Abstract
Objective
To investigate the cytotoxic activity of endophytic fungi isolated from mangrove fungi.
Methods
In the present study the DNA was isolated and the ITS region of 5.8s rRNA was amplified using specific primers ITS 1 and ITS4 and sequence was determined using automated sequencers. Blast search sequence similarity was found against the existing non redundant nucleotide sequence database thus, identified as Aspergilus flavus, Hyporcaea lixii, Aspergillus niger, Eutorium amstelodami, Irpex hydnoides and Neurospora crassa. Among the seven isolates, one fungi Irpex hydnoides was selected for further studies. The fungi were grown in sabouraud broth for five days and filtrate were separated and subjected to ethyl acetate for further studies.
Results
Nearly half (49.25%) of the extracts showed activity (IC50 of 125µg/mL). These values were within the cutoff point of the National Cancer Institute criteria for cytotoxicity (IC50<20 µg/mL) in the screening of crude plant extracts. The GC MS analysis revealed that the active principals might be Tetradecane (6.26%) with the RT 8.606.
Conclusions
It is clear from the present study that mangrove fungi with bioactive metabolites can be expected to provide high quality biological material for high throughout biochemical, anti cancer screening programmes. The results help us conclude that the potential of using metabolic engineering and post genomic approaches to isolate more novel bioactive compounds and to make their possible commercial application is not far off.
Keywords: Mangrove fungi, Cytotoxic activity, ITS sequencing, GC MS , Irpex hynoides, Characterization, Cytotoxic compound, Endophytic fungi, Mangrove, Bioactive metabolite
1. Introduction
Endophytes include an assemblage of microorganisms with different life history strategies: those that, following an endophytic growth phase, grow saprophytically on dead or senescing tissue, avirulent microorganisms, incidentals, but also latent pathogens and virulent pathogens at early stages of infection[1]. Their biological diversity, especially in temperate and tropical rainforests, is large and noticeable. Each plant species may be host to a number of endophytes[2]. Scientific studies have investigated the anticancer activity of several anthracenedione derivatives, which was separated from the secondary metabolites of the mangrove endophytic fungus Halorosellinia sp. (No. 1403) and Guignardia sp. (No. 4382)[3].
To date, endophytes have been most extensively studied for their ability to produce antibacterial, antiviral, anticancer, antioxidants, antidiabetic and immunosuppressive compounds. Cancer is a leading cause of death worldwide[4],[5]. As many anticancer drugs cannot discriminate cancer cells from non-cancer cells, many normal cells are also killed during the process of chemotherapy. Developing new anticancer drugs with a higher potency and specificity against cancer cells has therefore become an important goal in biomedical research and concern for the medical fraternity.
Their study is expected to become an important component in the production of new natural bioactive products. The current study was undertaken to investigate this biodiversity and to isolate and screen endophytic fungi with cytotoxic activities.
2. Materials and methods
2.1. Isolation of endophytic fungi
The leaves were washed with sterile seawater and grinded using distilled water and seawater in 1:1 ratio in a mortar and pestle under aseptic conditions. 1 mL of the above was mixed with 10 mL of sterile water (distilled water: seawater; 1:1) to get dilution 10−1 aseptically. The serial dilution was repeated till 10−6. From each dilution plating was done in sabouraud's agar by spread plate technique. The plates were then incubated at 27 °C for 5 days. After 5 days, the plates were examined and the pure culture was isolated on pure agar plate.
2.2. Preparation of extracts
The pure culture isolated by the above method was grown in sabouraud's dextrose broth. The flasks were incubated in the shaker-incubator at 200 rpm for 5 days. Then the mycelium and the filtrate were separately subjected to solvent extraction as follows:
The fresh mycelium of each fungus was washed three times with water (distilled water: sea water 1:1) to remove adherent filtrate, and then plotted between folds of whattman filter paper no 1. The plotted mycelium was crushed using mortar and pestle with ethyl acetate and methanol and subjected to sonication (Sartorious Labsonic) for 3-4 h to obtain intracellular metabolites. Centrifuged at 2 000-2 500 rpm for 5 min and the supernatant were used for further studies. The filtrate of each fungus was extracted several times with ethyl acetate (v/v) in a separating funnel.
The ethyl acetate extracts from both mycelia and filtrate were evaporated under vaccum at 50 °C till dryness. The obtained solid material was dissolved in ethyl acetate to form the crude extract and tested for bioassays.
