and SGH (Figure 2) showed some potential growth of the desired fungi P. chrysogenum. Other samples of soil from Victoria Hospital and Vanivilas Hospital showed no growth of the Penicillium spp. and the citrus samples had no desired mold (Penicillium spp.) growth either. The two samples of suspected Penicillium spp. were visualized under light microscope and confirmed as P. chrysogenum, based on the conidia and conidiophore arrangement of the fungi. These confirmed strains of Penicillium were further processed for pure culture used in the production and purification process.
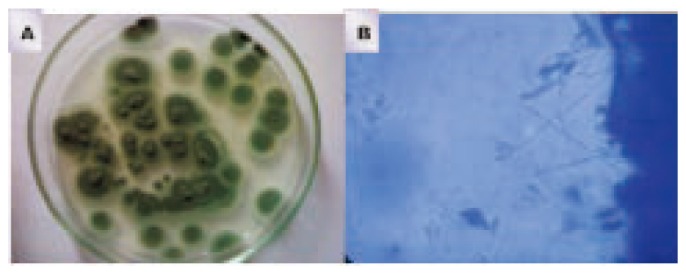
Figure 1. Pure culture of P. chrysogenum isolated from soil sample of GGH (A) and the microscopical identification of strain (B).
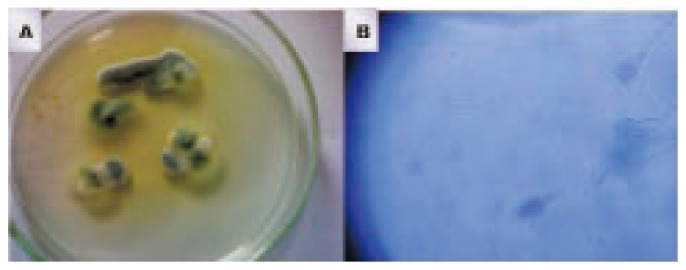
Figure 2. Pure culture of P. chrysogenum isolated from soil sample of SGH (A) and the microscopical identification of strain (B).
3.2. Production of penicillin
The production of penicillin was done in three shaking flasks with different production media and the carbohydrate sugar source alone was changed. All the three production media consisted of 0.68 mol/L KH2PO4, 0.242 mol/L NH4Cl, 0.06 mol/L phenyl acetic acid, 0.79 mol/L K2SO4, 0.05 mol/L MgSO4.7H2O, 6 mol/L ammonia, 2 mol/L citric acid. But carbohydrate source for media 1 was 2 g/200mL maltose, media 2 was 2 g/200mL dextrose and media 3 was 2 g/200mL lactose. The results of the penicillin production were estimated based on the routine antibiotic assay and carbohydrate utilization.
3.3. Antibiotic assay
Routine antibiotic assay was carried out to check the antibiotic sensitivity of the penicillin produced in the production media. The antibiotic assay was done every three days after inoculation of the production media and the zone of inhibition was observed. The data obtained during the routine assay of the strain obtained from SGH and GGH were presented in Table 1.
Table 1. Antibiotic routine assay of penicillin from SGH and GGH sample.
