Abstract
Objective
To compare the efficacy of three different tissue stains, namely haematoxylin and eosin (H&E), periodic-acid Schiff (PAS) and immunohistochemical (IHC) stains for detection of Entamoeba histolytica (E. histolytica) trophozoites in abscessed liver tissues of hamster.
Methods
Amoebic liver abscess was experimentally induced in a hamster by injecting 1 × 106 of axenically cultured virulent E. histolytica trophozoites (HM1-IMSS strain) into the portal vein. After a week post-inoculation, the hamster was sacrificed and the liver tissue sections were stained with H&E, PAS and IHC stains to detect the amoebic trophozoite.
Results
The three stains revealed tissue necrosis and amoebic trophozoites, but with varying clarity. H&E and PAS stained the trophozoites pink and magenta, respectively, however it was difficult to differentiate the stained trophozoites from the macrophages because of their similarity in size and morphology. On the other hand, IHC stain revealed distinct brown appearance of the trophozoites in the infected liver tissues.
Conclusions
It can be concluded that out of the three stains, IHC is the best for identification of E. histolytica trophozoites in tissue sections.
Keywords: Entamoeba histolytica, Amoebic liver abscess, H&E, PAS, IHC, Trophozoite
1. Introduction
Amoebic liver abscess (ALA) is the most common clinical presentation of extraintestinal infection of the intestinal protozoon, Entamoeba histolytica (E. histolytica). This illness is prevalent worldwide and endemic in tropical countries such as India, Bangladesh, tropical African countries, some areas in Brazil and Mexico, China and South-east Asia. Although less than 1% of patients infected with E. histolytica develop ALA, this still represents an alarming number. The ailment is easily acquired in poor sanitation area, via ingestion of infective E. histolytica cysts present in contaminated hands, food or water. Interestingly, the incidence rate is also increasingly reported in non-endemic and developed countries such as USA and European countries because of the ease of world travel and immigration of people from endemic areas[1]–[3].
Pathogenesis of ALA is known to be very complicated. It develops through the hematological dissemination of the pathogenic trophozoites into liver via the tributaries of portal vein after invasion of colonic mucosa, resulting in the formation of solitary or multiple abscesses regularly found in the right liver lobe[4]. The common virulence factors involved include Gal/GalNAc specific lectin, cysteine proteinases, amoebapores and lipophosphopeptidoglycan molecules[5],[6]. In the formation of ALA, the general sequence of morphological changes in liver tissues involves acute inflammation where the acute cellular infiltration is composed of polymorphonuclear leukocytes which surround the centrally located amoebas, then progress to granuloma formation after the leukocytes were being replaced by macrophages and epithelioid cells and subsequently followed by extensive necrosis with fused granulomas[7]–[9].
Several kinds of laboratory animal models have reportedly been used to study the formation of ALA. Since 1950s, inoculation routes such as direct intrahepatic, intracaecal, intraperitoneal and intraportal were performed to induce ALA in hamster, mouse and gerbil. Currently, the intraportal injection of E. histolytica trophozoites in hamster has been widely used to produce ALA[9],[10] and this technique is adopted in the present study.
A good staining method is pertinent in the pathogenesis study on ALA. An excellent stain facilitates visualization of the morphological changes in liver tissues and also differentiates the amoebas against surrounding cells such as hepatocytes, macrophages and other cell types[11]. The staining techniques reportedly used are haematoxylin and eosin (H&E), periodic-acid Schiff (PAS) and immunostaining. However, comparison of the efficiency of these staining methods in detecting amoebas has not been reported. Thus, this study was aimed to compare the efficacy of H&E, PAS and immunohistochemical (IHC) stains for detection of E. histolytica trophozoites in liver tissue of hamster with ALA.
