Abstract
Objective
To characterize the major allergens of Macrobrachium rosenbergii (giant freshwater prawn).
Methods
Raw and cooked extracts of the giant freshwater prawn were prepared. The IgE reactivity pattern was identified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting technique with the sera of 20 skin prick test (SPT) positive patients. The major allergen identified was then characterized using the proteomics approach involving a combination of two-dimensional (2-DE) electrophoresis, mass spectrometry and bioinformatics tools.
Results
SDS-PAGE of the raw extract showed 23 protein bands (15–250 kDa) but those ranging from 40 to 100 kDa were not found in the cooked extract. From immunoblotting experiments, raw and cooked extracts demonstrated 11 and 5 IgE-binding proteins, respectively, with a molecular mass ranging from 15 to 155 kDa. A heat-resistant 36 kDa protein was identified as the major allergen of both extracts. In addition, a 42 kDa heat-sensitive protein was shown to be a major allergen of the raw extract. The 2-DE gel fractionated the prawn proteins to more than 50 different protein spots. Of these, 10 spots showed specific IgE reactivity with patients' sera. Matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis led to identification of 2 important allergens, tropomyosin and arginine kinase.
Conclusions
It can be concluded that the availability of such allergens would help in component-based diagnosis and therapy of prawn allergies.
Keywords: Macrobrachium rosenbergii, Major allergen, MALDI-TOF, Tropomyosin, Arginine kinase, SDS-PAGE, Immunoblotting, 2-DE electrophoresis, IgE reactivity
1. Introduction
Shellfish is not only an important food source for man but also a frequent cause of adverse food hypersensitivity, which is commonly synonymous with IgE-mediated immediate-type I allergy[1]. Among shellfish allergies, prawn is the most frequent culprit[2],[3]. Sensitized individuals can develop urticaria, angioedema, laryngospasm, asthma and life-threatening anaphylaxis[4],[5].
So far, allergens of 34 to 38 kDa identified as tropomyosin have been established as the major allergens of a number of prawn, termed Pen i 1[6], Pen a 1[7] and Met e 1[8] depending on the species used. In addition, tropomyosin has also been identified as the major allergen of other crustaceans[9],[10], mollusks[11]–[15], house dust mites[16],[17] and cockroaches[18],[19]. Thus, it is currently accepted that tropomyosin is a cross-reactive pan allergen of invertebrates.
Besides tropomyosin, arginine kinase with a molecular mass of 40 kDa, termed as Pen m 2[20] and Lit v 2[21] has also been reported as prawn allergen. Also, arginine kinase has been described as a cross-reacting allergen among crustaceans and between crustaceans and insects[22]. In addition, a myosin light chain (MLC), Lit v 3, and sarcoplasmic calcium-binding protein (SCP), Lit v 4.0101, with molecular weight of 20 kDa and 22 kDa, respectively, were recently demonstrated to be new prawn allergens[23],[24].
For many years, reports on the identification of prawn allergens were limited to the family Penaeidae (seawater prawn). There are very few reports on allergen in Macrobrachium rosenbergii (M. rosenbergii) which is also known as the giant freshwater prawn or Malaysian freshwater prawn. Therefore, the aim of this study was to identify the major allergens of M. rosenbergii by using proteomic analysis.
2. Materials and methods
2.1. Preparation of allergen extracts
M. rosenbergii was obtained from the local market. Raw extract was prepared by washing giant freshwater prawn in purified water, followed by homogenization in phosphate buffered saline (PBS), pH 7.2 (1:10 weight/volume) using a Waring blender. Protein was extracted overnight by means of agitation at 4 °C. The homogenate was centrifuged at 4 500 rpm for 30 min at 4 °C and then at 14 000 rpm for 15 min at 4 °C. The clear supernatant was then filtered using a sterile 0.45 µm syringe filter. The lyophilized extracts were stored at -20 °C until use. Extract of cooked prawn was prepared by boiling for 15 min and homogenized according to the same protocol as above. For proteomic studies, extract of prawn was prepared by homogenization in distilled water, and processed as described above. The protein content of the extracts was estimated using Total Protein Kit (Sigma, USA).
2.2. Patients' sera
Sera of 20 patients with a history of prawn allergy and a positive skin prick test (SPT) to raw giant freshwater prawn extract were used in this study. This study was approved by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia.