| Sample | Day | Media | Zone of inhibition (mm) |
| SGH sample | Day 3 | Lactose | 21 |
| Maltose | 20 | ||
| Dextrose | 25 | ||
| Day 6 | Lactose | 17 | |
| Maltose | 24 | ||
| Dextrose | 25 | ||
| Day 9 | Lactose | 14 | |
| Maltose | 29 | ||
| Dextrose | 27 | ||
| GGH sample | Day 3 | Lactose | 25 |
| Maltose | 25 | ||
| Dextrose | 25 | ||
| Day 6 | Lactose | 26 | |
| Maltose | 28 | ||
| Dextrose | 27 | ||
| Day 9 | Lactose | 28 | |
| Maltose | 30 | ||
| Dextrose | 30 |
3.4. Sugar utilization analysis
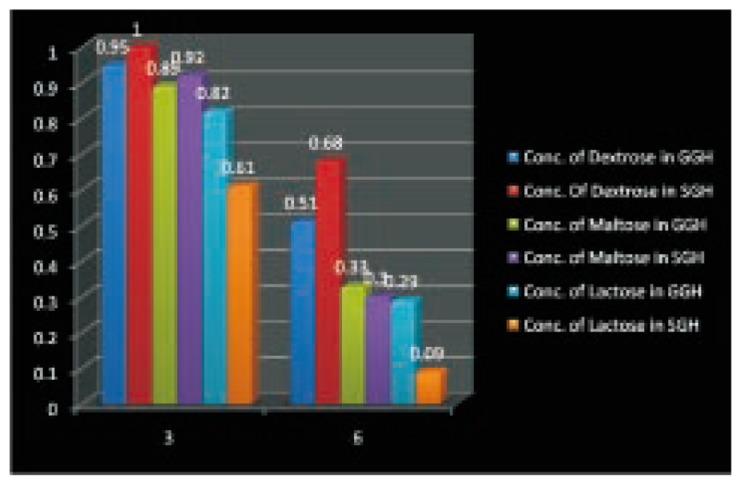
The sugar utilization in the culture media was quantified based on DNS method, which showed that the organism utilized the sugar as carbon source for their growth. The standard curve for the different concentrations of dextrose, maltose, and lactose was drawn based on the optical density (OD) value (Table 2). The OD of the sample from the dextrose media sample was read at 540 nm and tabulated in Table 3. The average OD value was calculated and compared with the standard curve to obtain the concentration of dextrose in the production media. Every three days the sugar utilization analysis was carried out and found that the growth of organism increased with the decrease of sugar. The sugar utilization assay was carried out in other media used for production (maltose, lactose). The concentrations of the respective sugars were calculated based on the comparison of OD value of sample with standard graph. The maltose and lactose concentrations were listed in Table 3. The basic idea of calculating the sugar concentration in the media was to determine the growth of the penicillium in the production media by depletion of carbohydrates. The growth of organism in the production media was inversely proportional to the sugar concentration present in the production media, i.e., growthα1/sugar concentration on media. Figure 3 depicted the chart comparing concentration of carbohydrate in penicillin production process.
Table 2. Standard concentration and OD values of dextrose, maltose, and lactose.
| Concentration of dextrose | OD | Concentration of maltose | OD | Concentration of lactose | OD |
| 0.2 | 0.09 | 0.2 | 0.14 | 0.2 | 0.23 |
| 0.4 | 0.16 | 0.4 | 0.28 | 0.4 | 0.36 |
| 0.6 | 0.23 | 0.6 | 0.35 | 0.6 | 0.49 |
| 0.8 | 0.34 | 0.8 | 0.49 | 0.8 | 0.64 |
| 1.0 | 0.54 | 1.0 | 0.60 | 1.0 | 0.78 |
| Blank | 0.00 | Blank | 0.00 | Blank | 0.00 |
Table 3. Analysis of dextrose concentration in media (540nm).
| Media | Duration | Conc. of sample | OD of GGH sample | Average OD value | Conc. in sample (g) | OD of SGH sample | Average OD value | Conc. in sample (g) |
| Dextrose | Day 3 | 0.1 | 0.317 | 0.404 | ||||
| 0.2 | 0.532 | 0.486 | 0.95 | 0.57 | 0.545 | 1 | ||
| 0.3 | 0.610 | 0.66 | ||||||
| Day 6 | 0.1 | 0.16 | 0.23 | |||||
| 0.2 | 0.19 | 0.2 | 0.51 | 0.278 | 0.276 | 0.68 | ||
| 0.3 | 0.25 | 0.319 | ||||||
| Maltose | Day 3 | 0.1 | 0.432 | 0.527 | ||||
| 0.2 | 0.572 | 0.54 | 0.89 | 0.594 | 0.573 | 0.92 | ||
| 0.3 | 0.618 | 0.6 | ||||||
| Day 6 | 0.1 | 0.23 | 0.119 | |||||
| 0.2 | 0.25 | 0.253 | 0.33 | 0.244 | 0.221 | 0.3 | ||
| 0.3 | 0.28 | 0.3 | ||||||
| Lactose | Day 3 | 0.1 | 0.59 | 0.42 | ||||
| 0.2 | 0.63 | 0.673 | 0.82 | 0.53 | 0.50 | 0.61 | ||
| 0.3 | 0.8 | 0.551 | ||||||
| Day 6 | 0.1 | 0.13 | 0.108 | |||||
| 0.2 | 0.35 | 0.293 | 0.29 | 0.19 | 0.179 | 0.09 | ||
| 0.3 | 0.4 | 0.24 |
Figure 3. Concentration of sugar in penicillin production process.
