Abstract
Objective
To evaluate the prevalence of multidrug resistant Staphylococcus aureus (S. aureus) in dairy products.
Methods
Isolation and identification of S. aureus were performed in 3 dairy-based food products. The isolates were tested for their susceptibility to 5 different common antimicrobial drugs.
Results
Of 50 samples examined, 5 (10%) were contaminated with S. aureus. Subsequently, the 5 isolates were subjected to antimicrobial resistance pattern using five antibiotic discs (methicillin, vancomycin, kanamycin, chloramphenicol and tetracycline). Sample 29 showed resistance to methicillin and vancomycin. Sample 18 showed intermediate response to tetracycline. The other samples were susceptible to all the antibiotics tested.
Conclusions
The results provide preliminary data on sources of food contamination which may act as vehicles for the transmission of antimicrobial-resistant Staphylococcus. Therefore, it enables us to develop preventive strategies to avoid the emergence of new strains of resistant S. aureus.
Keywords: Antimicrobial drug resistance, Staphylococcus aureus, Milk, Dairy product
1. Introduction
Staphylococcus aureus (S. aureus) is a versatile pathogen in humans and animals which is responsible for a diverse spectrum of diseases ranging from minor skin infections to life threatening diseases, such as pneumonia and meningitis[1]. It is also known as one of the most important agents of food poisoning globally[2]. It has been believed that all bacterial infections were treatable with a vast array of effective and antimicrobial agents. However, the emergence of resistance to multiple antibiotics among S. aureus has created breaking news for health practitioners and researchers[3]. It has been reported that shortly after the introduction of penicillin in 1940s, resistance developed in S. aureus, followed by resistance to methicillin and more recently to glycopeptides, e.g., vancomycin[4].
S. aureus can be transmitted to humans through contaminated and untreated milk and milk products[5]. S. aureus presents on the skin and mucosae of food producing animal reservoirs that include ruminants and it is frequently associated with sub-clinical or clinical mastitis leading to the contamination of dairy products[8]. This bacterium is considered as the most commonly occurring major pathogen of cow's mammary gland[6].
The objective of this study was to isolate S. aureus from dairy products which include fresh cow milk, pasteurized milk, goat milk, yogurt and cheese, to characterize S. aureus using conventional methods like Gram staining, coagulase test and catalase test and also to determine the prevalence of antimicrobial resistance among S. aureus isolated from dairy products.
2. Materials and methods
2.1. Study population and sample collection
A total of 50 dairy samples (12 fresh cow milk samples, 13 pasteurized milk samples, 13 goat milk samples, 6 yogurt samples and 6 cheese samples) from various places in Kedah, Malaysia, were analyzed from September 2007 to November 2007. The samples were collected at dairy farms, groceries and hypermarkets. The samples were refrigerated at 4 °C before subjected to microbiological analysis.
2.2. Bacteriological examinations
Isolation and identification of S. aureus were performed according to the National Mastitis Council recommendations on examination of quarter-milk samples[7]. Briefly, immediately after delivery, the milk samples were inoculated on blood agar plates (Difco, Detroit, MI) and then on mannitol salt agar (Difco, USA) plates, which were divided into 4 sections. A 10-µL loop was used to streak the milk sample, and 6 to 8 lines were made in one agar section. Samples were incubated for 24 to 72 h at 37 °C and examined for bacterial growth. Pure cultures were further examined for morphological (convex elevation and smooth margin), staining, and cultural characteristics, and for biochemical reactions according to standard keys. Staphylococci were studied in particular for hemolysis and coagulase production. Coagulase testing was performed according to a tube method using oxalated rabbit plasma in a 1:10 dilution in nutrient broth[8]. Only typical colonies identified as S. aureus were stored in cryogenic vials containing 1 mL of trypticase soy broth with 15% glycerin at -80 °C.