2.3. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was carried out with a 12% polyacrylamide separating gel and a stacking gel of 5%. Electrophoresis was performed using a Mini Protean 3 Apparatus (BioRad, USA) at 120 mA for 45 min. Each sample was dissolved in Laemmli sample buffer (BioRad, USA) in the presence of 5% 2-mercaptoethanol, heated at 97 °C for 4 min and subjected to electrophoresis. Precision plus protein standards (Bio-Rad, USA) were run as reference, along with samples. After running, the gel was stained with Coomassie brilliant blue R-250. Protein masses were estimated by comparing the prawn protein bands with the molecular weight markers using an Imaging Densitometer GS800 and Quantity One Software (BioRad, USA).
2.4. Immunoblotting
Following SDS-PAGE, the separated proteins were electrotransferred from the gel to a nitrocellulose membrane using Mini Transblot System (BioRad, USA) at 100 V for 70 min. Immunoblot detection for IgE binding was done with extracts of raw and cooked giant freshwater prawn. The non-specific sites were blocked with 5% non-fat milk in TBS. Following washing with Tris-buffered saline (TBS) containing 0.05% Tween 20 (TTBS), the membrane was incubated with individual patient's serum overnight at 4 °C. IgE binding proteins were detected using biotinylated goat antihuman IgE antibody (Kirkergaard and Perry Laboratories, UK) followed by incubation with streptavidin-conjugated alkaline phosphatase (BioRad, USA) for 30 min at room temperature. Finally, Alkaline Phosphatase Conjugate Substrate Kit (BioRad, USA) was used for color development. Serum from a non-allergic subject was used as negative control.
2.5. Two-dimensional (2-DE) gel electrophoresis
Protein extract was suspended in rehydration buffer containing 8 M urea, 50 mM DTT, 4% chaps, 0.2% carrier ampholyte pH 3–10, 0.000 2% bromophenol blue. Then 50 µg of prawn extract was applied to immobilized non-linear pH 3–10 gradient strip of 7 cm length for rehydration overnight (12–14 h). Isoelectric focusing was run at 20 °C using the Protean IEF Cell Apparatus (BioRad, USA) with the following voltage/time gradient: 100 V for 1 min, 250 V for 30 min, 4 000 V for 2 h and 4 000 V for 10 000 V-h. Before transferring the IPG strip onto the second dimension, the strip was equilibrated sequentially for 10 min in a buffer containing 65 mM dithio-threitol and then 135 mM iodoacetamide in 125 mM Tris-HCl, pH 6.8, 6 M urea, 2% SDS, 30% glycerol and 0.01% bromophenol blue. After equilibration, the strips were then placed on 12% polyacrlamide separating gels and sealed in place using ReadyPrep Overlay Agarose (BioRad, USA). The proteins were then separated using the Mini Protean 3 Apparatus (BioRad, USA) for 45 min or until the bromophenol blue dye reached the bottom of the gel. The protein spots profile was stained with Coomassie brilliant blue (CBB) R-250 and then scanned using an Imaging Densitometer GS800 with PDQuest software (BioRad, USA).
2.6. 2-DE immunoblotting
A nitrocellulose membrane blotted with 2-DE-separated giant freshwater prawn components was subjected to a procedure similar to immunoblotting analysis.
2.7. Identification of major allergenic spots
The Coomassie-stained protein spots corresponding to those recognized by the above sera were manually excised and transferred to microcentrifuge tubes. Protein samples were trypsin digested and peptides extracted according to standard techniques. Peptides were analysed by matrix-assisted laser desorption-ionization time of flight (MALDI-TOF) mass spectrometer using a 4800 Proteomics Analyzer. Spectra were analysed to identify protein of interest using Mascot sequence matching software (Matrix Science) with Ludwig NR Database and taxonomy set to other metazoa.
3. Results
3.1. SDS-PAGE of giant freshwater prawn extracts
SDS-PAGE of a raw giant freshwater prawn extract revealed at least 23 protein bands with molecular masses ranging from 15 to 250 kDa. Fewer bands were detected in the cooked extract as several protein bands between 40 to 100 kDa were sensitive to heat denaturation and thus were no longer detected in the gel.
