Abstract
Objective
To investigate the antimicrobial activity of the tissue extracts of Babylonia spirata (B. spirata) against nine bacterial and three fungal pathogens.
Methods
Crude extract of gastropod was tested for inhibition of bacterial and fungal growth. Antibacterial assay was carried out by disc diffusion method and in vitro antifungal activity was determined against Czapex Dox agar. The antimicrobial activity was measured accordingly based on the inhibition zone around the disc impregnated with gastropod extract. Molecular size of muscle protein was determined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). And fourier transform infrared spectroscopy (FTIR) spectro photometry analysis was also studied.
Results
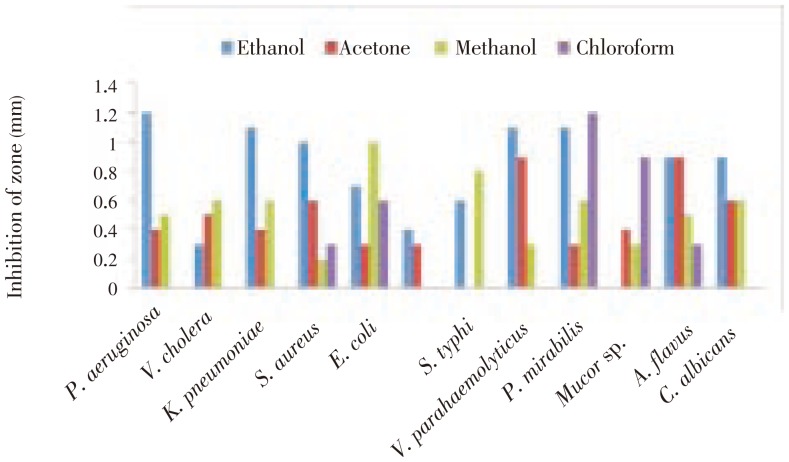
The maximum inhibition zone (12 mm) was observed against Pseudomonas aeruginosa in the crude ethanol extract of B. spirata and the minimum inhibition zone (2 mm) was noticed against Staphylococcus aureus in the crude methanol extract of B. spirata. Water extract of B. spirata showed the highest activity against Vibrio parahaemolyticus, Staphylococcus aureus and Candida albicans. Ethanol, acetone, methanol, chloroform and water extracts showed antimicrobial activity against almost all the bacteria and fungus. Compared with water extracts, ethanol and methanol extracts showed higher activity against all pathogens. The molecular weight of protein of the gastropod sample ranged from 2-110 kDa on SDS-PAGE. FTIR analysis revealed the presence of bioactive compounds signals at different ranges.
Conclusions
The research shows that the great medicinal value of the gastropod muscle of B. spirata may be due to high quality of antimicrobial compounds.
Keywords: Gastropod, Babylonia spirata, Antimicrobial activity, SDS-PAGE, FTIR, Antibacterial activity, Antifungal activity, Inhibition zone, Muscle protein, Bioactive compound
1. Introduction
The marine environment is a huge source to discover bioactive natural products. A wide variety of bioactive substances are being isolated and characterized from the food that is derived from the marine environment, several with great promise for the treatment of human and fish disease. For the past two decades, pharmaceutical industry has been relatively successful in overcoming problems due to single resistant determinants. However, the advent of multiple resistant mechanism has limited the use of many major classes of antimicrobial compounds. The demand for effective and non-toxic antibacterial therapeutics has become even greater with the increased incidence of bacterial infections. There is a vital interest in discovering new antimicrobial compounds with fewer environmental and toxicological risks and no resistance developed by the pathogens[1]. In marine invertebrates so far approximately 7 000 marine natural products have been reported, 33% from sponges, 18% from coelenterates (sea whips, sea fans and soft corals), and 24% from representatives of other invertebrate phyla such as ascidians (also called tunicates), opisthobranch mollusks (nudibranchs, sea hares, etc), echinoderms (starfish, sea cucumbers, etc) and bryozoans (moss animals). In India, till today, 5 070 species of Mollusca have been recorded, among which 3 370 species are from marine environment[2], while rest from the fresh water and terrestrial environment.
