Abstract
Objective
To investigate the toxicity of methanol extract of various parts (Root, Stem, Leaf, Flower and Fruit) of Lantana camara (L. Camara) in Artemia salina.
Methods
The methanol extracts of L. camara were tested for in vivo brine shrimp lethality assay.
Results
All the tested extract exhibited very low toxicity on brine shrimp larva. The results showed that the root extract was the most toxic part of L. camara and may have potential as anticancer agent.
Conclusions
Methanolic extract of L. camara is relatively safe on short-term exposure.
Keywords: Lantana camara, Artemia salina, Toxicity
1. Introduction
Folk healers in Asia and South America have used lantana species including Lantana camara(L. camara) for centuries to treat various human ailments such as dermatological and gastrointestinal diseases, tetanus, malaria and tumors[1]. Traditional healers have used lantana species for centuries to treat various diseases. Different parts of L. camara is used for the treatment of various human ailments such as itches, cuts, ulcers, swellings, bilious fever, catarrh, eczema, tetanus, malaria, tumors and rheumatism[2]. Considering both the ethnobotanical and pharmacological applications of the plant, the aim of this study was to investigate the possible toxic effects of the leaf extract of L. camara agaist Artemia salina.
Estimation of toxicity of a natural product or other compounds usually passes through three levels of observations. First level includes the test of the alterations in morphology, cell growth and metabolism using normal human or animal cell lines. At the second level, lower animals such as fishes and Artemia salina are exploited for close monitoring of toxic changes in eggs or whole body. Brine shrimp Artemia salina, also known as sea monkey, is a marine invertebrate about 1 mm in size[3]. It is used as a “benchtop bioassay” for the discovery and purification of bioactive natural products and is an excellent choice for elementary toxicity investigations of consumer products. The shrimp lethality assay is based on the ability to kill laboratory-cultured Artemia nauplii (animal's eggs)[4].
2. Material and methods
2.1. Plant samples
Different parts of L. camara were collected from Amanjaya, Kedah, Malaysia, on February 2008. The identity of plant was confirmed by Dr. S. Sudhakaran, associate professor in faculty of applied sciences, AIMST University, Kedah, Malaysia. A voucher with number 11008 was deposited in the herbarium of Biology School, Universiti Sains Malaysia, Penang, Malaysia.
2.2. Extraction procedure
In the laboratory, the different parts of L. camara sample were washed with freshwater and brushed with a soft brush before drying. Clean plant material was transferred to oven (ECOCELL) in 50 °C temperature for 96 h for drying. Then they were powdered by electric blender. Approximately 100 g of different parts of L. camara powder was added to 400 mL methanol and soaked for 4 days. Removal of the plant material from solvents was done by filtration through cheesecloth, and the filtrate was concentrated using a rotary evaporator.
2.3. Hatching shrimp
Brine shrimp eggs, Artemia salina were hatched in artificial seawater which was prepared by dissolving 38 g sodium chloride in 1 liter distilled water. After 36-h incubation at room temperature (22-29 °C) under a light source, the larva (nauplii) were separated from shells and unhatched eggs by siphoning with a plastic tube.
2.4. Brine shrimp assay
Bioactivity of the L. camara extracts was measured by the brine shrimp lethality test[5]. Samples were dissolved in DMSO and diluted with artificial seawater (concentration 100 µg/mL). Two mL artificial seawater was placed in all the universal bottles. A two-fold dilution was carried out to obtain a concentration of 50-0.195 mg/mL. The last bottle was left with salt water and DMSO to serve as the drug free control. Vitamin C and chloramphenicol were used as reference drugs. Hundred microliters of suspension of nauplii containing about 10-15 larva was added into each bottle and incubated at room temperature. After 6 h and 24 h, the number of dead nauplii and the total number in each bottle was counted. The experiment was done in triplicate.
2.5. Data Analysis
To ensure that the mortality is attributed to bioactive compounds and not to starvation or DMSO effect, the dead larva in each treatment was compared with the dead larva in the control. The percentage of mortality (% M) was calculated as:
% M = percentage of survival in the control - percentage of survival in the treatment.
The best equation of fit curve was obtained by preliminarily comparison of R squares achieved by linear and nonlinear regression analysis by SPSS 16.0.0 program (SPSS Inc. TEAM EQX). Lethality concentration fifty(LC50) values were determined at 70% confidence intervals. Following one way ANOVA, the results were evaluated by Spearman's rho to find out the potential correlation between observations at different times.