3.2. Reactivity of IgE in patients' sera with M. rosenbergii extracts
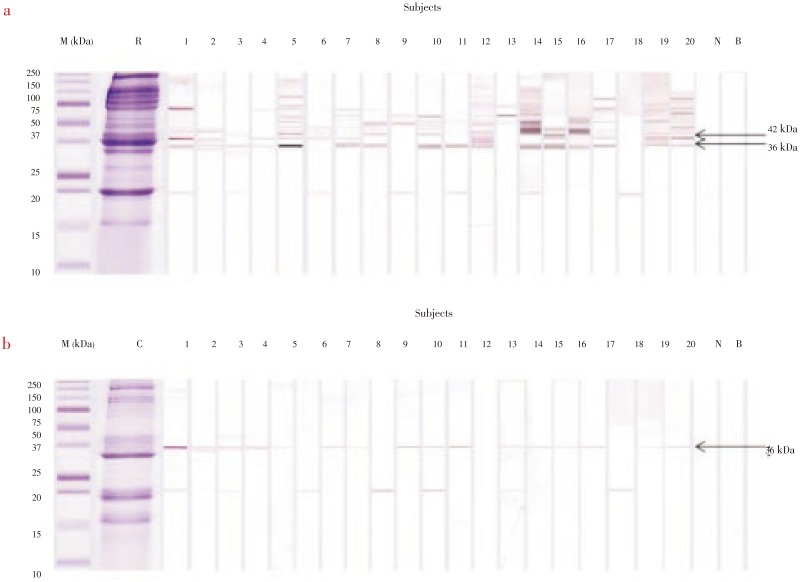
To identify IgE-binding proteins, we analyzed both raw and cooked extracts of M. rosenbergii by using SDS-PAGE and immunoblotting. When serum samples from giant freshwater prawn allergic patients were tested on immunoblots for IgE binding to raw extract, all the patient serum samples exhibited IgE binding to eleven protein bands with apparent molecular masses of 15–250 kDa. Of these, two components of 36 and 42 kDa were IgE reactive to more than 50% of the patient's sera. Further, allergens with molecular masses of 47, 50, 65 and 75 kDa were present in 40%–45% of the patients, compared with 20 and 100 kDa allergens which were seen in only 25%–35% of the patients. The remaining IgE binding components of 15 and 155 kDa were detected at lower frequencies of 5%. The cooked immunoblots showed that the sera of 16 (80%) of 20 patients reacted with a 36 kDa band, that the sera of 7 (35%) of 20 recognized a 20 kDa protein and that only the sera of 1 (5%) of 20 reacted with 15, 34 and 155 kDa. No IgE binding was observed when healthy donor serum was used (Figure 1). For our analysis, we selected the 36 and 42 kDa protein because it is a novel IgE binding protein identified in the sera of patients at a considerably high frequency.
Figure 1. SDS-PAGE and immobloting profiles of raw (a) and cooked extracts (b) of M. rosenbergii (giant freshwater prawn) using sera frcm 20 patients with giant frehwater prawn allergy.
Lane M: Molecular mass markers; Lane R: Coomassie blue staining of the raw extract; Lane C: Coomassie blue staining of the cooked extract; Lanes 1–20: Immonoblots showing binding of IgE from different serun samples; Lane N: Immunoblot using serum from a non-allergic individual; Lane B: Blank.
