Abstract
Objective
To observe three species of Kalicephalus found in three species of snake (Ophiophagus hannah, Ptyas mucosus, and Naja Sputatrix) during research on Capture Snake for Trading in Java and Snake Biodiversity in Kalimantan Islands.
Methods
Specimens for light microscopy examination were fixed with warm 70% alcohol, cleared and mounted in lactophenol for wet mounting. Drawings were made with the aid of a drawing tube attached to a Nikon compound microscope. Measurements were given in micrometers (µ) as the average of findings, followed by the range in parentheses, unless otherwise stated.
Results
Kalicephalus (Costatus) indicus was found from 7 Ptyas mucosus, Kalicephalus bungari from 2 Naja sputatrix and 1 Kalicephalus (Costatus) indicus and Kalicephalus assimilis found from 1 Ophiophagus hannah. The morphology and measurement of three species of Kalicephalus found in this study were close to those described before.
Conclusions
New finding of host of Kalicephalus (Costatus) indicus and Kalicephalus bungari was a snake species of Naja sputatrix. New records of locality were Kalimantan island as the new locality of Kalicephalus assimilis, and Java island was new locality of Kalicephalus (Costatus) indicus.
Keywords: Strongyloidea, Kalicephalus, Snakes, New record, Indonesia, Intestinal nematode, New host
1. Introduction
Nematode parasites of wild animal have important role to human health because some species are zoonotic[1]. During research on Capture Snake for Trading in Java and Snake Biodiversity in Kalimantan Islands, three species of nematode of genus Kalicephalus were found in intestine of 11 of snakes. They are Kalicephalus assimilis (K. assimilis) Wu et Hu (1938), Kalicephalus (Costatus) indicus Ortlepp (1923) and Kalicephalus bungari Mac Callum (1918).
The genus Kalicephalus Molin (1861) is a strongylid nematode of the family Diaphanocephalidae in the intestine of reptiles and lizards[2]. A total of 50 species of Kalicephalus have been reported in many regions of the world. Schad[3] revised the Kalicephalus spp and recognized from 50 to 23 species.
The infection of Kalicephalus caused mild enteritis in snakes and raised to secondary bacterial infection that caused of death on host[4], but its infection has never been reported in human.
Although Kalicephalus have been reported from many countries, its information from Indonesia is very limited. The species from Java has been reported as Kalicephalus fimbriatus[2] and Kalicephalus willeyi from Komodo Island[5] and adjacent area, Papua New Guinea[6]. Morphology of three species found in this study and the new findings of host and locality records are presented herein.
2. Materials and methods
Nematodes were collected in 2002 from three species of 11 snakes 1 Ophiophagus hannah(O. hannah), 7 Ptyas mucosus(P. mucosus) and 3 Naja sputatrix(N. sputatrix) during the Research on Captured Snakes for Trading in Wonogiri and Klaten Districts of Java Island and Snake Biodiversity in Kayan Mentarang District of Kalimantan Island.
Specimens for light microscopy examination were fixed with 70% warm alcohol, cleared and mounted in lactophenol for wet mounting. Drawings were made with the aid of a drawing tube attached to a Nikon compound microscope. Measurements were given in micrometers (µ) as the average of findings, followed by the range in parentheses, unless otherwise stated. All specimens described here were deposited in the Nematode Collection Section of Bogor Zoological Museum, Research Center for Biology - Indonesian Institute of Sciences, Cibinong City, Indonesia. The anterior end morphological terminology followed what Schad had reported [3].
3. Results
Kalicephalus (Costatus) indicus (K. indicus) was found from 7 P. mucosus, Kalicephalus bungari from 2 N. sputatrix and 1 K. indicus and K. assimilis found from 1 O. hannah.
A new finding was reported that N. sputatrix was the new host for K. (Costatus) indicus and Kalicephalus bungari (K. bungari). Other new findings were recorded that K. island was new locality for Kalicephalus assimilis and Java Island was new locality for Kalicephalus (Costatus) indicus and K. bungari.