Molluscs are widely distributed throughout the world and have many representatives such as slugs, whelks, clams, mussels, oysters, scallops, squids and octopods in the marine and estuarine ecosystem. Many classes of bioactive compounds exhibiting anti-tumor, anti-leukemic, antibacterial and antiviral activities have been reported world wide[3]–[5]. Among the molluscs some have pronounced pharmacological activities or other properties which are useful in the biomedical arena. It is surprising to find that some of the pharmacological activities are attributed to the presence of polysaccharides particularly sulphated muco polysaccharide. Antimicrobial peptides are important in the first line of the host defense system of many animal species[6]. Their value in innate immunity lies in their ability to function without either high specificity or memory. Moreover, they are synthesized without dedicated cells or tissues and they can rapidly diffuse to the point of infection. The potential of marine gastropod as a source of biologically active products is largely explored in India. Therefore, the aim of the present study was to evaluate the antimicrobial activity of the tissue extracts of gastropod Babylonia spirata (B. spirata) against different pathogenic bacterial and fungal strains.
2. Materials and methods
2.1. Collection and extraction of samples
Live specimens of B. spirata (Family: Buccinidae) were collected from Thazhanguda coastal waters (latitude: 110 45′ 0N; longitude: 790 45′ 0E) Cuddalore, southeast coast of India. They were immediately brought to the laboratory and their soft bodies were removed by breaking the shells. The whole body muscle of the sample (50 g) was cut into small pieces and the tissue sample was used for extraction using different solvents such as ethanol, acetone, methanol, chloroform and water. The extracts were cold steeped over night at -18 °C and filtered with Whatman No. 1 filter paper. The filtrate was poured in previously weighted Petri plate and evaporated to dryness in rotary evaporator[7],[8]. The dried crude extracts were used for antimicrobial assay against human pathogens [Pseudomonas aeruginosa (P. aeruginosa), Vibrio cholera (V. cholera), Vibrio parahaemolyticus (V. parahaemolyticus), Klebsiella pneumoniae (K. pneumoniae), Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Streptococcus pneumoniae (S. pneumoniae), Salmonella typhi (S. typhi), Proteus mirabilis (P. mirabilis), Aspergillus flavus (A. flavus), Candida albicans (C. albicans) and Mucor sp.]. All the pathogenic bacterial and fungal strains were obtained from Raja Muthiah Medical College, Annamalai University.
2.2. Antimicrobial assay
Gastropod crude extract was tested for inhibition of bacterial and fungal growth against human pathogenic bacteria and fungi. Microbial assay was carried out by disc diffusion technique followed by Kelman et al[9]. Pathogenic bacterial strains were inoculated in sterile nutrient broth and incubated at 37 °C for 24 h. Pathogens were swabbed on the surface of the Muller Hinton agar plates and discs (Whatman No.1 filter paper with 9 mm diameter) impregnated with 50 µL of gastropod extracts placed on the surface. In vitro antifungal activity of gastropod crude extract was determined against Czapex Dox agar. Inoculums of 24 h old culture of A. flavus well drained spores were distributed uniformly on the surface of the agar plates with the help of sterile cotton swab. Mucor sp. and C. albicans fungal strains were inoculated by taking a piece of fungal colony using a sterile cotton swab and gently swabbed on the surface of the medium. Control discs were with water and solvents to assess the effect of water and solvents on pathogens. The plates were incubated at 37 °C for 24 h and the antimicrobial activity was measured accordingly based on the inhibition zone around the disc impregnated with gastropod extract.
2.3. Molecular size of muscle protein by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Molecular size of muscle protein was determined using SDS-PAGE gel following the procedure by Sambrro et al[10]. Glass plates were assembled and 20 mL of 15% resolving gel was prepared and poured immediately to the notch plate. It was over laid with butanol. After polymerization was completed, it was poured off and the top layer was washed with deionized water. Then it was over laid with 8 mL of stock gel. Approximately 1 mL of 1% SDS gel loading buffer and sample were taken and it was heated at 100 °C for 3 min. Then it was assembly fixed in electrophoresis apparatus. 15 µL of samples with different molecular weight markers (6.5–97.4 kDa) were loaded, respectively in the well, run in the gel and stained with coomassie brilliant blue.