2.3. Cytotoxic activity (MTT assay)
Cytotoxicity of extracts at various concentrations (15-1 000 µg/mL ) was assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (Sigma) assay[12] but with minor modification, following 72 h of incubation. Assay plates were read using a spectrophotometer at 520 nm. Data generated were used to plot a dose-response curve of which the concentration of extract required to kill 50% of cell population (IC50) was determined.
2.4. GC Ms analysis
The crude extract exhibiting activity was subjected to GC-MS equipped with Agilent 5975 inert XL MSD to find out the active principle of the extracts.
2.5. Fungal isolation, identification
The total deoxyribonucleic acid (DNA) of marine-derived fungus GIBH-Mf082 was extracted using the EZNA kit (Omega). The internal transcribed spacers (ITS) of ribosomal DNA (rDNA) were amplified employing the combination of a conserved forward primer ITS1 (50- TCCGTAGGTGAACCTGCGG-30) and reverse primer ITS4 (50- TCCTCCGCTTA TTGATATGC-30). The polymerase chain reaction product is about 0.7 kb. The purified ITS rDNA was sequenced. The sequence data have been submitted to GenBank with an accession number. The sequences were aligned manually using CLUSTAL X version 1.8 with sequences of representative strains retrieved from the DNA Data Bank of Japan/European Molecular Biology Laboratory/GenBank databases.
3. Results
Mangroves and their associates are a source of novel medicines, agrochemicals and many biologically active compounds[5]. However, investigations on mangrove endophytic fungi metabolisms are scarce. In the present investigation seven fungal strains were isolated from the leaves of Rhizophora mucronata and Avicenna officinalis. In the present study the DNA was isolated and the ITS region of 5.8s rRNA was amplified using specific primers ITS1 and ITS4 and sequence was determined using automated sequencers. Blast search sequence similarity was found against the existing non redundant nucleotide sequence database thus, identified as Aspergilus flavus, Trichoderma hypericum, Hyporcaea lixii, Aspergillus niger, Eutorium amstelodami, Irpex hydnoides and Neurospora crassa. Among the seven isolates, one fungi Irpex hydnoides was selected for further studies. The fungi were grown in sabouraud broth for five days and filtrate were separated and subjected to ethyl acetate for further studies.
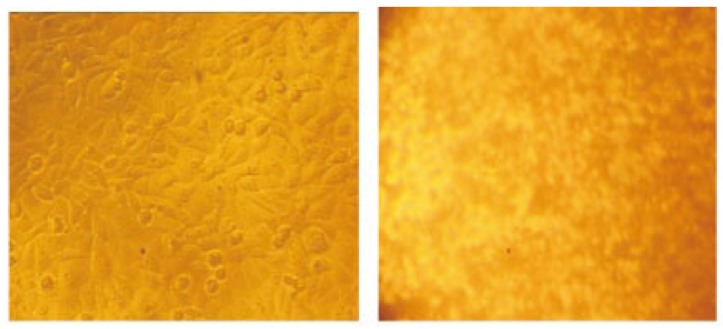
Cytotoxicity of the extracts against Hep2 cell lines is shown in Table 1. Generally, the extracts were found to be more effective against this cell line. Nearly half (49.25%) of the extracts showed activity (IC50 of 125 µg/mL). These values were within the cutoff point of the National Cancer Institute criteria for cytotoxicity (IC50 < 20 µg/mL) in the screening of crude plant extracts (Figure 1). The GC MS analysis revealed that the active principals might be tetradecane (6.26%) with the RT 8.606 (Figure 2).
Table 1. Cytotoxicity checked against Hep2 cell line.
| Concentration (µg/mL) | Dilutions | Absorbance | Cell viability |
| 1 000 | Neat | 0.11 | 21.15 |
| 500 | 1:1 | 0.19 | 36.53 |
| 250 | 1:2 | 0.23 | 44.23 |
| 125 | 1:4 | 0.31 | 59.61 |
| 62.5 | 1:8 | 0.39 | 75.00 |
| 31.25 | 1:16 | 0.48 | 92.30 |
| 15.62 | 1:32 | 0.50 | 96.15 |
| Cell control | - | 0.52 | 100.00 |
Figure 1. Hep 2 cell line treated with crude extract.
Figure 2. GC MS analysis spectrum.