3.5. TLC analysis of purified penicillin
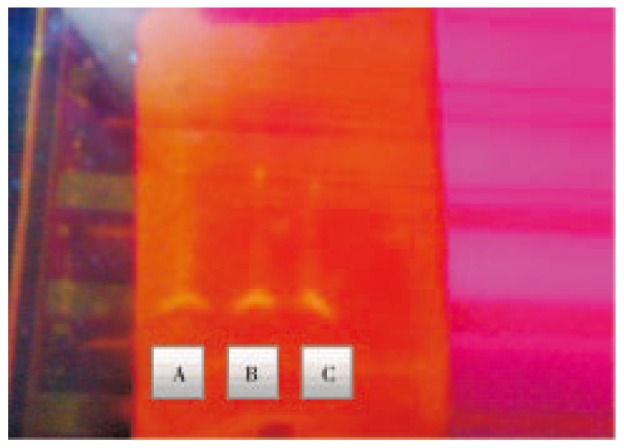
The purified penicillin was obtained in a clear solution after concentrating. The penicillin in its pure form was confirmed using analytical TLC. The samples showed clear fluorescent bands on illumination with UV illuminator (Figure 4).
Figure 4. Analytical TLC image of GGH maltose (A), SGH maltose (B), SGH dextrose (C) samples of penicillin.
3.6. Characterization of purified penicillin
The characterization studies on different microbes gave good results, as the penicillin showed clear inhibition on Klebsiella spp. and E. coli. But no inhibition was noticed on MRSA. The penicillin produced in maltose media gave optimum inhibition compared with other samples.
3.7. HPLC analysis of purified penicillin
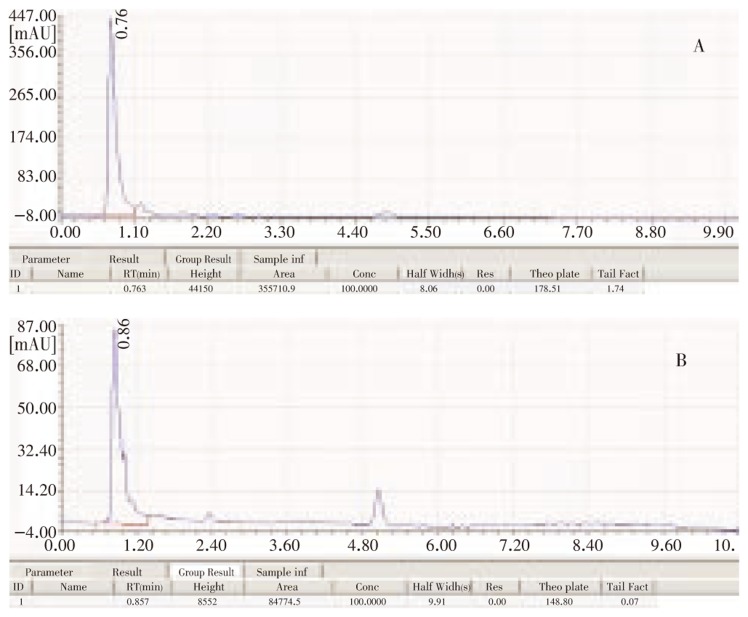
On standard run in HPLC using Pencom®13, the results obtained were 756 mAU at a retention time of 0.62. The HPLC results of penicillin from GGH sample and SGH sample showed a sharp peak at the retention time of 0.763 min and 0.857 min, respectively, with an absorbance value of 441.5 mAu and 85.52 mAu, respectively (Figure 5).
Figure 5. HPLC data of penicillin.
A: GGH sample; B: SGH sample.
4. Discussion
From various samples of soil and citrus fruits analyzed throughout the city of Bangalore, the good source of penicillin was found to be the soil sample collected from the GGH which is consistent with the results of Sir Alexander Fleming's that the hospital contaminants are rich penicillin source. During the routine antimicrobial assay the zone of inhibition were measured to be 30 mm (optimum) diameter on the 9th day of assay. Based on the works of Knudsen and Randall the maximum zone diameter was measured to be 21 mm. Raahave, from University of Copenhagen, noticed the zone of inhibition in his version of work on penicillin assay by paper diffusion method as 33.5 mm. Accordingly, the zone formed by the strain isolated in this study is considered to be highly potential against pathogens during characterization.