2.4. Fourier transform infrared spectroscopy (FTIR) spectral analysis
The lyophilized samples of B. spirata (10 mg) were mixed with 100 mg of dried potassium bromide (kbr) and compressed to prepare as a salt disc. The disc was then read spectro photometerically (Bio-Rad FTIR-40-model, USA). The frequencies of different components present in each sample were analyzed.
3. Results
3.1. Antimicrobial activity
Totally five crude extracts from one species of gastropod B. spirata were screened against nine human pathogenic bacteria and three fungal pathogens for testing their antimicrobial activities. The inhibition zones of ethanol, acetone, methanol, and chloroform extracts as compared with water extract against the specific test organisms were given in Figure 1. The maximum inhibition zone (12 mm) was observed against P. aeruginosa in the crude ethanol extract of B. spirata and the minimum inhibition zone (2 mm) was noticed against S. aureus. As for fungi only A. flavus and C. albicans showed more activity on ethanol, acetone crude extract. One fungal pathogen showed negative results on ethanol crude extract. The acetone and methanol extracts were able to produce a zone of 6 mm against S. aureus, C. albicans, V. cholera, K. pneumoniae and P. mirabilis. However, only slight activity was shown by the crude extract of chloroform (Figure 1).
Figure 1. Diameter of inhibition zone of molluscs B. spirata against each test microorganism.
3.2. SDS-PAGE
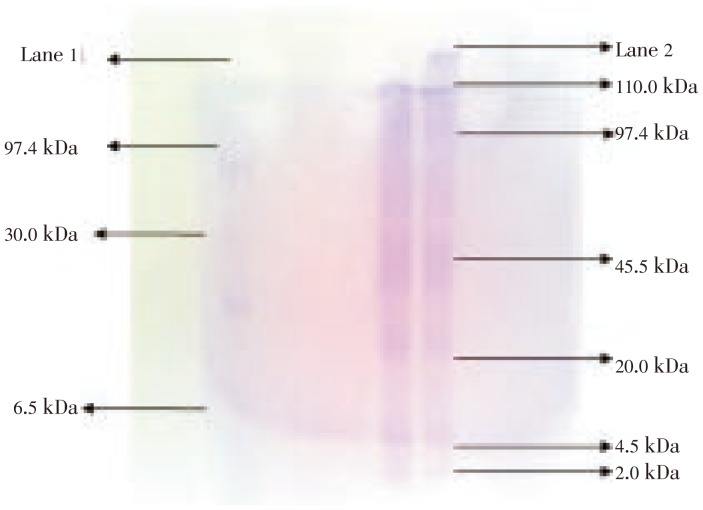
Crude protein sample of B. spirata yielded 6 bands ranging from 2.0–110.0 kDa with well defined. The bands were at 110.0, 97.4, 45.5, 20.0, 4.5 and 2.0 kDa, respectively. Gastropod sample was compared with the standard protein molecular weight marker (6.5–97.4 kDa) (Banglore Genei, India) (Figure 2).
Figure 2. Molecular size of B. spirata muscle protein determined by SDS-PAGE.
Lane 1: Standard protein molecular weight marker; Lane 2: Crude protein profiles in B. spirata.
3.3. FTIR spectral analysis
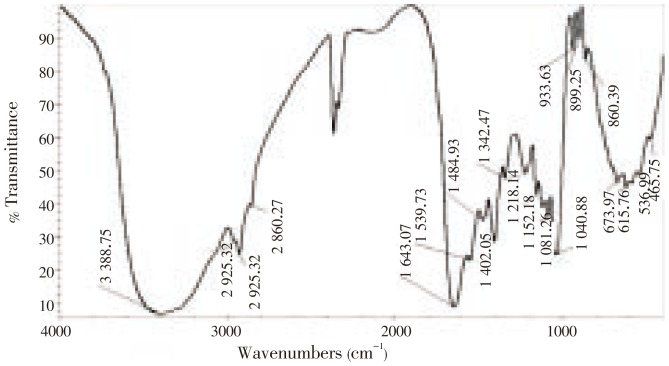
The FTIR spectra of the lyophilized sample of the 8 major peaks were at 2 860.27, 1 484.93, 1 342.47, 1 218.14, 1 152.18, 933.63, 899.25 and 860.39 cm−1, whereas the spectra of the sample of B. spirata showed all peaks with very close values at 3 388.75, 2 958.90, 2 925.32, 1 643.07, 1 539.73, 1 402.05, 673.97, 615.76, 536.99 and 465.75 cm−1 (Figure 3).