2. Materials and methods
2.1. Development of ALA in experimentally induced hamster
ALA was induced in a Syrian golden hamster as described by Olivos-Garcia and Weber et al[12],[13]. Briefly, 1×106 of axenically cultured virulent strain E. histolytica trophozoites (HM1-IMSS) was suspended in 0.2 mL phosphate buffer saline (PBS) and then inoculated into the portal vein of an anesthetized male hamster. After one week post-inoculation, the animal was sacrificed with a three-time overdose of pentobarbital. Immediately after the animal became unconscious, cardiac puncture was performed to collect the blood, then transferred into a sterile 1.5 mL microfuge tube and allowed to clot. The hamster serum containing polyclonal antibody against E. histolytica was then stored at -20 °C until used. The liver was removed aseptically, followed by fixation in 10% formalin. The same procedures were performed in the control healthy hamster, except that the injection fluid comprised 0.2 mL PBS. The animal experimentation was approved by USM Animal Research Ethics Committee [No. Animal Ethics Approval: USM/Animal Ethics Approval/2008/(40)(129)].
2.2. Tissue processing
Both infected and healthy formalin-fixed livers were cut into small pieces and kept in separate cassettes. The tissues were then processed overnight in an automated tissue processer (Leica TP 1020, Germany), which involved 1 h fixation, dehydration through graded alcohols for a total of 6 h, followed by 3 h clearing with xylene and 4 h tissue impregnation with embedding medium. The processed liver tissues were then embedded in paraffin wax to produce tissue blocks. Four µm thick formalin-fixed, paraffin-embedded tissue sections were cut with a microtome (Microm HM 325 Rotary Microtome, Germany) and subsequently stained with the three stains. Triplicate tissue sections were prepared for each stain.
2.3. Histochemical staining methods
2.3.1. H&E stain
Staining of the processed tissue sections was performed according to the standard protocol as described by Bancroft and Gamble with some modifications[14]. In brief, processed tissues were deparaffinized with two changes of xylene for 2 min each, rehydrated with two changes of absolute, 95% and 80% alcohols for 2 min each, followed by washing in running tap water for 5 min. Then, the tissues were stained with Harris's haematoxylin (Sigma-Aldrich, USA) for 20 min and washed in running tap water. Differentiation with 1% acid alcohol was carried out for 10 sec, followed by washing and bluing by dipping the tissues in ammonia water for 10 sec. After a washing step, the tissues were counterstained with eosin Y (Sigma-Aldrich, USA) for 2 min, dehydrated with increasing graded of alcohols for 2 min each, cleared with two changes of xylene for 2 min each and finally mounted with dibutyl phthalate xylene (DPX).
2.3.2. PAS stain
Slides were prepared based on the conventional protocol described by Bancroft and Gamble[14]. Briefly, processed tissues underwent the same deparaffinization, rehydration and washing steps as mentioned in the H&E stain. Next, the tissues were treated with periodic acid solution (Sigma-Aldrich, USA) for 5 min and washed with distilled water for 5 min. The tissues were then covered with Schiff's reagents for 10 min, followed by washing in running tap water for 5 min. Counterstaining was performed with Harris's haematoxylin (Sigma-Aldrich, USA) for 1 min, then washed in running tap water for 5 min and differentiated with 1% acid alcohol. Subsequently, the tissues were dipped in ammonia water for 10 sec until the sample turned blue, washed in running tap water for 5 min, followed by dehydration with increasing graded of alcohols, cleared with xylene and mounted with DPX.
