Abstract
Objective
To evaluate the antinociceptive activity of the methanol extract of Ricinus communis leaves (MRCL).
Methods
Antinociceptive activity was evaluated using acetic acid induced writhing test, formalin induced paw licking and tail immersion method in mice at doses of 100, 125 and 150 mg/kg bw.
Results
The results indicated that MRCL exhibited considerable antinociceptive activity against three classical models of pain in mice. Preliminary phytochemical analysis suggested the presence of saponin, steroids and alkaloids.
Conclusions
It can be concluded that MRCL possesses antinociceptive potential that may be due to saponin, steroids and alkaloids in it.
Keywords: Ricinus communis, Analgesic, Antinociceptive, Writhing, Formalin
1. Introduction
Ricinus communis Linn (Euphorbiaceae) (R. comolunis) is a soft-wooded small tree widespread throughout tropics and warm temperature regions of the world. In the Indian system of medicine, the leaf, root and seed oil of this plant have been used for the treatment of inflammation and liver disorders[1]. It is reported that this plant possesses hepatoprotective[2],[3], antidiabetic[4], laxative[5], and antifertility[6] activities. Methanol extract of root shows anti-inflammatory and free radical scavenging activity[7]. The gallic acid, quercetin, gentisic acid, rutin, epicatechin and ellagic acid are the major phenolic compounds isolated from leaves possessing antioxidant activity[8]. Flavonoids kaempferol-3-O-beta-d-rutinoside and kaempferol- 3-O-beta-d-xylopyranoid[9],[10] and tannins[11] have been isolated from the leaves. Indole-3-acetic acid has been extracted from the roots[12]. In the present study efforts were made to evaluate the antinociceptive activity of methanol extract of R. communis leaves (MRCL).
2. Material and methods
2.1. Plant material
Fresh leaves of R. communis were collected from Baramati localities, Pune district (Maharashtra), and dried in the shade at room temperature. The plant was authenticated by Professor Deshmukh RB, Head of Botany Department, Shardabai Pawar Mahila Mahavidyalaya, Shardanagar, Baramati. The voucher specimen (PASR-115) was deposited in herbarium.
2.2. Preparation of extract
Dried and powdered leaves (100 g) were extracted by petroleum ether in Soxhlet extractor, and remaining marc was extracted by cold maceration with methanol for 48 h. After filtration, the filtrate was evaporated to dryness at (35-40) °C to yield MRCL of 9.5% w/w.
2.3. Animals
Albino mice (25-30) g of either sex were housed under standard laboratory conditions. The animals had free access to food and water. The Animal Ethical Committee of the Institute approved all the protocols of the study (Registration No.1214/ac/08/CPCSEA).
2.4. Preliminary phytochemical screening
To determine the chemical constituents, qualitative phytochemical screening of MRCL was carried out for alkaloids, flavonoids, saponins, steroids and glycoside following standard procedure[13],[14].
2.5. Antinociceptive activity
2.5.1. Acetic acid induced writhing
Mice were divided into five groups with five in each group. Control group was intraperitoneally treated with 1% Tween-80 solution (5 mL/kg), test groups received MRCL at doses of 100-150 mg/kg, and standard group received diclofenac sodium at a dose of 50 mg/kg. After 30 min, all groups were administered with 0.6% acetic acid at a dose of 10 mL/kg intraperitoneally. The number of writhing for each mouse was counted for 20 min starting 10 min after injection of acetic acid and the percent inhibition of writhing was calculated[15].
2.5.2. Formalin induced paw licking
The mice were divided into four groups with five mice in each group. Control group was intraperitoneally treated with 1% Tween-80 solution (5 mL/kg), test groups received MRCL at doses of 100-150 mg/kg, and standard group received diclofenac sodium at a dose of 50 mg/kg. 30 min after administration of test and standard drugs, all groups received 10 µL of 2.5% formalin in the sub-plantar region of right hind paw using a micro syringe. The number of paw licking was monitored 0-5 min (phase-I) and 20-25 min (phase-II) after injection of formalin. Percent inhibition of paw licking was calculated by comparing test group with control group[16].
2.5.3. Tail immersion method
The mice were divided into five groups with five in each group. Control group was intraperitoneally treated with 1% Tween-80 solution (5 mL/kg), test groups received MRCL at doses of 100-150 mg/kg, and standard group received aspirin at a dose of 50 mg/kg. 1-2 cm of the mice tail was immersed in warm water and kept constant at 55 °C. The reaction time was the time taken by the mice to deflect their tails. The first reading was discarded and the reaction time was recorded as a mean of the next three readings. A latent period of 20 sec was defined as complete analgesia and the measurement was then stopped to avoid injury to mice. The latent period of the tail-flick response was determined before and 0, 30, 60 and 90 min after the administration of test drugs.