2.3. Antibiotic susceptibility testing
Before antibiotic susceptibility testing[9], the isolates were revived by subculturing on blood agar base (Difco) at 35 °C for 24 h. The isolates were tested for their susceptibility to 5 different antimicrobial drugs: methicillin (Met), vancomycin (Van), kanamycin (Kan), chloramphenicol (Chl) and tetracycline (Tet). The antibiotic disks (Oxoid, Amsterdam, The Netherlands) were gently pressed to ensure their contact with the inoculated Mueller-Hinton agar surface, and the plates were incubated at 35 °C. The plates were examined after 18 h and the zones of inhibition were measured to the nearest millimeter. The interpretive breakpoints for resistance were determined according to the standard table provided by the manufacturer of the antibiotic disks, and the isolates were reported as susceptible, intermediate, or resistant. For quality control, S. aureus ATCC 25923 was used as control strain. It should be pointed out that the interpretive breakpoints used were originally developed for human infections.
2.4. Statistical analysis
Analysis of susceptibility testing was by category agreement, where the zone diameters were divided into different categories (susceptible, intermediate, and resistant). Statistical significance of differences in resistance was evaluated using SPSS software (version 12). Univariant analysis was done to verify if the zone of inhibition means were affected by a single antibiotic. Comparison of the zones of inhibition between the samples was done. A P value <0.05 was considered statistically significant.
3. Results
A total of 50 milk samples from various sources were cultured for S. aureus, and (12, 24.0%) of the samples tested were from fresh cow milk samples, (13, 26.0%) from pasteurized milk, (13, 26.0%) from goat milk, (6, 12.0%) from yogurt and (6, 12.0%) from cheese samples. S. aureus was isolated from a total of 5 (10.0%) of the 50 samples.
3.1. Isolation and biochemical characterization of S. aureus
From all the 50 samples, 35 samples had growth on tryptic blood agar (Figure 1a). When those colonies were subcultured, 21 samples had growth on the mannitol salt agar (Figure 1b) which is a selective media for S. aureus. When those colonies were subjected to biochemical characterization which involves Gram staining, hemolysis coagulase and catalase test, only 5 samples showed positive result for all the three tests. The samples that were positive for S. aureus included 4 fresh cow milks and 1 pasteurized milk.
Figure 1. S. aureus culture.
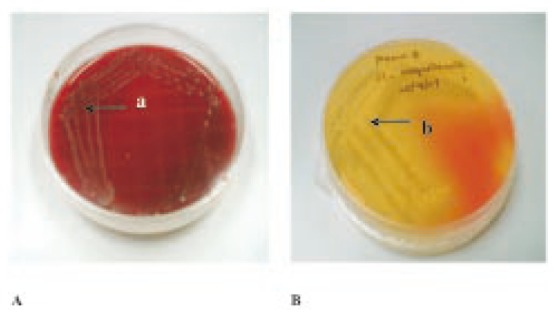
a: Golden yellow colonies of S. aureus; b: Mannitol fermentation caused by S. aureus.
3.2. Susceptibility of S. aureus isolates
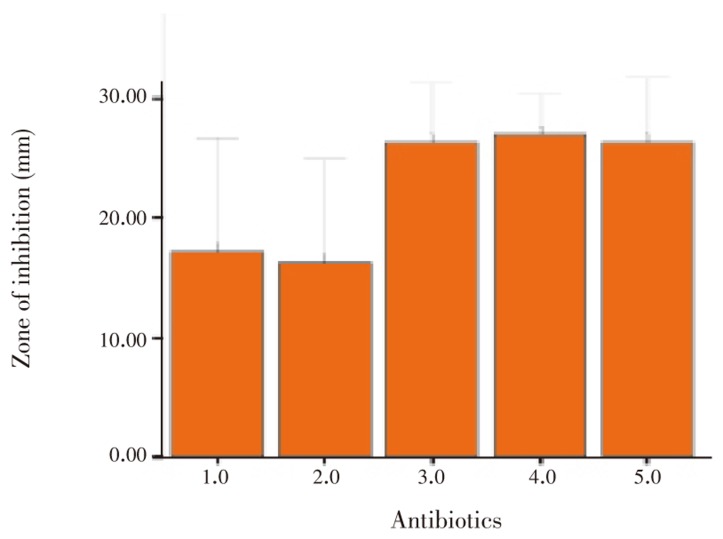
Overall, 6.0% of the S. aureus isolates were susceptible to all of the antimicrobial agents tested (Table 1). All the S. aureus isolates from sample 2, 10 and 14 were susceptible to methicillin, vancomycin, kanamycin, chloramphenicol and tetracycline. Of the 5 S. aureus isolates, isolate from sample 29 was resistant to 2 antimicrobials drugs (methicillin and vancomycin) and isolate from sample 18 showed intermediate response to tetracycline (Table 1 and Figure 2).