2.4. Immunohistochemical staining method (IHC stain)
Indirect staining was performed on processed tissue sections with some modifications of the standard protocol as described by Bancroft and Gamble[14]. First, the tissues were deparaffinized with two changes of xylene for 5 min each, followed by rehydration with two changes of absolute, 70% and 50% alcohols for 3 min each and washing in running tap water for 5 min. Tissues were then blocked with 3% hydrogen peroxide for 5 min, dipped in distilled water for 5 min and followed by 30 min incubation with 1:100 dilution of the corresponding polyclonal hamster serum sample i.e. sera from the ALA-induced hamster and control hamster used for the infected and control tissues, respectively. Washing steps were then carried out five times with PBS-Tween 20 (PBST), 2 min each. Tissues were incubated with 1:1 000 dilution of HRP-conjugated anti-hamster antibody (Sigma-Aldrich, USA) for 30 min and again washed with PBST. After washing, the tissues were developed with 3,3′-diaminobenzidine (DAB) substrate solution for 3 min and again washed with PBST. Finally, the tissues were counterstained with Harris's haematoxylin (Sigma-Aldrich, USA) for 1 min, followed by washing, differentiation with 1% acid alcohol, bluing with ammonia water, another washing step, dehydration with increasing graded alcohols, clearance with xylene and then mounted with DPX.
Finally, the three differently stained tissues were observed under a light microscope at different magnifications (40×, 100× and 400×) and the images were captured using image analysis system (Nikon eclipse 80i, Japan). Comparisons on the ease and clarity of E. histolytica trophozoites detection were then made based on the captured images.
3. Results
Gross examinations of both the infected and non-infected liver tissues were performed prior to processing for histology. The infected liver was found to be enlarged and studded with multiple small yellow-white abscesses, whereas the non-infected liver was normal in size with a smooth clean surface (Figure 1). All the triplicate stained tissue slides revealed similar overall appearance. The healthy liver tissue sections revealed intact hepatic lobules with central veins and cords of radiating hepatocytes surrounded by the portal triads. On the contrary, in sections from infected liver tissue, a well defined endothelial layer of central vein was not observed as seen in normal tissue section (Figure 2). The abscesses in the infected tissue were seen as foci of extensive necrolysis and degenerative changes. Efficacy of each staining method was compared in terms of the ease and clarity of trophozoites detection from tissue sections. With H&E stain, the trophozoites were stained pink whereas the PAS stain outlined the trophozoites magenta in colour. Both the stains could not differentiate the trophozoites clearly, as the amoebas resembled the macrophages. However, with the immunostain, the trophozoites were stained brown in colour, an end-product of the enzymatic reaction between DAB and horseradish peroxidase. Consequently, the appearances of IHC-stained trophozoites were easily identified from the background of inflamed and necrotic tissues (Figure 3). In Figure 4, the images captured from IHC stained slides clearly revealed central necrotic region in liver tissue surrounded by scanty inflammatory cells with amoebic trophozoites along the margins. Islands of better preserved liver tissue were also seen scattered among the necrotic foci.
Figure 1. Gross appearance of hamster livers.
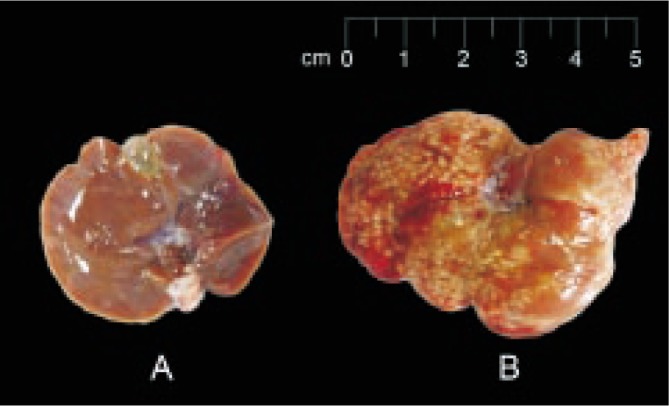
A: Non-infected healthy liver with a smooth and clean surface; B: One-week post inoculation abscessed liver with multiple tiny whitish spots.
Figure 2. Photomicrographs showing normal liver tissues (left) and infected liver tissues (right) using three different staining techniques. 1A, 1B: H&E stain; 2A, 2B: PAS stain; 3A, 3B: IHC stain (100×); CV: Central vein. E. histolytica trophozoites are indicated with arrows. All the sections from non-infected liver show normal liver architecture with intact central vein and cords of hepatocytes. Sections from the infected liver show necrolytic tissues with distorted central vein.