The ITS region is now perhaps the most widely sequenced DNA region in fungi. It has typically been most useful for molecular systematics at the species level, and even within species (e.g., to identify geographic races). The percentage of similarity between the fungi and database suggests it as novel strain. Thus, the novel strain was named as Irpex hydnoides VB4 strain and made publically available in GenBank with an assigned accession number HQ271348.
4. Discussion
Marine fungi have been reported to harbour potential antitumor value. As an evidence to the previous statement, a new Topo isomerase inhibitor, (+)-3, 3′,7,7′,8,8′-hexahydroxy-5,5′dimethylbianthraquinone (2240A) was isolated from mangrove endophytic fungus no: 2240[6]. Thus endophytic fungal strains displaying both topoisomerase I inhibitory and antitumour activity together may indicate their potential ability of producing antitumour compounds targeting at topoisomerase I[7]. Antitumor and cytotoxic compounds from marine fungi are summarized by Mayer and Gustafson[8]. Cytotoxicity assays are a widely used method in in vitro toxicology studies. The antimicrobial potential of 71 endophytic fungi isolated from mangrove plants towards selected bacteria (Bacillus subtilis, Pseudomonas. aeruginosa, Escherichia coli and Staphylococcus aureus) was tested using ethyl acetate extracts of fungi cultivated under static conditions[9]. Moreover three metabolites named phomopsin A, B and C together with two compounds cytosporone B and C were isolated from the mangrove endophytic fungus Phomopsis sp. ZSU H76 from the South China Sea. Cytosporone B and C inhibited the growth of two fungi Candida albicans and Fusarium oxysporum with an MIC ranging from 32 to 64 microg/mL[10]. The structure activity analysis of secondary metabolites of mangrove fungi indicated that the hydroxyl group was important for their cytotoxic activity and that bulky functional groups such as phenyl rings could result in a loss of biological activity, which will direct further development of marine product based derivatives[11]. Thus mangrove fungi possess active metabolites with some novel chemical structures that belong to diverse chemical classes such as alkaloids, phenol, steroids, terpenoids, tannins, etc. Also two compounds namely Sch54796[1] and Sch54794[2] isolated from the metabolites of a marine mangrove fungus(Penicillium sp.No.2556) from the fermentation liquid remarkably inhibited the growth of cancer cell lines hep2 and hepG2[12]. Apart from the variety of bioactive compounds, mangrove endophytic fungi are also known to produce anthraquinones, eniatin G and xyloketals[13].
Emergence of antibiotic resistance among pathogenic microorganisms limits treatment options. Antibiotic resistance genes, in addition to clinical pathogens are also present in environmental isolates, which are horizontally transferred to other microorganisms[14]. Hence natural products and their analogs or molecules derived thereof comprise approximately 50% of the effective drugs presently used for clinical purposes[15]. It is estimated that there may be as many as 1 million different endophyte species; however, only handful of them are isolated and studied[16]. Of late the FDA (Food and Drug Administration) has approved Taxol from endophyte Taxomyces andreanae for the treatment of advanced breast cancer, lung cancer, and refractory ovarian cancer[17]. Anticancer activity of 14 anthracenedione derivatives separated from the secondary metabolites of the mangrove endophytic fungi Halorosellinia sp. (No. 1403) and Guignardia sp. (No. 4382) that inhibited potently the growth of KB and KBv200 cells is also reported[18].
Problems related to human health such as the development of drug resistance in human pathogenic bacteria, fungal infections, and life threatening virus claim for new therapeutic agents for effective treatment of diseases in human, plants, and animals that are currently unmet[19].
In the present study, the crude extracts of fungi were made with only ethyl acetate ruling out a comparative analysis. Different species of marine fungi may show different degrees of sensitivity against various cancer cell lines. Thus, it should be useful if future cytotoxic tests these of marine fungi are carried out on other cancer cell lines.