2.6. Statistical analysis
All observations were presented as mean±SEM. The data were analyzed by one way ANOVA and followed by Newman keuls' test. P< 0.05 was considered as significant.
3. Results
3.1. Preliminary phytochemical test
Preliminary phytochemical test showed the presence of alkaloids, saponins and steroids.
3.2. Acetic acid induced writhing
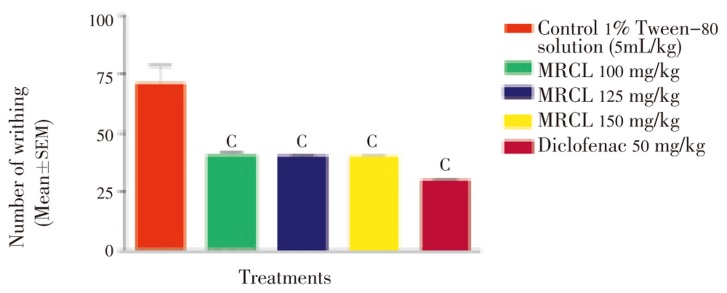
Control group showed maximum writhing (70.200±8.108) while MRCL reduced acetic acid induced writhing significantly (P<0.001) in the test group. MRCL at doses of 100, 125 and 150 mg/kg inhibited writhing in a dose-dependent manner as shown in Figure 1.
Figure 1. Effect of MRCL on acetic acid induced writhing test in mice.
c: P<0.001 when compared with control group.
3.3. Formalin induced paw licking test
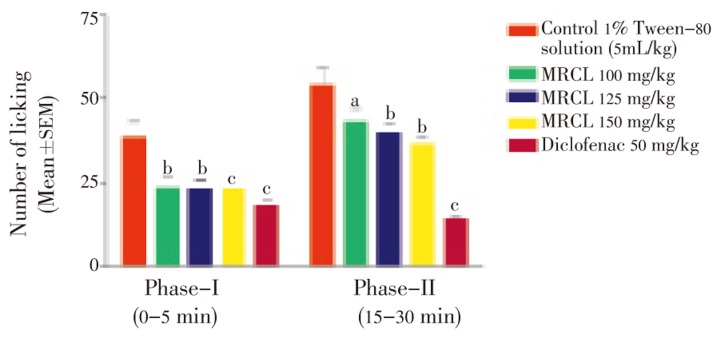
MRCL showed analgesic effect on phase-I and phases-II of formalin induced pain. The phases corresponded to neurogenic and inflammatory pains, respectively. MRCL inhibited significantly neurogenic and inflammatory phase in a dose-dependent manner (Figure 2). MRCL at dose of 150 mg/kg showed significant (P<0.001) inhibition of neurogenic and inflammatory phase comparable to standard drug.
Figure 2. Effect of MRCL on formalin induced paw licking test in mice.
a: P<0.05; b: P<0.01; c: P<0.001 when compared with control group.
3.4. Tail immersion method
MRCL showed significant increase in latent time after 90 min at a dose of 150 mg/kg as shown in Table 1.
Table 1. The effect of MRCL on tail immersion method (Mean±SEM).
| Treatment | Dose mg/kg | Tail flick time in sec |
|||
| After 0 min | After 30 min | After 60 min | After 90 min | ||
| Control | - | 2.900±0.194 | 2.900±0.034 | 2.500±0.030 | 3.040±0.050 |
| MRCL (i.p.) | 100 | 2.330±0.590 | 2.848±0.430 | 3.108±0.190 | 4.240±0.620 |
| 125 | 2.810±2.025 | 2.108±1.800 | 4.600±0.630c | 5.310±1.360 | |
| 150 | 2.640±0.205 | 3.590±1.120 | 5.200±0.060c | 6.30±0.110b | |
| Aspirin (i.p.) | 50 | 2.830±0.060 | 7.170±0.300a | 8.500±0.150c | 10.800±0.210c |
aP<0.05; bP<0.01; cP<0.001 when compared with control group.
4. Discussion
Results of the present study indicated that MRCL possesses significant antinociceptive activity. The acetic acid induced abdominal contraction and the tail immersion methods elucidated peripheral and central activity, while the formalin test investigated both[17]. Drugs that act primarily on the central nervous system inhibit both phases equally while peripherally acting drugs inhibit the late phase[18]. The mechanism of analgesic effect of MRCL could probably be due to inhibition of the effect or release of endogenous substances that induces pain nerve endings similar to that of NSAIDs. In the formalin test, the pain in the early phase was due to the direct stimulation of the sensory nerve fibers by formalin, whereas the pain in the late phase was due to the inflammatory mediators, like histamine, prostaglandin, serotonin, and bradykinin[19]. It is reported that NSAIDs reduce both phases of the formalin test[20]. MRCL inhibits significantly acetic acid induced writhing and formalin induced paw licking in both neurogenic and inflammatory pain in a dose-dependent manner. Tail immersion model is a chronic pain model which is sensitive to centrally acting analgesic agents. MRCL significantly increases latent time after 90 min of drug treatment.