Figure 3. Micrographs indicating the different staining of E. histolytica trophozoites.

A: H&E stain; B: PAS stain; C: IHC stain (400×); CV: Central vein. E. histolytica trophozoites are indicated with arrows. (A) Trophozoites (arrow) are visible as round, oval to pear shaped cells lying in lacunar spaces with occasional ingested red blood cells inside, very similar to macrophages in morphology. (B) PAS stained section showing the trophozoites (arrow) with magenta coloured cell membrane in a necrotic background. (C) IHC stain showing brown coloured trophozoites (arrow) with a distinct cell membrane easily identifiable against a background of necrosis and inflammation.
Figure 4. Photomicrograph from IHC stained liver tissue from infected hamster showing extensive necrosis representing coalescing microabscesses.

E. histolytica trophozoites (brownish, marked with arrow) are seen along the abscess margins invading the better preserved liver tissue. Magnification: 40×. N: necrotic area; LC: well-preserved liver cells.
4. Discussion
ALA has been known to be a potentially fatal extraintestinal infection of amoebiasis. Multiple factors involving parasite and the host have been reported to be involved in the development of ALA. The general concept of development of ALA involves the adaptation and survival of amoebas in liver tissue[9]. Rigothier et al[11], reported that there was massive death of parasites after a few hours of post-infection and inflammation in the hamster liver tissue was caused. After 12 h, the parasites started multiplying and the size of inflammation foci increased. In addition, other factors such as oxygen reduction ability, complement resistant, ROS and NOS scavenger capacity and immune evasion of the parasites also contribute to the parasites survival. Once the parasites are able to adapt to the environment in the liver, inflammation will be stimulated and followed by extensive tissue destruction[8],[15],[16].
In this study, the results showed that tissue destruction and amoebae in the tissue sections can be visualized by all the three stains, but with varying clarity. H&E and PAS stains required high technical expertise to identify and interpret the staining results. Even though H&E stain is the most widely employed histology stain to demonstrate the morphology of different cells and tissue[14], it has been reported to be not ideal for detection of amoebic trophozoites especially in the examination of fixed and stained biopsy samples due to the difficulty in differentiating the stained trophozoites from the surrounding tissues. PAS stains tissue carbohydrates magenta, and it is commonly used to stain liver glycogen[14]. The problem arises because E. histolytica trophozoites are also magenta in colour when stained with PAS, possibly due to the presence of glycoprotein in the amoeba cell membrane[17]. Thus, with both the H&E and PAS stains, amoebic trophozoites were difficult to differentiate from macrophages because of their similarities in size and morphology[18].
In comparison, IHC is presumed to be more specific as it is the consequence of specific reactions between antigens of amoebic trophozoite and antibodies against them. In this study, immunostaining gave more distinct and easily identifiable appearance of the trophozoites in a background of necrosis and inflammation as compared with the other two staining techniques. Even though numerous reported studies on amoebic pathogenesis utilized H&E and PAS stains, this study showed that IHC stain was more superior than the two stains. As was previously described for hamster and human ALA[9],[19], the images captured from IHC stained slides clearly revealed central necrotic region in liver tissue surrounded by scanty inflammatory cells with amoebic trophozoites along the margins. Islands of better preserved liver tissue were also seen scattered among the necrotic foci. Moreover, serum sample could easily be obtained from 5–7 days post-infected hamster, and contained sufficient polyclonal antibodies that recognize E. histolytica trophozoites[20].
A previous study has reported that monoclonal antibody can be used in cryopreserved tissue section to stain amoeba but not in formalin-fixed, paraffin-embedded tissue[21]. However, this study showed that amoeba in paraffin-embedded tissue can be visualized when polyclonal antibody was employed. The use of polyclonal antibody may be able to show stronger antigen recognition on amoebas in the formalin fixed tissue sections as compared with monoclonal antibodies which only recognize single epitopes. Also, processed tissue is favored to cryopreserve tissue because the structures of amoeba are physically supported by the embedding medium, while amoebic structure might be lost with frozen treatment due to the water crystallization.