3.3. 2-DE immunoblotting
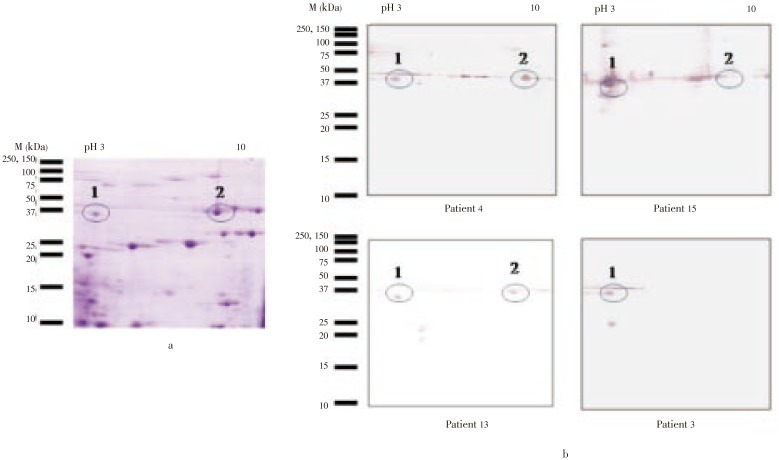
To better characterize the M. rosenbergii allergens recognized by sera from patients with prawn sensitivity, the proteins in a raw extract were subjected to 2-DE gel electrophoresis and subsequently, to immunoblotting. Figure 2a showed the 2-DE gel profile of the M. rosenbergii raw extract, in which > 50 distinct protein spots were detectable by Coomassie blue staining. To identify the allergenic spots, 2-DE separated components were immunoblotted with individual sera from prawn allergic patients, and at least 10 different reactive spots with molecular masses of 25–50 kDa and isoelectric point (pI) values ranging from 3.0 to 10.0 were demonstrated (Figure 2b). Highly reactive protein spots with molecular masses of 36–42 kDa were observed in all of the serum samples, including one with a molecular mass of 36 kDa and a pI of 4.6 and another with a molecular mass of 42 kDa and pI of 6.5. None of the proteins showed reactivity with control serum (data not shown).
Figure 2. Two dimensional electrophoresis and immunoblot analysis of M. rosenbergii.
a: Coomassie blue stained blot; b: Immunoblot with individual patients' sera. The circle shows the spot sent for MALDI/MS analysis. M: Molecular mass macker; pI: Isoelectric point.
3.4. Matrix-assisted laser desorption-ionization/mass spectrometry (MALDI/MS)
To identify the IgE reactive spot, it was excised and digested in-gel with trypsin and analyzed by MALDI-TOF. The MS profile of the peptides from spot 1, the 36 kDa protein with a pI of 4.6 was found to be tropomyosin from prawn Metapenaeus ensis, which corresponded to 33% sequence coverage. The MS profiles of spot 2 showed high similarity with arginine kinase from prawn Neocaridina denticulate, with the sequence coverage of 21% (Table 1).
Table 1. Identities of protein spots of M. rosenbergii identified by MALDI-TOF.
| Spot No. | Protein identification | Organism | Accession No. | No. match peptides/Total signal | Coverage of protein structure |
| 1 | Tropomyosin | Metapenaeus ensis | Q25456 | 10/635 | 33% |
| 2 | Arginine kinase | Neocaridina denticulate | C0STY2 | 7/417 | 21% |
4. Discussion
Previous research has shown that the prevalence of shellfish allergy is common among patients with allergic rhinitis and asthma[25].
Using SDS-PAGE, protein bands between 40–100 kDa in size were absent in cooked extract when compared with the SDS-PAGE of the raw extract. The disappearance of bands in the cooked extract may be due to a loss of some of the protein structure as a consequence of high temperature[26].
Major allergens are defined on the basis of frequency of recognition by serum IgE antibodies; that is, a frequency of greater than 50% justifies a designation as a major allergen[27]. In immunoblotting of the prominent bands, allergens with molecular mass of 36 kDa were detected by 80% (16 of 20) of the tested sera for both raw and cooked extracts. This protein is heat stable because it retains its IgE reactivity in immunoblots in which the protein is boiled. We also detected other heat stable protein at 20 kDa but as a minor allergen in both extracts. Aside from 36 kDa, an IgE binding protein with a molecular mass of 42 kDa was also noted as a major allergen of the raw extract. Several studies on various food proteins reported that cooking may reduce allergenicity of some food allergens[28]. As observed in our study, this 42 kDa major allergen and several additional minor allergens were detected only in the raw extract. Moreover, patient 13 had no detectable specific IgE to the 36 kDa which had only IgE-binding proteins to the raw extract. These findings show that all those allergens were heat-sensitive proteins and boiling has decreased the allergenicity of the allergen extract.
The IgE reactive bands identified by 1-DE may be a mixture of proteins appearing at the same molecular weight and hence the need for 2-DE immunoblotting was necessary to determine the true number of allergens[29],[30]. Several studies have demonstrated the major IgE reactive component in prawn is a 34 to 38 kDa protein of pI 4.5 identified as a tropomyosin[6]–[8]. It binds approximately 75% of the prawn-specific IgE from prawn-sensitive patients[6]–[8]. In our study, the 36 kDa protein (spot 1, pI 4.6) showed a significant homology with a tropomyosin. Tropomyosins are a diverse group of proteins with distinct isoforms found in muscle (skeletal, cardiac, smooth), brain and non-muscle cells. In muscles, tropomyosin interacts with actin and troponin T in regulating muscle contraction[31],[32].