3. Results
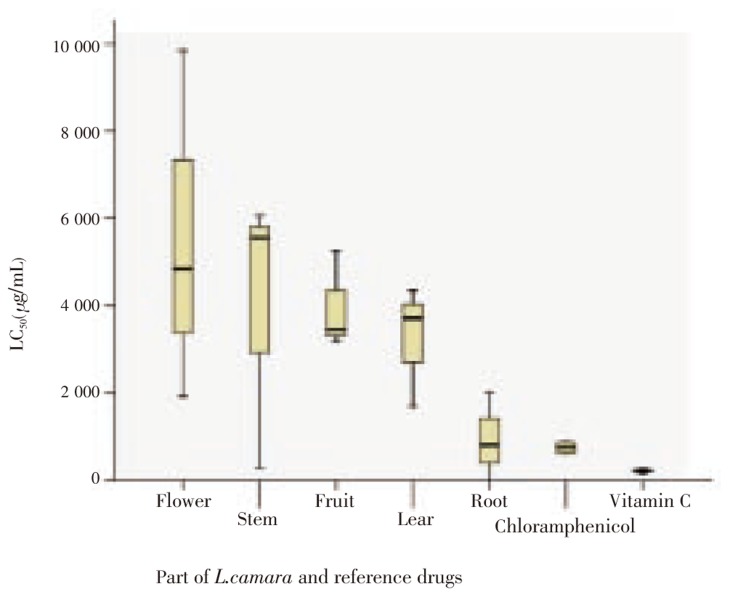
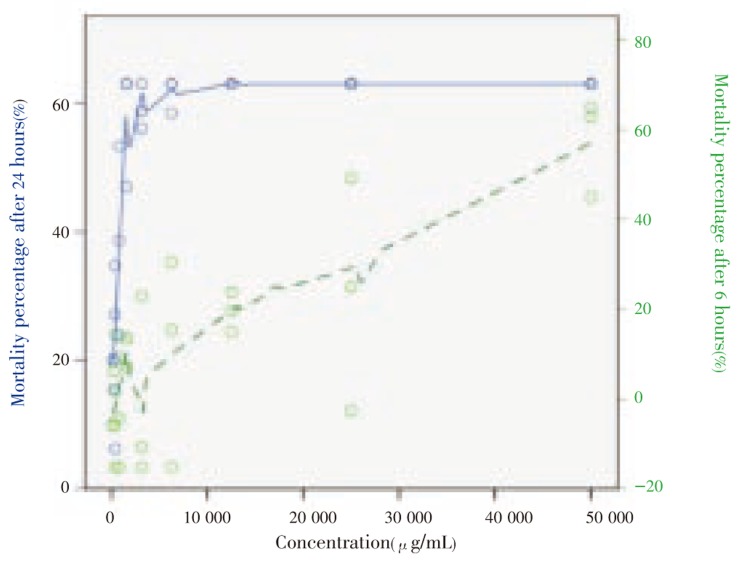
The LC50 values of different extracts of L. camara and reference drugs with by 70% confidence intervals are shown in Table 1. The results showed that root extract was the most toxic part followed by leaves, flower, fruits and stem in descending order. Figure 1 summarized LC50 values in boxplot bars. Effects of exposure time on mortality percentage were depicted in Figure 2. After exposure to the extract for 6 h, LC50 of leaf was 6 0419.1 µg/mL(70% CI=7 281.2-113 557.0), and the profile had a sharp rise in mortality at lower concentrations and a slowly increase at higher doses, while after exposure for 24 h the profile showed a gradual increase of mortality at lower concentrations and become constant at 5 000 µg/mL.
Table 1. LC50 values for different parts of L. camara and reference drugs (Vitamin C and chloramphenicol) based on brine shrimp lethality assay.
| Part of L. camara and reference drugs | LC50 after 24 h (µg/mL) | 70% CI after 24 h |
| Root | 940.7 | 128.8 - 1 752.5 |
| Stem | 3 966.0 | 1 402.9 - 6 529.0 |
| Leaf | 3 251.8 | 2 142.1 - 4 361.5 |
| Flower | 5 536.6 | 2 326.5 - 8 746.7 |
| Fruit | 3 964.7 | 3 057.8 - 4 871.6 |
| Vitamin C | 221.7 | 175.2 - 268.2 |
| Chloramphenicol | 748.1 | 495.9 - 1 000.3 |
Figure 1. LC50 boxplot for different parts of L. camara, vitamin C and chloramphenicol tested by brine shrimp lethality assay.
Figure 2. Mortality percentages after treatment of leaf extract of L. camara at different concentrations.
4. Discussion
The brine shrimp lethality test is used to evaluate different pharmacological activities of natural remedies, taking into account the basic premise that pharmacology is simply toxicology at a lower dose. Hence in this study we used the test to study the toxicity of various part of L. camara extract. Although the toxicities of root extract, vitamin C and chloramphenicol are higher than other extracts in the experiment, based on one way ANOVA analysis, the differences in LC50 among various parts of L. camara and also two reference drug are not significant (P>0.05). But if 100 µg/mL is regarded as an approximate border line for toxicity[6] all the tested compounds and extract exhibit very low toxicity on brine shrimp larva.
Some other toxicological researches using brine shrimp bioassay confirm that the lethality of L. camara extracts of various parts are considerably lower than studied plants. Adoum[7] reported that Sclerocarya birrea, Momordica charantia, Boerhaavia diffusa and Nauclea aculeata extracts have exhibited potent activity at LC50 values <60µg/mL. Moshi[8] found that the intermediate and polar extracts of Terminalia sericea roots were toxic to brine shrimps with LC50 values ranging from 3.5-26.5µg/mL, while that of cyclophosphamide, a standard anticancer drug, was 10.6-25.2 µg/mL.