Table 1. Susceptibility (resistant, intermediate, and susceptible) of S. aureus isolates from dairy products samples.
| Sample | Zones of inhibition (mm) |
||||
| ME | VA | K | C | TE | |
| 2 | 23.7 | 19.7 | 30.7 | 27.3 | 31.3 |
| (S) | (S) | (S) | (S) | (S) | |
| 10 | 25.3 | 21.7 | 22.0 | 23.3 | 23.7 |
| (S) | (S) | (S) | (S) | (S) | |
| 14 | 16.3 | 20.0 | 29.0 | 29.7 | 30.7 |
| (S) | (S) | (S) | (S) | (S) | |
| 18 | 20.7 | 20.3 | 19.3 | 23.0 | 17.3 |
| (S) | (S) | (S) | (S) | (I) | |
| 29 | - | - | 30.7 | 31.3 | 28.3 |
| (R) | (R) | (S) | (S) | (S) | |
ME: Methicillin; VA: Vancomycin; K: Kanamycin; C: Chloramphenicol; TE: Tetracycline: R: Resistant; I: Intermediate; S: Susceptible.
Figure 2. Susceptibility of S. aureus against various antibiotics.
ME: Methicillin; VA: Vancomycin; K: Kanamycin; C: Chloramphenicol; TE: Tetracycline.
4. Discussion
Food-borne diseases are an important public health problem as it not only affects human health, but also has a significant impact on economic and trade issues. The global changes affecting population growth, lifestyle, international food trade, food production and processing, agricultural and animal husbandry practices and antimicrobial resistance, have posed a threat to the emergence of food borne diseases. Food-borne diseases, especially dairy products infections, are not limited to the third world countries. Even in developed countries it has been reported that around 2%-6% of the bacterial outbreaks, in which the food vehicle is known, were related to milk and dairy products[10].
Improper food handling and unhygienic practices among food handlers during production, processing and distribution, have contributed to food poisoning episodes[11]. In Malaysia, the incidence of notifiable food-borne diseases, namely, cholera, typhoid, food poisoning, hepatitis A and dysentery, is less than 5/100 000 population sporadic in nature, and outbreaks are confined to certain areas only[12]. Hence this study was conducted to isolate S. aureus and determine the prevalence of antimicrobial resistance among the isolates from the local dairy products.
S. aureus exhibits some problematic features which are not found in other relevant bacteria. This bacterium is capable of expressing a variety of virulence factors and thus is considered medically relevant when encountered in dairy products. S. aureus continues to demonstrate the ability to develop and expand resistance to a broad array of antimicrobial classes[13]. It is a prominent pathogen in both the hospital and the community settings.
With respect to the 5 dairy products studied, it showed that contamination occurs in fresh cow milk and pasteurized milk. Milking operations, including storage, handling and transportation, are considered as critical points that contaminate milk products. Susceptible populations of bacteria may become resistant to antimicrobial agents through mutation and selection or by the acquisition of new genetic material from other resistant organisms through transformation, transduction and conjugation. In sample number 29, S. aureus was resistant to multiple classes of antibiotics (methicillin and vancomycin), which can cause serious health problems[14]. The fact that resistance is high in environmental isolates is mainly because antimicrobials are frequently prescribed by veterinarians as treatment for gram-negative bacterial infections on farms[15]. Thus, the indiscriminate use of those antimicrobial agents might account, at least in part, for such a high resistance[15]. However, the assessment of cheeses and yogurt indicated that they do not contain satisfactory levels of S. aureus at the time of consumption. Staphylococcal food poisoning due to cheese and yogurt has not been reported to any great extent to the authorities[16].