In a recent study, an arginine kinase has also been identified as a prawn allergen[20],[21]. In addition to tropomyosin, the tryptic peptide fragments isolated from the digested 42 kDa of spot 2 is similar to arginine kinase. Arginine kinase belongs to a class of kinases or phosphagens that play a role in the maintenance of ATP levels in invertebrates by the phosphorylation[15]. In conclusion, 2-DE immunoblotting and proteomic analysis have enabled identification of important IgE reactive proteins from the prawn extract of M. rosenbergii. Two important allergens reacting with more than 50% patents' sera were identified using mass spectrometric approach. The major allergens identified can contribute to designing safer and more effective immunotherapeutic antigens.
Acknowledgments
The authors wish to thank the Director General of Health for his permission to publish this paper.
Footnotes
Foundation Project: Supported by a research grant from UPSI (grant No. UPSI 2011-0018-102-01).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Lu Y, Ohshima T, Ushio H, Hamada Y, Shiomi K. Immunological characteristics of monoclonal antibodies against shellfish major allergen tropomyosin. Food Chem. 2007;100:1093–1099. [Google Scholar]
- 2.Samson KTR, Chen FH, Miura K, Odajima Y, Iikura Y, Rivas MN, et al. IgE binding to raw and boiled shrimp proteins in atopic and nonatopic patients with adverse reactions to shrimp. Int Arch Allergy Immunol. 2004;133:225–232. doi: 10.1159/000076828. [DOI] [PubMed] [Google Scholar]
- 3.Jirapongsananuruk O, Sripramong C, Pacharn P, Udomputunurak S, Chinratanapisit S, Piboonpocanun S, et al. Specific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic children. Clin Exp Allergy. 2008;38:1038–1047. doi: 10.1111/j.1365-2222.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–1024. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 5.Bush RK. Approach to patients with symptoms of food allergy. Am J Med. 2008;121:376–378. doi: 10.1016/j.amjmed.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J Immunol. 1993;151:5354–5363. [PubMed] [Google Scholar]
- 7.Daul CB, Slattery M, Reese G, Lehrer SB. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. 1994;105:49–55. doi: 10.1159/000236802. [DOI] [PubMed] [Google Scholar]
- 8.Leung PSC, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, et al. Cloning, expression and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol. 1994;94:882–890. doi: 10.1016/0091-6749(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 9.Motoyoma K, Suma Y, Ishizaki S, Nagashima Y, Shiomi K. Molecular cloning of tropomyosins identified as allergens in six species of crustaceans. J Agric Food Chem. 2007;55:985–991. doi: 10.1021/jf062798x. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Cao M, Su W, Zhang L, Huang Y, Liu G. Identification and characterization of the major allergens of Chinese mitten crab (Eriocheir sinensis) J Food Chem. 2008;111:998–1003. [Google Scholar]
- 11.Miyazawa H, Fukamachi H, Inagaki Y, Reese G, Daul CB, Lehrer SB, et al. Identification of the first major allergen of a squid (Todarodes pacificus) J Allergy Clin Immunol. 1996;98:948–953. doi: 10.1016/s0091-6749(96)80011-x. [DOI] [PubMed] [Google Scholar]
- 12.Chuo KH, Wong SH, Leung PSC. Tropomyosin is the major mollusk allergen: reverse transcriptase polymerase chain reaction, expression and IgE reactivity. Mar Biotechnol. 2000;2:499–509. doi: 10.1007/s101260000035. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama K, Ishizaki S, Nagashima Y, Shiomi K. Cephalopod tropomyosins: identification as major allergens and molecular cloning. Food Chem Toxicol. 2006;44:1997–2002. doi: 10.1016/j.fct.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Taylor SL. Molluscan shellfish allergy. Adv Food Nutr Res. 2008;54:139–177. doi: 10.1016/S1043-4526(07)00004-6. [DOI] [PubMed] [Google Scholar]