3.1. Descriptions
3.1.1. Kalicephalus assimilis Wu et Hu, 1938
General: Body attenuated posteriorly, maximum width at level behind the anterior of intestine, head diameter was as large as maximum body width. Face curved but not tilted, mouth crowned with rudimentary leaf. Buccal capsule was supported by two lateral valve, consisting of several chitinoid pieces. Anterior chitinious pieces curved, small, posterior chitinious pieces stretching posteriorly to anterior end of esophagus (Figure 1A). Esopahgus was as short as about 1/15 body length, enlarge posteriorly, ended in rounded bulb.
Figure 1. Kalicephalus asimilis.
1A: Anterior end of male, lateral view; 1B: Posterior end of female, lateral view; 1C: Copulatory bursa, dorsal view.
Female: Body length 8.51 (7.35-9.57) mm, width at head 198 (170-220)µ, maximum width 443 (420-450)µ. Buccal capsule 147 (140-190)µlong. From anterior to nerve ring 320 (340-390) µ, to excretory pore 345 (310-480)µ, esophagus short, 555 (510-580)µ, tail short, conical end (Figure 1B), length 340 (330-380)µ. Vulva protruding prominently 2701 (2 630-3 090)µ from posterior end. Uteri opposed ovejector with short vagina vera, vestibule longer, spincter and infundibulum about same length, egg oval, thin shelled, (71 × 63)µ [(68-75) µ× (51-71)µ].
Male:Body length 7.29 (8.18-6.29) mm, width at head 176(160-180)mm, maximum width 443 (420-450) µ. Buccal capsule was 121(110-150) µ long. From anterior end to nerve ring 298 (270-320) µ, to excretory pore 440 (410-480) µ, length of esophagus 543 (520-580) µ, spicule short, equal and similar, alate, broad spatulated at tip, length 454 (410-550) µ, gubernaculum present. Copulatory bursa type III[3], ventral lobe is the shortest, lateral and ventral lobes were at the same length. Ventro and lateroventral united almost their length, and bent anteriorly. Lateral rays thick, externolateral rays divergent, shorter than other laterals, medioand posterolateral same length. Externodorsal rays is the thickest rays, arising from 1/3 proximal of dorsal trunk, dorsal ray devided into two branches at the tip, each branch tridigitated type (Figure 1C).
3.1.2. Kalicephalus (Costatus) indicus Ortlepp, 1923
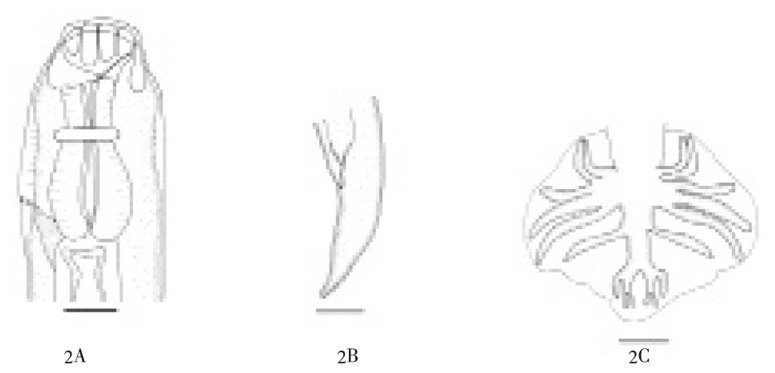
General: Body attenuated posteriorly, maximum width at level behind the anterior of intestine. Face rounded anteriorly, mouth without rudimentary leaf crown. Anterior chitinoid pieces wide, posterior chitinoid pieces rounded posteriorly. Nerve ring at about a half of oesophagus, excretory pore posterior to its. Esopahgus was as short as about 1/15 body length, anlarge anteriorly and posteriorly, ended in an elongated bulb (Figure 2A).
Figure 2. Kalicephalus (Costatus) indicus.