Nowadays, in the diagnosis of amoebiasis, stool, blood, liver pus, urine and saliva samples are often investigated with various molecular-based and immunological-based techniques[22]–[25], whereas staining techniques are hardly reported. However, IHC is still relevant for confirmation of numerous pathogenic diseases[14], but rarely reported for use in the investigation of invasive amoebiasis. Thus, it is potentially important as a confirmatory test for ALA if sample from aspiration of liver abscess, liver biopsy or autopsy is available.
In conclusion, in this study, IHC stain was found to be more superior than H&E and PAS stains for detection of E. histolytica trophozoites in the infected tissues because the IHC allowed easy identification of brown-stained amoebas among the inflamed and necrotic liver cells.
Acknowledgments
This study was funded by a research university grant from Universiti Sains Malaysia (1001/PPSK/813009). The first two authors received financial support through the USM Fellowship.
Footnotes
Foundation Project: Supported by a grant from Universiti Sains Malaysia (grant No. 1001/PPSK/813009).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Rao S, Solaymani-Mohammadi S, Petri WA, Parker SK. Hepatic amebiasis: a reminder of the complications. Curr Opin Pediatr. 2009;21(1):145–149. doi: 10.1097/MOP.0b013e32831ef249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parija SC. Textbook of medical parasitology protozoology & helminthology. 3rd ed. New Delhi: All India Publishers & Distributors; 2008. [Google Scholar]
- 3.Seeto RK, Rockey DC. Amebic liver abscess: epidemiology, clinical features, and outcome. West J Med. 1999;170(2):104–109. [PMC free article] [PubMed] [Google Scholar]
- 4.Salles JM, Moraes LA, Salles MC. Hepatic amebiasis. Braz J Infect Dis. 2003;7(2):96–110. doi: 10.1590/s1413-86702003000200002. [DOI] [PubMed] [Google Scholar]
- 5.Ackers JP, Mirelman D. Progress in research on Entamoeba histolytica pathogenesis. Curr Opin Microbiol. 2006;9(4):367–373. doi: 10.1016/j.mib.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Baxt LA, Singh U. New insights into Entamoeba histolytica pathogenesis. Curr Opin Infect Dis. 2008;21(5):489–494. doi: 10.1097/QCO.0b013e32830ce75f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa CA, Fonseca TH, Oliveira FM, Santos JF, Gomes MA, Caliari MV. Influence of inflammation on parasitism and area of experimental amoebic liver abscess: an immunohistochemical and morphometric study. Parasit Vectors. 2011;4(1):27. doi: 10.1186/1756-3305-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo X, Houpt E, Petri WA. Crosstalk at the initial encounter: interplay between host defense and ameba survival strategies. Curr Opin Immunol. 2007;19(4):376–384. doi: 10.1016/j.coi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santi-Rocca J, Rigothier MC, Guillen N. Host-microbe interactions and defense mechanisms in the development of amoebic liver abscesses. Clin Microbiol Rev. 2009;22(1):65–75. doi: 10.1128/CMR.00029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsumi V, Shibayama M. Experimental amebiasis: a selected review of some in vivo models. Arch Med Res. 2006;37(2):210–220. doi: 10.1016/j.arcmed.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Rigothier MC, Khun H, Tavares P, Cardona A, Huerre M, Guillen N. Fate of Entamoeba histolytica during establishment of amoebic liver abscess analyzed by quantitative radioimaging and histology. Infect Immun. 