In conclusion, the present study revealed that MRCL possesses antinociceptive activity that may be due to the presence of alkaloids and steroids.
Acknowledgments
The authors are thankful to the Management SVPM's College of Pharmacy, Malegaon (Bk), Baramati, for providing necessary facilities and also to the Professor Deshmukh RB, Head Department of Botany, Shardabai Pawar Mahila Mahavidyalaya, Shardanagar for the authentication of the plant.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Kirtikar KR, Basu BD. Indian medicinal plants. 2nd ed. Dehradun: International Book Distributor; 1985. pp. 2274–2277. [Google Scholar]
- 2.Yanfg LL, Yen KY, Kiso Y, Kikino H. Antihepatotoxic actions of formosan plant drugs. J Ethanopharmacol. 1987;19:103–110. doi: 10.1016/0378-8741(87)90142-5. [DOI] [PubMed] [Google Scholar]
- 3.Visen P, Shukla B, Patnaik G, Tripathi S, Kulshreshtha D, Srimal R, et al. Hepatoprotective activity of Ricinus communis leaves. Int J Pharmacogn. 1992;30:241–250. [Google Scholar]
- 4.Shokeen P, Anand P, Krishna YM, Tandon V. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol. 2008;46:3458–3466. doi: 10.1016/j.fct.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Capasso F, Mascolo N, Izzo AA, Gaginella TS. Dissociation of castor oil induced diarrhoea and intestinal mucosal injury in rat: effect of NG-nitro-l-arginine methyl ester. Br J Pharmacol. 1994;113:1127–1130. doi: 10.1111/j.1476-5381.1994.tb17113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandhyakumary K, Bobby RG, Indira M. Antifertility effects of Ricinus communis Linn. on rats. Phytother Res. 2003;17:508–511. doi: 10.1002/ptr.1308. [DOI] [PubMed] [Google Scholar]
- 7.Ilavarasan R, Mallika M, Venkataraman S. Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract. J Ethnopharmacol. 2006;103:478–480. doi: 10.1016/j.jep.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Singh PP, Chauhan Ambika SMS. Activity guided isolation of antioxidants from the leaves of Ricinus communis L. Food Chem. 2009;114:1069–1072. [Google Scholar]
- 9.Khafagy SM, Mahmoud ZF, Salam NAE. Coumarins and flavanoids of Ricinus communis growing in Egypt. Planta Medica. 1979;37:191. [Google Scholar]
- 10.Kang SS, Cordell A, Soejarto DD, Fong HHS. Alkaloids and flavonoids from Ricinus communis. J Nat Prod. 1985;48:155–156. [Google Scholar]
- 11.Khogali A, Barakat S, Abou-Zeid H. Isolation and identification of the phenolics from Ricinus communis L. Delta J Sci. 1992;16:198–211. [Google Scholar]
- 12.Hall SM, Medlow GC. Identification of IAA in phloem and root pressure saps of Ricinus communis by mass spectrometry. Plant Physiol. 1975;56:177. doi: 10.1007/BF00429049. [DOI] [PubMed] [Google Scholar]
- 13.Khandelwal KR. Practical pharmacognosy technique and experiments. 13th ed. Pune: Nirali Prakashan; 2005. pp. 146–159. [Google Scholar]
- 14.Evans WC. 15th ed. London: Sounders Company Ltd; 2005. Trease and evans' pharmaconosy. [Google Scholar]
- 15.Koster R, Anderson M, Olbur EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:41–44. [Google Scholar]
- 16.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 17.Amresh G, Singh PN, Rao CV. Antinociceptive and antiarthritic activity of Cissampelos pareira roots. J Ethnopharmacol. 2007;111:531–536. doi: 10.1016/j.jep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Chen YF, Tsai HY, Wu TS. Antiinflammatory and analgesic activity from roots of Angelica pubescens. Planta Medica. 1995;61:2–8. doi: 10.1055/s-2006-957987. [DOI] [PubMed] [Google Scholar]
- 19.Murray CW, Porreca F, Cowan A. Methodological refinements in the mouse paw formalin test: an animal model of tonic pain. J Pharmacol Methods. 1988;20:175–186. doi: 10.1016/0160-5402(88)90078-2. [DOI] [PubMed] [Google Scholar]
- 20.Martindale J, Bland-Ward PA, Chessell IP. Inhibition of C-fibre mediated sensory transmission in the rat following intraplantar formalin. Neurosci Lett. 2001;316:33–36. doi: 10.1016/s0304-3940(01)02362-x. [DOI] [PubMed] [Google Scholar]