2A: Anterior end of male, lateral view; 2B: Posterior end of female, lateral view; 2C: Copulatory bursa, dorsal view.
Female: Body length 7.209 (6.462-7.890) mm, width at head 166 (150-178)µ, maximum width 342 (320-355) µ. Buccal capsule 157 (150-170 )µ long. From anterior to nerve ring 227 (220-230)µ, to excretory pore 440 (430-450)µ, esophagus short 477 (460-500)µ, tail short tapering (Figure 2B), length 144 (122-165) µ. Cuticle of vulva protruding, 1 662 (1 442-1 830) µ from posterior end. Uteri opposed ovejector with short vagina vera, vestibule, spincter, and infundibulum about same length, no egg were found.
Male: Body length 5.79 (5.43-6.16) mm, width at head 126 (120-130) µ, maximum width 262 (260-265) µ. Buccal capsule 106 (102-110 ) µ long. From anterior end to nerve ring 226 (220-240) µ, to excretory pore 395 (380-410) µ, length of esophagus 403 (370-450)µ, spicule short, equal, similar, ended in elongated spatula, length 386 (370-410) µ, gubernaculum present. Copulatory bursa type III[3]: ventral lobe is the shortest, united almost their length, elbow curved anteriorly. Externolateral rays divergent, shorter than other lateral medio and posterolateral same length, parallel. Externodorsal ray slender, long arising from 1/3 proximal of dorsal trunk, dorsal ray divided into two branches at the tip, each branch tridigitated (Figure 2C).
3.1.3. Kalicephalus bungari (MacCallum, 1918)
General: Body attenuated posteriorly, maximum width at level behind the anterior of intestine. Face cuticle inflated, angular at dorsal and ventral, mouth without rudimentary leaf crown absent. Anterior chitinoid pieces narrow, posterior citinoid pieces triangular posteriorly. Nerve ring at about a half of esophagus, excretory pore posterior to its. Esopahgus short about 1/15 body length, anlarge anteriorly and posteriorly, ended in an elongated bulb (Figure 3A).
Figure 3. Kalicephalus bungari.
3A: Anterior end of male, lateral view; 3B: Posterior end of female, lateral view; 3C: Copulatory bursa, dorsal view Scale bars: Figures 1-9 = 100 micrometers (µ).
Female: Body length 6.83 (5.99-7.89) mm, width at head 121(102-139) µ, maximum width 326 (280-350) µ. Buccal capsule 185 (120-190) µ long. From anterior to nerve ring 298 (200-320) µ, to excretory pore 575 (421-680) µ, esophagus short 694 (450-700) µ, tail short with spike at tip (Figure 3B), length 144 (122-165) µ. Anterior and posterior cuticle of vulva protuberant 1 662 (1 442-1 830) µ from posterior end. Uteri opposed ovejector with short vagina vera, vestibule, spincter and infundibulum about same length, no egg were found.
Male: Body length 5.21 (4.79-5.83) mm, width at head 162 (130-190) µ, maximum width 285 (260-360) µ. Buccal capsule 175 (140-180) µ long. From anterior end to nerve ring 313 (220-360 ) µ, to excretory pore 480 (350-560) µ, length of esophagus 576 (420-680) µ, spicule, anequal and dissimilar, long spicule alate, extending almost to tip, 590 (514-666) µ. Short spicule stout, in front of distal end with burb, ended in pointed tip, length 359 (330-400) µ, gubernaculum present. Copulatory bursa type I[3], ventral lobes is the shortest, lateral and dorsal lobes same length. Ventro and lateroventral united almost their length, curved anteriorly. Externolateral divergent, shorter than other laterals, medio and posterolateral same length. Externodorsal arising from the base of dorsal trunk, dorsal ray giving off lateral branches near the base, devided into two branche sat the tip, each branch tridigitated (Figure 3C).