In conclusion, it is believed that a rich source of anticancer drug candidates could be obtained from marine organisms or their metabolites. This preliminary screening of fungal endophytes revealed their potential to yield potent bioactive compounds for drug discovery programmes. Extract showed very potent cytotoxic effect indicating its possible potential for development as an anti-cancer drug and warrants further scientific investigation.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Suriyanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Sasse F, Jansen R, Murali TS. Fungal endophytes and bioprospecting. Fungal Biol Rev. 2009;23:9–19. [Google Scholar]
- 2.Strobel GA. Endophytes as sources of bioactive products. Microbes Infect. 2003;5:535–544. doi: 10.1016/s1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang JY, Tao LY, Liang YJ, Chen LM, Mi LM, Zheng LS, et al. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Mar Drugs. 2010;8(4):1469–1481. doi: 10.3390/md8041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian HL, Luo H, Chang DC, Luo KQ. A high throughput drug screen based on fluorescence resonance energy transfer. Br J Pharmacol. 2007;3:321–334. doi: 10.1038/sj.bjp.0706988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patra JK, Thatoi HN. Metabolic diversity and bioactivity screening of mangrove plants:A review. Acta Physiologiae Plantarum. 2010;11:1–11. doi: 10.1007/s11738-010-0667-7. [DOI] [Google Scholar]
- 6.Tan N, Cai XL, Wang SY, Pan JH, Tao YW, She ZG, et al. A new hTopo I isomerase inhibitor produced by a mangrove endophytic fungus no:2240. J Asian Nat Prod Res. 2008;10:607–610. doi: 10.1080/10286020802133167. [DOI] [PubMed] [Google Scholar]
- 7.Cai X, Liang X, Zhou S, Gao J, Wu S, Lai X, et al. Cytotoxic and topoisomerase I inhibitory activities from extracts of endophytic fungi isolated from mangrove plants in Zhuhai, China. J Ecol Nat Environment. 2010;2(2):17–24. [Google Scholar]
- 8.Mayer AMS, Gustafson KR. Marine pharmacology in 2003-2004: Anti- tumour and cytotoxic compounds. Eur J Cancer. 2006;42:2241–2270. doi: 10.1016/j.ejca.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Sukanyanee C, Jittra P, Anthony JS, Whalley, Prakitsin S. Endophytic fungi from mangrove plant species of Thailand: their antimicrobial and anticancer potentials. Botanica Marina. 2010;53:555–564. [Google Scholar]
- 10.Huang Z, Cai X, Shao C, She Z, Xia X, Chen Y, et al. Chemistry and weak antimicrobial activities of phomopsins produced by mangrove endophytic fungus Phomopsis sp. ZSU-H76. Phytochemistry. 2008;69(7):1604–1608. doi: 10.1016/j.phytochem.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JY, Tao LY, Liang YJ, Chen LY, Mi YJ, Zheng LS, et al. Anticancer effect and structure activity analysis of marine products isolated from metabolites of mangrove fungi in South China sea. Ar Drugs. 2010;8:1094–1105. doi: 10.3390/md8041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CY, Ding WJ, Shao CL, She ZG, Lin YC. Secondary metabolites of a marine mangrove fungus (Penicillium sp.No.2556) from South China sea. J Chin Med Mater. 2008;7:960–962. [PubMed] [Google Scholar]
- 13.Chen ZS, Pan JH, Tang WC, Chen QC, Lin YC. Biodiversity and biotechnological potential of mangrove associated fungi. J For Res. 2009;20:63–72. [Google Scholar]
- 14.Gayathri S, Saravanan D, Radhakrishnan M, Balagurunathan R, Kathiresan K. Bioprospecting potential of fast growing endophytic bacteria from leaves of mangrove and salt marsh plant species. Indian J Biotechnol. 2010;9:397–402. [Google Scholar]
- 15.Almeida Ana Paula, Dethoup Tida, Singburaudom Narong, Lima Raquel, Vasconcelos Maria Helena, Pinto Madalena, et al. The in vitro anticancer activity of the crude extract of the sponge-associated fungus Eurotium cristatum and its secondary metabolites. J Nat Pharm. 2010;1(1):25–29. [Google Scholar]
- 16.Guo B, Wang Y, Sun X, Tang K. Bioactive natural products from endophytes: A review. Appl Microbiol Biotechnol. 2008;44(2):136–142. [PubMed] [Google Scholar]
- 17.Cremasco MA, Hritzko BJ, Linda Wang NH. Experimental purification of paclitaxel from a complex mixture of taxanes using a stimulated moving bed. Braz J Chem Eng. 2009;26(1):207–218. [Google Scholar]
- 18.Zhang JY, Tao LY, Liang YJ, Chen LM, Mi YJ, Zheng LS, et al. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Mar Drugs. 2010;8(4):1469–1481. doi: 10.3390/md8041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, An R, Wang J, Sun N, Zhang S, Hu J, et al. Exploring novel bioactive compounds from marine microbes. Curr Opin Plant Biol. 2005;8(3):276–281. doi: 10.1016/j.mib.2005.04.008. [DOI] [PubMed] [Google Scholar]