- 15.Sereda MJ, Hartmann S, Lucius R. Helminths and allergy: the example of tropomyosin. Trends Parasitol. 2008;24:272–278. doi: 10.1016/j.pt.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Wayne RT, Belinda JH, Wendy AS. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010;16:321–328. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Helton CS, Sasisekhar B, Alexis B, Mark E, Thomas BN. Strutural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan MS, Arlian LG, Bernstein JA, Yoder JA. Allergenicity of the Madagascar hissing cockroach. Ann Allergy Asthma Immunol. 2007;98:258–261. doi: 10.1016/S1081-1206(10)60715-6. [DOI] [PubMed] [Google Scholar]
- 19.Santos AB, Rocha GM, Oliver C, Ferriani VPL, Lima RC, Palma MS, et al. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008;121:1040–1046. doi: 10.1016/j.jaci.2007.12.1147. [DOI] [PubMed] [Google Scholar]
- 20.Yu CJ, Lin YF, Chiang BL, Chow LP. Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol. 2003;170:445–453. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Orozco KD, Aispuro-Hernandez E, Yepiz-Plascencia G, Calderon-de-la-Barca AM, Sotelo-Mundo RR. Molecular characterization of arginine kinase, an allergem from the shrimp Litopenaeus vannamei. Int Arch Allergy Immunol. 2007;144:23–28. doi: 10.1159/000102610. [DOI] [PubMed] [Google Scholar]
- 22.Binder M, Mahler V, Hayek B, Sperr WR, Scholler M, Prozell S, et al. Molecular and immunological characterization of arginine kinase from the Indian meal moth, Plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 2001;167:5470–5477. doi: 10.4049/jimmunol.167.9.5470. [DOI] [PubMed] [Google Scholar]
- 23.Ayuso R, Grishina G, Bardina L, Carrilo T, Blanco C, Dolores M, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 2008;122:795–802. doi: 10.1016/j.jaci.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Ayuso R, Grishina G, Ibáñez MD, Blanco C, Carrilo T, Bencharitiwong R, et al. Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. J Allergy Clin Immunol. 2009;124(1):114–120. doi: 10.1016/j.jaci.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Shahnaz M, Gendeh BS, Nasuruddin A. Skin test reactivity to inhalant and food allergens in patients with allergic rhinitis. Int Med Res J. 2001;5(2):69–73. [Google Scholar]
- 26.Williams D. Protein modification by thermal processing. Allergy. 1998;53(Suppl 46):S102–S105. doi: 10.1111/j.1398-9995.1998.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 27.King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TA, Thomas W. Allergen nomenclature. WHO/IUIS Allergen Nomenclature Subcommittee. Int Arch Allergy Immunol. 1994;105:224–233. doi: 10.1159/000236761. [DOI] [PubMed] [Google Scholar]
- 28.Porcel S, Leon F, Valero AM, Martín-Calderin P, Cuevas M, Alvarez-Cuesta E. Occupational rhinitis and asthma by Lathyrus sativus flour: characterization of allergens. J Allergy Clin Immunol. 2001;107:743–744. doi: 10.1067/mai.2001.113567. [DOI] [PubMed] [Google Scholar]
- 29.Dixit AB, Lewis WH, Wedner HJ. The allergens of Epicoccum nigrum link: I. Identification of the allergens by immunoblotting. J Allergy Clin Immunol. 1992;90:11–20. doi: 10.1016/s0091-6749(06)80006-0. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Buitrago JM, Ferreira L, Isidoro-Garcia M, Sanz C, Lorente F, Davila I. Proteomic approaches for identifying new allergens and diagnosing allergic diseases. Clin Chim Acta. 2007;385:21–27. doi: 10.1016/j.cca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Smillie LB. Preparation and identification of alpha- and beta-tropomyosins. Methods Enzymol. 1982;85:234–241. doi: 10.1016/0076-6879(82)85023-4. [DOI] [PubMed] [Google Scholar]
- 32.Stanislava VA, Nikolay SS, Yurii SB. A new property of twitchin to restrict the ‘rolling’ of mussel tropomyosin and decrease its affinity for actin during the actomyosin ATPase cycle. Biochem Biophys Res Commun. 2010;394:126–129. doi: 10.1016/j.bbrc.2010.02.128. [DOI] [PubMed] [Google Scholar]