Figure 3. The FTIR spectra of lyophilized sample in B. spirata.
4. Discussion
There is a growing interest in marine natural products or marine secondary metabolites. This field of research receives the attention of investigators from various fields such as marine biology, marine ecology, biochemistry, chemistry, pharmacology and biotechnology. In the industrialized countries, about 25% of all prescription drugs contain active principles that are extracted from higher plants. In traditional Indian medicine, especially Sidha medical preparations, the opercula of gastropods are used as an ingredient to combat different diseases. B. spirata are organisms of muddy bottom of shallow waters. In the present study, a pronounced antimicrobial activity has been observed against some bacterial and fungal strains. The ethanol, acetone, methanol, chloroform and water crude extracts of B. spirata show activity against both bacterial and fungal strains. In antimicrobial activity the maximum zone of inhibition was recorded in P. aeruginosa strain and minimum zone inhibition was observed in S. pneumonia strain. The maximum antifungal activity was observed against A. flavus and minimum activity was recorded in Mucor sp. Similar result was reported in the antibacterial activities of ethanol extracts of gastropods B. spirata and Turbo brunneus showed the maximum activity against E. coli, K. pneumoniae, P. vulgaris and S. typhi[4]. The maximum antibacterial activity against S. aureus and E. coli by Trochus radiates was also reported[11]. These investigations support the present findings of the antimicrobial activity of muscle extraction of B. spirata. The antimicrobial activity was reported in four bivalves against few pathogens and found that extracts showed significant activity against Bacillus subtillus[12]. This study corroborates the results of the present investigation. The antimicrobial activity from the gill extraction of Perna viridis (Linnaeus, 1758)[13], antimicrobial activities of bivalve mollusk Meretrix meretrix (Linnaeus, 1758) and Meretrix casta (Gemelin, 1791)[14], antibacterial activities of green mussel (Perna viridis) and edible oyster (Crassostrea madrasensis)[15] were reported. It is also reported that the acetone extract of the winged oyster Pteria chinensis was found to have a broad spectral activity inhibiting all the fish pathogenic strains tested and the extract of chloroform inhibited eight pathogens. These also support the present study on antimicrobial activity of gastropod extracts[1]. Difference in the antibacterial activity found in gastropod extract may depend on extracting capacity of the solvents and the compounds extracted.
The first attempt to locate antimicrobial activity in marine organisms was initiated around 1950's[16]. Since this time, a large number of marine organisms from a wide range of phyla have been screened for antimicrobial activity[17]. Many of these organisms have antimicrobial properties, although most of the antibacterial agents that have been isolated from marine sources have not been active enough to complete with classicical antimicrobial activity against microorganisms[18]. The presence of antimicrobial activity in mollusca has been reported from the mucus of the giant snail Achantina fulica[19],[20]. The methanol extract of Hemifusus pugilinus possessed the highest activity against E. coli and the lowest activity was observed against Klebsiella oxytoca. The methanol extracts of Anadara granosa showed the highest activity against E. coli and the lowest activity against S. typhi[21]. The maximum antibacterial inhibition zone was exhibited from acetone crude extract of Trochus tentorium against human pathogen S. pneumonia[22]. The flesh of Meretrix meretrix was used widely in India and China as a fisher folk medicine to treat several liver diseases like jaundice, hepatitis-A&B[23]. Likewise the steroid extract of Meretrix meretrix can inhibit the cell growth and induction of G1-phase cell cycle arrested in hepatoma cells[24]. Two edible bivalve species of Perna viridis and Meretrix casta showed the antifungal activities[25]–[39]. Gonzalez et al[40] reported the mantle tissues of the oyster Crassostrea gigas. A polyproline-type AMP (47 residues) isolated from the Chilean scllop (Argopecten purpuratus) showed antifungal activity against Fusarium oxisporum and Saprolegnia parasitic[41]. The methanol extract of Sepia officinalis showed the maximum inhibition zone against E. coli and Lactobacillus vulgaris and the minimum inhibition zone was recorded against Salmonella paratyphi[42]. The antibacterial and antifungal activities of various concentrations of the polysaccharides extracted from the cuttle bone of Sepia aculeate and Sepia brevimana were reported by Shanmugan et al[43]. The antibacterial activity of crude extracts of Murex virgineus exhibited the zone of inhibition ranged between 2 mm and 10 mm. Methanolic extract of Murex virgineus was estimated significant effective antifungal activity against all the tested strains[44]. Commercial antibiotics are highly effective to kill the bacterial and fungal pathogens involved in common infection. Water, ethanol, acetone, methanol and chloroform extracts of gastropod used in the present study showed significant antimicrobial activity compared with others solvents. It is worthy to note that the product from natural source is good for health and devoid of side effects. In the present investigation, muscle extraction that showed antimicrobial activity was subjected to SDS-PAGE to estimate the number and molecular weight of proteins present. After electrophoresis clear bands were detected in the gel which represented molecular weight of proteins ranging from 2 to 110 kDa. FTIR analysis reveals the presence of bioactive compounds signals at different ranges. The research shows that the medicinal value of the gastropod B. spirata muscle may be due to high quality of antimicrobial compounds.