2002;70(6):3208–3215. doi: 10.1128/IAI.70.6.3208-3215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivos-Garcia A, Nequiz-Avendano M, Tello E, Martinez RD, Gonzalez-Canto A, Lopez-Vancell R, et al. Inflammation, complement, ischemia and amoebic survival in acute experimental amoebic liver abscesses in hamsters. Exp Mol Pathol. 2004;77(1):66–71. doi: 10.1016/j.yexmp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Weber C, Blazquez S, Marion S, Ausseur C, Vats D, Krzeminski M, et al. Bioinformatics and functional analysis of an Entamoeba histolytica mannosyltransferase necessary for parasite complement resistance and hepatical infection. PLoS Negl Trop Dis. 2008;2(2):e165. doi: 10.1371/journal.pntd.0000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancroft JD, Gamble M. Theory and practice of histological techniaques. 5th ed. China: Churchill Livingstone. :2002. [Google Scholar]
- 15.Olivos-Garcia A, Saavedra E, Ramos-Martinez E, Nequiz M, Perez-Tamayo R. Molecular nature of virulence in Entamoeba histolytica. Infect Genet Evol. 2009;9(6):1033–1037. doi: 10.1016/j.meegid.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Wong-Baeza I, Alcantara-Hernandez M, Mancilla-Herrera I, Ramirez-Saldivar I, Arriaga-Pizano L, Ferat-Osorio E, et al. The role of lipopeptidophosphoglycan in the immune response to Entamoeba histolytica. J Biomed Biotechnol. 2010;2010:254521. doi: 10.1155/2010/254521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aley SB, Scott WA, Cohn ZA. Plasma membrane of Entamoeba histolytica. J Exp Med. 1980;152(2):391–404. doi: 10.1084/jem.152.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins & Cotran: pathologic basis of disease. 8th ed. Philadelphia: Saunders Elsevier. :2010. [Google Scholar]
- 19.Costa CA, Nunes AC, Ferreira AJ, Gomes MA, Caliari MV. Entamoeba histolytica and E. dispar trophozoites in the liver of hamsters: in vivo binding of antibodies and complement. Parasit Vectors. 2010;3(1):23. doi: 10.1186/1756-3305-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Compton SR, Riley LK. Detection of infectious agents in laboratory rodents: traditional and molecular techniques. Comp Med. 2001;51(2):113–119. [PubMed] [Google Scholar]
- 21.Sherchand JB, Thammapalerd N, Riganti M, Tharavanij S, Punpoowong B. Monoclonal antibody-based immunohistochemical demonstration of Entamoeba histolytica in liver tissues of experimentally infected hamster (Mesocricetus auratus) Int J Parasitol. 1994;24(6):909–916. doi: 10.1016/0020-7519(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 22.Haque R, Kabir M, Noor Z, Rahman SM, Mondal D, Alam F, et al. Diagnosis of amebic liver abscess and amebic colitis by detection of Entamoeba histolytica DNA in blood, urine, and saliva by a real-time PCR assay. J Clin Microbiol. 2010;48(8):2798–2801. doi: 10.1128/JCM.00152-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khairnar K, Parija SC. Detection of Entamoeba histolytica DNA in the saliva of amoebic liver abscess patients who received prior treatment with metronidazole. J Health Popul Nutr. 2008;26(4):418–425. doi: 10.3329/jhpn.v26i4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parija SC, Khairnar K. Detection of excretory Entamoeba histolytica DNA in the urine, and detection of E. histolytica DNA and lectin antigen in the liver abscess pus for the diagnosis of amoebic liver abscess. BMC Microbiol. 2007;7:41. doi: 10.1186/1471-2180-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Othman N, Mohamed Z, Verweij JJ, Huat LB, Olivos-Garcia A, Yeng C, et al. Application of real-time polymerase chain reaction in detection of Entamoeba histolytica in pus aspirates of liver abscess patients. Foodborne Pathog Dis. 2010;7(6):637–641. doi: 10.1089/fpd.2009.0427. [DOI] [PubMed] [Google Scholar]