Different production media showed varied range of growth of Penicillium. This shows that the carbon source is the main factor in determining the Penicillium growth. Since the other entire source is maintained in uniformity, their effect on the growth of Penicillium was not determined. The sugar utilization data obtained during the penicillin production shows growth of Penicillium spp. in the production media. According to the data obtained the sugar utilization in the production media except for that having lactose as carbon source (that decreases rapidly) was decreasing constantly. Also during determination of zone of inhibition lactose media sample showed less zone of inhibition, which showed that penicillin production was instable in lactose media. The exact reason behind why there is no penicillin production is not clear but there should be some factor inhibiting the production in lactose media. Optimum production of penicillin was obtained in maltose which proved maximum zone of inhibition during assay. Characterization of penicillin on pathogens like wild E. coli. strain, Klebsiella spp. and MRSA gave quite interesting results, such as no activity on the later strain as it is resistant, but good activity was noticed on the earlier two pathogens. The MRSA is usually dominant against penicillin and its derivatives, so the trials of exploring penicillin active against MRSA do not provide good result. Further characterizations against other pathogens were restricted due to safety hazards. HPLC data provided the analytical and confirmation details of the penicillin produced. Accordingly, the penicillin produced from the soil sample of GGH has a high milli absorbance unit of 441.5 mAu compared with that of the penicillin produced from SGH sample, 85.52 mAu. Therefore, there is a considerable change in the quantity of penicillin produced from both the samples.
In conclusion, the Penicillium spp. could be possibly rich in hospital contaminants and its environments. This study focuses on various unexplored sources of medical ailments. The result also shows that the growth of penicillin is high in maltose rich media that could possibly enhance the growth. Even though the study showed no inhibition against MRSA, there could be some wild source or mutated source for penicillin to act on MRSA. Nevertheless, this could be a challenge. On further strain improvement, we would possibly get high rate of penicillin production.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ainsworth GC, Bisby GR. Dictionary of the fungi. 8th ed. Wallingford: CABI; 1995. p. 445. [Google Scholar]
- 2.Diana WF. Soil biodiversity: its importance to ecosystem processes. Fort Collins: Colorado State University; 1994. [Google Scholar]
- 3.Hawks worth DL, Rossman AY. Where are all the undescribed fungi? Phytopathology. 1997;87:888–891. doi: 10.1094/PHYTO.1997.87.9.888. [DOI] [PubMed] [Google Scholar]
- 4.Waksman SA. A method for counting the number of fungi in the soil. J Bacteriol. 1922;7:339–341. doi: 10.1128/jb.7.3.339-341.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raper KB, Thom C. A manual of the penicillia. 1949:875. [Google Scholar]
- 6.Raahave D. Antimicrobial agents and chemotherapy. American Society for Microbiology. 1974;6(5):603–606. doi: 10.1128/aac.6.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaroopa RA, Jetty A, Ramakrishna SV. Kinetic studies of penicillin production during batch and repeated batch in fluidized bed bioreactor with agar immobilized Penicillium chrysogenum cells. Indian J Biotechnol. 2004;3:394–399. [Google Scholar]
- 8.Tan XD, Ji QR, Chang ZD. Partition behavior of penicillin in three-liquid-phase extraction system. The Chinese Journal Process Engineering. 2006;6(3):363–368. [Google Scholar]
- 9.Suhail M, Akhund S, Tahira JA, Mangrio M, Abro H. Isolation and identification of Penicillium spp from the river indus bed at Kotri. Pak J Bot. 2006;38(4):1289–1292. [Google Scholar]
- 10.Haider M, Hamzah, Anwar HL, Ali Hamid G, Hassan Physiological regulation of protease and antibiotics in Penicillium spp. using submerged and solid state fermentation techniques. J Eng Sci Technol. 2009;4(1):81–89. [Google Scholar]
- 11.Baghel US, Singhal M, Gupta MM, Singh HP, Shuchi D, Sahu M. Analytical method validation for tablet of phenoxymethyl penicillin potassium by HPLC method. J Chem Pharm Res. 2009;1(1):271–275. [Google Scholar]