The present study revealed that the species of B. spirata showed antimicrobial activities against the pathogenic microbial forms. So they possess potential pharmacological action. However, some novel and uncharacterized mechanisms of action that might ultimately benefit the ongoing global search for clinically useful antimicrobial agents need to be explored to explain the characteristic of antimicrobial activity of B. spirata.
Acknowledgments
Authors are thankful to Professor Balasubaramanian T, Dean and Director, Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences, Annamalai University for giving facilities and encouragement during the study period.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest
References
- 1.Chellaram C, Gnanambal KME, Edward JKP. Antibacterial activity of the winged oyster Pteria chinensis (Pterioida: Pteridae) Indian J Mar Sci. 2004;33(4):369–372. [Google Scholar]
- 2.Venkataraman K, Wafer M. Coastal and marine biodiversity of India. Indian J Mar Sci. 2005;34(1):57–71. [Google Scholar]
- 3.Kamiya H, Muramoto K, Ogata K. Antibacterial activity in the egg mass of a sea hare. Cell Mol Life Sci. 1984;40:947–949. [Google Scholar]
- 4.Anand PT, Rajaganapathy J, Edward P. Antibacterial activity of marine mollusks from Porto Nova region. Indian J Mar Sci. 1997;26:206–208. [Google Scholar]
- 5.Rajaganapathy J, Thyagarajan SP, Edward JKP. Study on cephalopod ink for antiretroviral activity. Indian J Exp Biol. 2000;38:519–520. [PubMed] [Google Scholar]
- 6.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 7.Becerro MA, Lopez NI, Turon X, Uniz MJ. Antimicrobial activity and surface bacterial film in marine sponges. J Exp Mar Biol Ecol. 1994;179:195–205. [Google Scholar]
- 8.Wright AE. Isolation of marine natural products. In: Cannell RPJ, editor. Methods in biotechnology, natural products isolation. New Jersey: Humana Press Inc; 1998. pp. 305–408. [Google Scholar]
- 9.Kelman D, Kashman Y, Rosenberg E, Ilan M, Iirach I, Loya Y. Antimicrobial activity of the reef sponge Amphimedon viridis from the Red Sea evidence for selective toxicity. Aquat Microb Ecol. 2001;24:9–16. [Google Scholar]
- 10.Sambrro JE, Fritsch E, Maniatis T. Appendix-8. In: Russel T, editor. Molecular cloning. New York: Cold Spring Harbour Laboratory Press; 2006. [Google Scholar]
- 11.Elezabeth MKG, Chellaram C, Jamila P. Antimicrobial activity of reef associated gastropod, Trochus radiatus. National Seminar on Ecosystem, Remediation; 2003. p. 68. [Google Scholar]
- 12.Jayaseli AA, Prem Anand T, Murugan D. Antimicrobial activity of four bivalves from Gulf of Mannar, Phuket. Mar Biol Cent Spec Publ. 2001;25(1):215–217. [Google Scholar]
- 13.Chandran B, Rameskumar G, Ravichadren S. Antimicrobial activity from the gill extraction of Perna viridis (Linnaues, 1758) Glob J Biotech Biochem. 2009;4(2):88–92. [Google Scholar]
- 14.Sugesh S. Antimicrobial activities of bivalve Mollusca Meretrix meretrix (Linnaeus, 1758) and Meretrix casta (Gmelin, 1791) (M. Phily thesis) Parangipettai: Annamalai University; 2010. p. 65. [Google Scholar]
- 15.Annamalai N, Anburaj R, Jayalaksmi S, Thavasi R. Antibacterial activities of green mussel (Perna vridis) and edible oyster (Crassostrea madrasensis) Res J Microbiol. 2007;2(12):978–982. [Google Scholar]