The range of LC50 value for leaf extract is more than 2 000 µg/mL (confidence interval, 2 142.1-4 361.5 µg/mL) that is much higher than vitamin C (less than 300 µg/mL) and chloramphenicol (equal or less than 1 000 µg/mL). This is a privilege for leaf extract over mentioned reference drugs regarding antioxidant and antimicrobial activities. Spearman's rho statistical test shows a weak bivariate correlation between mortality percentage after 6 h and 24 h of treating brine shrimp larva with leaf extract (Correlation coefficient = 0.432). Figure 2 also illustrates that while the relation between increasing leaf extract concentration and mortality percentage is more linear in the acute lethality observation (after 6 h treatment), it's completely logarithmic in the lag lethality observation (after 24 h treatment). It indicates that the lethality effect of leaf extract is time dependent, increasing significantly after 24 h exposure. At this time nauplii are mainly in instar II/III[4].
From a pharmacological point, a good relationship has been found with the brine shrimp lethality test to detect antitumoral compounds in terrestrial plant extracts[5], [9], [10]. Then, root extract as the most toxic part of L. camara is more potential to anticancer phytochemicals. However, further toxicity studies are needed to determine the effects of this extract on chronic oral toxicity, animal foetus, pregnant animals, and their reproductive capacity, and to complete the safety profile of this extract since the L. camara possesses various useful biological activities as described in introduction.
Footnotes
Foundation Project: Supported by a grant form Universiti Sains Malaysia University.
Conflict of interest statement: We delcare that we have no conflict of interest.
References
- 1.Badakhshan MP, Sasidharan S, Rameshwar NJ, Ramanathan S. Comparative study: antimicrobial activity of methanol extracts of Lantana camara various parts. Pharmacogn Res. 2009;1:348–351. [Google Scholar]
- 2.Abou-Karam M, Shier WT. A simplified plaque reduction assay for antiviral agents from plants. Demonstration of frequent occurrence of antiviral activity in higher plants. J Nat Prod. 1990;53:340–344. doi: 10.1021/np50068a011. [DOI] [PubMed] [Google Scholar]
- 3.Moshafi MH, Sharififar F, Dehghan GR, Ameri A. Bioassay screening of the essential oil and various extracts of fruits of Heracleum persicum desf. and rhizomes of Zingiber officinale rosc. using brine shrimp cytotoxicity assay. Iran J Pharmaceutical Res. 2009;8:59–63. [Google Scholar]
- 4.Carballo JL, Hernández-Inda ZL, Pérez P, García-Grávalos MD. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002;2(17) doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Plant med. 1982;45:31–34. [PubMed] [Google Scholar]
- 6.Mbwambo ZH, Moshi MJ, Masimba PJ, Kapingu MC, Nondo RSO. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement Altern Med. 2007;7(9 ) doi: 10.1186/1472-6882-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adoum OA. Determination of toxicity effects of some savannah plants using brine shrimp test (BST) Int Jor P App Scs. 2008;2(3):1–5. [Google Scholar]
- 8.Moshi MJ, Mbwambo ZH. Some pharmacological properties of extracts of Terminalia sericea roots. J Ethnopharmacol. 2005;97(1):43–47. doi: 10.1016/j.jep.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 9.Solís PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD. A microwell cytotoxicity assay using Artemia salina. Plant Med. 1993;59:250–252. doi: 10.1055/s-2006-959661. [DOI] [PubMed] [Google Scholar]
- 10.Mackeen MM, Ali AM, Lajis NH, Kawazu K, Hassan Z, Amran M, et al. Antimicrobial, antioxidant, antitumour-promoting and cytotoxic activities of different plant part extracts of Garcinia atroviridis Griff. Ex T. Anders. J Ethnopharmacol. 2000;72:395–402. doi: 10.1016/s0378-8741(00)00245-2. [DOI] [PubMed] [Google Scholar]
- 11.Aiyalu R, Ramasamy A, Shanmugasundaram M. Evaluation of antipyretic activity of ethyl acetate extract of Adenema hyssopifolium G. Don in a rat model. Asia Pac J Trop Med. 2010;3(7):523–526. [Google Scholar]
- 12.Diganta P, Ashok KB, Bhilegaonkar KN. Characterization of toxin from Verocytotoxigenic Ecscherichia coli(VTEC) strains isolated from neonatal calves in India. Asia Pac J Trop Med. 2009;2(2):35–38. [Google Scholar]
- 13.Nwodo NJ, Omeje EO, Brun R. In vitro in vivo studies on anti-trypanosomal potentials of Zapoteca portoricensis. Asia Pac J Trop Med. 2009;2(1):25–29. [Google Scholar]
- 14.Oyero OG, Oyefolu AOB. Fungal contamination of crude herbal remedies as a possible source of mycotoxin exposure in man. Asia Pac J Trop Med. 2009;2(5):38–43. [Google Scholar]