This research provides some important preliminary data about the contamination status of dairy-based food products in Kedah of Malaysia and the patterns of resistance of S. aureus isolates towards commonly used antimicrobials. The presence of multi-drug resistant strains is alarming, because such strains are considered a serious danger to public health. Additional research is required to better understand the ecology and evolution of bacterial resistance to antimicrobial agents in the environment as a whole.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Jangra P, Singh A. Staphylococcus aureus β-hemolysin-neutralizing single-domain antibody isolated from phage display library of Indian desert camel. Asian Pac J Trop Med. 2010;3(1):1–7. [Google Scholar]
- 2.Genigeorgis CA. Present state of knowledge on Staphylococcal intoxication. Int J Food Microbiol. 1989;9:327–360. doi: 10.1016/0168-1605(89)90100-1. [DOI] [PubMed] [Google Scholar]
- 3.Hossein M, Hadis M, Tahere S. Determining of antibiotic resistance profile in Staphylococcus aureus isolates. Asian Pac J Trop Med. 2010;3(9):734–737. [Google Scholar]
- 4.Monroe S, Polk R. Antimicrobial use and bacterial resistance. Curr Opin Microbiol. 2000;3:496–501. doi: 10.1016/s1369-5274(00)00129-6. [DOI] [PubMed] [Google Scholar]
- 5.Seifu E, Buys EM, Donkin EF, Petzer IM. Antibacterial activity of the lactoperoxidase system against food-borne pathogens in Saanen and South African indigenous goat milk. Food Control. 2004;15:447–452. [Google Scholar]
- 6.Chaffer M, Leitner G, Winkler M, Glickman A, Krifucks O, Ezra E, et al. Coagulase-negative Staphylococci and mammary gland infections in cows. Vet Med. 1999;10:707–712. doi: 10.1046/j.1439-0450.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 7.National Mastitis Council . Laboratory handbook on bovine mastitis. Madison: National Mastitis Council Inc; 1992. [Google Scholar]
- 8.Arbeit D. Laboratory procedures for the epidemiological analysis of Staphylococci. In: Archer G, Crossley T, editors. Staphylococci and Staphylococci Diseases. New York: Churchill Livingstone; 1988. pp. 203–286. [Google Scholar]
- 9.NCCLS . Performance standards for antimicrobial susceptibility testing. Pennsylvania: NCCLS; 2002. pp. M100–S12. [Google Scholar]
- 10.De Buyser ML, Dufour B, Maire M, Lafarge V. Implication of milk and milk products in food-borne diseases in France and in different industrialized countries. Int J Food Microbiol. 2001;67:1–17. doi: 10.1016/s0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 11.Angelillo IF, Viggiani NMA, Rizzo L, Bianco A. Food handlers and foodborne diseases: knowledge attitudes and reported behavior in Italy. J Food Prot. 2000;3:381–385. doi: 10.4315/0362-028x-63.3.381. [DOI] [PubMed] [Google Scholar]
- 12.Morteza SM, Susan M, Esmaeil D, Hossein M, Seyyed MSN. Antibacterial activity of eight Iranian plant extracts against methicillin and cefixime restistant Staphylococcous aureus strains. Asian Pac J Trop Med. 2010;3(4):262–265. [Google Scholar]
- 13.Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:3–10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Zouhairi O, Saleh I, Alwan N, Toufeili I, Barbour E, Harakeh S. Antimicrobial resistance of Staphylococcus species isolated from Lebanese dairy-based products. East Mediterr Health J. 2010;12:1221–1225. doi: 10.26719/2010.16.12.1221. [DOI] [PubMed] [Google Scholar]
- 16.Lindqvist R, Andersson Y, Lindbäck J, Wegscheider M, Eriksson Y, Tidestrim L, et al. A one year study of foodborne illnesses in the municipality of Uppsala, Sweden. Emerg Infect Dis. 2001;7:588–592. doi: 10.3201/eid0707.010742. [DOI] [PMC free article] [PubMed] [Google Scholar]