- 16.Berkholder PR, Burkholder LM. Antimicrobial activity of horny corals. Science. 1958;127:1174. doi: 10.1126/science.127.3307.1174. [DOI] [PubMed] [Google Scholar]
- 17.Shaw PD, McClure WO, Van Blaricom G, Sims J, Fenical W, Rude J. Antimicrobial activities from marine organisms. In: Webber HH, Ruggieri GD, editors. Fooddrugs from the sea. Washington DC: Marine Technology Society; 1976. pp. 55–60. [Google Scholar]
- 18.Rinehart KL, Shaw PD, Shield LS, Gloer JB, Harbour GC, Koker MES, et al. Marine natural products as a source of antiviral, antimicrobial and antineoplastic agents. Pure Appl Chem. 1981;53:795–817. [Google Scholar]
- 19.Kubota Y, Watanabe Y, Otsuka H, Tamiya T, Tsuchiya T, Matsumoto JJ. Purification and characterization of an antibacterial factor from snail mucus. Comp Biochem Physiol C. 1985;82:345–348. doi: 10.1016/0742-8413(85)90173-2. [DOI] [PubMed] [Google Scholar]
- 20.Lguchi SM, Aikawa T, Matsumoto JJ. Antibacterial activity of snail mucus mucin. Comp Biochem Physiol A Comp Physiol. 1982;72:571–574. doi: 10.1016/0300-9629(82)90123-2. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan G. Studies on antimicrobial properties of a gastropod Hemifusus pugilinus and a bivalve Anadara granosa (MSc thesis) Parangipettai: Annamalai University; 2008. p. 27. [Google Scholar]
- 22.Anbuselvi S, Chellaram C, Jonesh S, Jayanthi L, Edward JKP. Bioactive potential of coral associated gastropod, Trochus tentorium of Gulf of Mannar, Southeastern India. J Med Sci. 2009;9(5):240–244. [Google Scholar]
- 23.Wang GD, Liu BZ, Tang BJ, Zhang T, Xiang JH. Pharmacological and immunocytochemical investigation of the role of catecholamines on larval metamorphosis by β-adrenergic-like receptor in the bivalve Meretrix meretrix. Aquaculture. 2006;258:611–618. [Google Scholar]
- 24.Wu TH, Yang RL, Xie LP, Wang HZ, Chen L, Zhang SY, et al. Inhibition of cell growth and induction of G1-phase cell cycle arrest in hepatoma cells by steroid extract from Meretrix meretrix. Cancer Lett. 2006;232:199–205. doi: 10.1016/j.canlet.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Sumita S, Chatterji A, Das P. Effect of different extraction procedures on antimicrobial activity of marine bivalves: a comparison. Pertan J Trop Agric Sci. 2009;32(1):77–83. [Google Scholar]
- 26.Darabpour E, Motamedi H, Nejad SMS. Antimicrobial properties of Teucrium polium against some clinical pathogens. Asian Pac J Trop Med. 2010;3(2):124–127. [Google Scholar]
- 27.Saraswathi R, Krishnan PN, Dilip C. Antimicrobial activity of cotton and silk fabric with herbal extract by micro encapsulation. Asian Pac J Trop Med. 2010;3(2):128–132. [Google Scholar]
- 28.Madhumitha G, Saral AM. Preliminary phytochemical analysis, antibacterial, antifungal and anticandidal activities of successive extracts of Crossandra infundibuliformis. Asian Pac J Trop Med. 2011;4(3):192–195. doi: 10.1016/S1995-7645(11)60067-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M, Wesely EG, Kavitha MS, Uma V. Antibacterial activity of leaves and inter-nodal callus extracts of Mentha arvensis L. Asian Pac J Trop Med. 2011;4(3):196–200. doi: 10.1016/S1995-7645(11)60068-0. [DOI] [PubMed] [Google Scholar]