4. Discussion
The morphology and measurements of K. assimilis found in O. hannah was as same as that found in the same species of snake reported by Zoological Garden, London, that was originally from Celebes (Sulawesi Island) Indonesia. Kayan Mentarang District in Kalimantan Island was the new locality record of K. assimilis, previously this species has been reported found in Hainan (China), Malaya and Celebes. Species of K. assimilis was found only in O. hannah. K. assimilis. The synonym of K. assimilis was Occipitodontus edesoni Yeh, 1956, that described also from O. hannah in Kuantan, Malaya[3].
K. (C) indicus found in P. mucosus in this study was also same in morphology and measurement as that found in the same species of snake recorded by Zoological Garden, London. Schad (1962) found morphological variation in female tail of K. (C) indicus in 5 species of snake: Boiga kraepeleni, P. mucosus, Liopeltis major, Zaoshisozymae and Natrix stolata. The species of snake; N. sputatrix was recorded as a new host of K. (C) indicus. The female tail of K. (C) indicus found in this study was similar to that found in P. mucosus by Schad [3], and synonym to K. indicus Ortlepp, 1923, Kalicephalus bengalensis Maplestone, 1929, Kalicephalus parvus Maplestone, 1932, Kalicephalus maplestonei Chaterji 1935, and Kalicephalus obeus Baylis 1935. Kalicephalus (C) indicus is the subspecies of K. cosatatus that geographically distributed in Orient and Australasia (Schard, 1962). The finding of locality of Wonogiri and Klaten Districts, in Central Java Province, Java Island was recorded as new locality for K. (C) indicus. Before this finding, K. (C) indicus was found in Japan, Malaya, China and Australia from many species of host (25 species of snake and 2 species of lizard).
There were no differences between morphology and measurement of K. bungari found in this study and those of K.bungari Mac Callum (1918). The locality of Wonogiri and Klaten Districts of Central Java Province in Java Island were also new locality for K. bungari. Previously, K. bungari was reported found in Thailand, Sumatra, Japan and China. The Naja sputatrix was found as new host of K. bungari recorded in this study. K. bungari was firstly described as Camallanus bungari Mac Callum, 1918 found in Bungarus fasciatus snake in Bogor, Indonesia. Schad (1962) recognized Camallanus bungari synonym to K. bungari, Diaphanocephalus minutes Ortlepp, 1923, Kalicephalus naiae Maplestone, 1931, and Kalicephalus minutes Yeh 1956.
Acknowledgments
We wish to thank Dr. Hideo Hasegawa for collaborating in identification the specimens from genus to species.This research was financially supported by DIPA-LIPI Program, 2009.
Footnotes
Foundation Project: Supported by DIPA-LIPI Program.
Conflict of interest atatement: We declare that we have no conflict of interest.
References
- 1.Anderson RC. Nematode parasites of vertebrates: their development and trasmission. 2nd ed. Wallingford: CABI Publishing; 2000. [Google Scholar]
- 2.Yamaguti S. Nematode parasites of vertebrates. Systema helminthum. London: Interscience Publisher; 1961. p. 1261. [Google Scholar]
- 3.Schad GA. Studies on the genus Kalicephalus (Nematode: Diaphanocephalidae) Can J Zool. 1962;40(6):1035–1065. [Google Scholar]
- 4.Junker K, Lane EP, Dlanini B, Boomker KA. Post mortem identifiction of Kalicephalus colubri (Nematode: Diaphanocephalidae) i captive mole snakes (Pseudaspes cana) in South Africa. J South Afr Vet Assoc. 2009;80(1):54–56. doi: 10.4102/jsava.v80i1.170. [DOI] [PubMed] [Google Scholar]
- 5.Pinnell JL, Schmidt GD. Helmint of reptiles from Komodo Islands, Indonesia, with descriptions of two new species. J Parasitol. 1977;63(2):337–340. [PubMed] [Google Scholar]
- 6.Sami B, Ghaleb A. Prevalence of intestinal parasitic infections in Jenin Governorate, Palestine: a 10-year retrospective study. Asian Pac J Trop Med. 2010;3(9):745–747. [Google Scholar]