- 30.Peixoto JRO, Silva GC, Costa RA, Fontenelle JRLDS, Vieira GHF, Filho AAF, et al. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med. 2011;4(3):201–204. doi: 10.1016/S1995-7645(11)60069-2. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee SK, Bhattacharjee I, Chandra G. Isolation and identification of bioactive antibacterial components in leaf extracts of Vangueria spinosa (Rubiaceae) Asian Pac J Trop Med. 2011;4(1):35–40. doi: 10.1016/S1995-7645(11)60028-X. [DOI] [PubMed] [Google Scholar]
- 32.Kannan RR, Arumugam R, Anantharaman P. Antibacterial potential of three seagrasses against human pathogens. Asian Pac J Trop Med. 2010;3(11):890–893. [Google Scholar]
- 33.Johnson M, Wesely EG, Hussain ZMI, Selvan N. In vivo and in vitro phytochemical and antibacterial efficacy of Baliospermum montanum(Wïlld.) Muell. Arg. Asian Pac J Trop Med. 2010;3(11):894–897. [Google Scholar]
- 34.Kaur J, Rathinam X, Kasi M, Leng KM, Ayyalu R, Kathiresan S, Subramaniam S. Preliminary investigation on the antibacterial activity of mango (Mangifera indica L: Anacardiaceae) seed kernel. Asian Pac J Trop Med. 2010;3(9):707–710. [Google Scholar]
- 35.Naik MI, Fomda BA, Jaykumar E, Bha JA. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac J Trop Med. 2010;3(7):535–538. [Google Scholar]
- 36.Bhattacharjee I, Chatterjee SK, Chandra G. Isolation and identification of antibacterial components in seed extracts of Argemone mexicana L. (Papaveraceae) Asian Pac J Trop Med. 2010;3(7):547–551. doi: 10.1016/S1995-7645(11)60028-X. [DOI] [PubMed] [Google Scholar]
- 37.Seyyednejad SM, Koochak H, Darabpour E, Motamedi H. A survey on Hibiscus rosa-sinensis, Alcea rosea L. and Malva neglecta Wallr as antibacterial agents. Asian Pac J Trop Med. 2010;3(5):351–355. [Google Scholar]
- 38.Koochak H, Seyyednejad SM, Motamedi H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran) Asian Pac J Trop Med. 2010;3(3):180–184. [Google Scholar]
- 39.Jarrar N, Abu-Hijleh A, Adwan K. Antibacterial activity of Rosmarinus officinalis L. alone and in combination with cefuroxime against methicillin–resistant Staphylococcus aureus. Asian Pac J Trop Med. 2010;3(2):121–123. [Google Scholar]
- 40.Gonzalez M, Gueguen Y, Desserre G, de Lorgeril J, Romestand B, Bachere E. Molecular characterization of two isoforms of defensin from hemocytes of the oyster Crassostrea gigas. Dev Comp Immunol. 2007;31:332–339. doi: 10.1016/j.dci.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Arenas G, Guzman F, Cardeness C, Mercado L, Marshall SH. A novel antifungal peptide designed from primary structure of natural antimicrobial peptides from Argopecten purpuratus hemocyes. Peptides. 2009;30:1405–1411. doi: 10.1016/j.peptides.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Reddy NSV. Antibacterial activity and bioactive stuff from the ink gland of cuttle fish Sepia officinalis (Linnaeus, 1758) (MSc thesis) Parangipettai: Annamalai University; 2008. p. 37. [Google Scholar]
- 43.Shanmugan A, Mahalakshmi TS, Vino BA. Antimicrobial activity of poly saccharide isolated from the cuttle bone of Sepia aculeate (Orbingy, 1848) and Sepia brevimana (Steenstyup, 1872) an approach to selected antimicrobial actibvity for human pathogenic microorganisms. J Fish Aquat Sci. 2008;3(5):268–274. [Google Scholar]
- 44.Lenin T. Biochemical composition and antibacterial activity of marine gastropod Murex virgineus (MSc thesis) Parangipettai: CAS in Marine Biology, Annamalai University; 2011. p. 30. [Google Scholar]