Abstract
Objective
To evaluate the acaricidal activity of extracts of three essential oils of chamomile, marjoram and Eucalyptus against Tetranychus urticae (T. urticae) Koch.
Methods
Extracts of three essential oils of chamomile, marjoram and Eucalyptus with different concentrations (0.5%, 1.0%, 2.0%, 3.0% and 4.0%) were used to control T. urticae Koch.
Results
The results showed that chamomile (Chamomilla recutita) represented the most potent efficient acaricidal agent against Tetranychus followed by marjoram (Marjorana hortensis) and Eucalyptus. The LC50 values of chamomile, marjoram and Eucalyptus for adults were 0.65, 1.84 and 2.18, respectively and for eggs 1.17, 6.26 and 7.33, respectively. Activities of enzymes including glutathione-S-transferase, esterase (α-esterase and β-esterase) and alkaline phosphatase in susceptible mites were determined and activities of enzymes involved in the resistance of acaricides were proved. Protease enzyme was significantly decreased at LC50 of both chamomile and marjoram compared with positive control. Gas chromatography-mass spectrometer (GC-MS) proved that the major compositions of Chamomilla recutita are α-bisabolol oxide A (35.251%), and trans-β-farersene (7.758%), while the main components of Marjorana hortensis are terpinene-4-ol (23.860%), p-cymene (23.404%) and sabinene (10.904%).
Conclusions
It can be concluded that extracts of three essential oils of chamomile, marjoram and Eucalyptus possess acaricidal activity against T. urticae.
Keywords: Tetranychidae, Plant essential oils, Enzymes, Glutathione-S-transferase, Non specific esterase, Alkaline phosphatase, Protease, Chamomile, Marjoram, Eucalyptus
1. Introduction
The two-spotted spider mite, Tetranychus urticae (T. urticae) Koch is one of the most important pests responsible for yielding losses to many horticultural ornamental and agronomic crops. A major problem in the control of T. urticae is the response to develop resistance to many acaricides due to an approximate 5-fold increase in the mixed function of oxidase activity[1]. For several years, chemical control of mites has been extensively practiced[2]–[4]. In Egypt, different extracts from Syzygium cumini (S. cumini) L. Skeels (Pomposia) against T. urticae Koch were used to control mite population and ethanol extract showed the most potent acaricidal activity[5]. Resistance problems and high residual levels of botanical insecticides have long been touted as attractive alternatives to synthetic chemical insecticides for pest management because botanicals reputedly pose little threat to the environment or human health[6]. Therefore, methods used to detect and determine these multiresidue in food products by LC-MS-MS tandem spectroscopy[7] may hinder its marketing. T. urticae females were repelled by spinosad and largely oviposited and fed on nonspinosad treated areas. Spinosad did not affect the behavior of Panonychus ulmi (P. ulmi) females. When T. urticae females were released on potted bean plants (two-leaf stage) in which leaves received spinosad sprays on the adaxial or abaxial leaf surfaces, or complete spinosad coverage on one or two of the leaves, mite population increase lagged significantly behind those released on control plants. These results indicate that S. cumini L. Skeels (Pomposia) and spinosad have significant acaricidal effects against T. urticae but not P. ulmi. Therefore, the use of essential oils of plant extracts in pest management programs has recently attracted the attention of many scientists. Pesticides of plant origin seem to be recommended as they generally have a very short persistence in the plant[6],[8]. However, the selectivity of these products has to be strictly evaluated for different species of natural enemies as deleterious or sometimes positive effects were recorded among the natural enemies complex[9],[10].
In insects, glutathione-S-transferase represents a very interesting enzyme carrying out detoxification mechanism due to their involvement in tolerance to acaricides[5],[11]. It is reported that most xenobiotics are subjected to enzymatic modification after penetration through protein binding and transportation in biological system like insects and acaricide. It had been clearly demonstrated that several enzymatic system such as esterase (α and β), and phosphatase (alkaline and acid) can play a vital role in the detoxification of xenobiotics to nontoxic materials[12].
Herein, this study was aimed to evaluate the acaricidal activity of extracts of essential oils of chamomile, marjoram and Eucalyptus against T. urticae. The biochemical changes due to treatment of T. urticae by LC50 of the tested oils were evaluated. In this respect, some detoxifying enzymes such as glutathione-S-transferase, nonspectific esterase (α and β), phosphatase (alkaline and acid) and protease enzymes were manipulated. The chemical compositions of the essential oils were studied with gas chromatography-mass spectrometer (GC-MS).
2. Materials and methods
2.1. Test mite
T. urticae was collected from infested cucumber plants (Cucumis sativus L.). Bean (Phaseolus vulgaris L.) seeds were planted in plastic pots (14 cm in diameter) at a rate of 4–5 seeds per pot, and seedlings were infested with T. urticae adults. From this culture, adult mites were transferred to aluminum pans (30 cm × 20 cm × 70 cm) on fresh leaves of beef steak (Acalypha wilkesiana L.) placed upside down on wet cotton pads. Water was added when needed. Mites were maintained at suitable moisture and kept in incubator at (25±2) °C and (70±5)% relative humidity.
2.2. Source of sample
Marjorana hortensis (M. hortensis) (Family: Lamiaceae) and Chamomilla recutita (C. recutita) (Family: Asteraceae) were collected from Sekam farm in Belbase (Sharqia Governorate). Leaves of Eucalyptus (Eucalyptus sp.) (Family: Myrtaceae) were collected from the Faculty of Agriculture Farm of Cairo University.
2.3. Preparation of essential oils
The whole plants (herbs) of marjoram and chamomile and leaves of Eucalyptus were dried for a week at room temperature, and then crushed according to the method of Calmasur et al[13]. Essential oils were extracted by hydro distillation (deionized water for 4 h) under vacuum according to the method of Aroiee et al[14]. Essential oils and components were kept under freezing until used. Series of aqueous concentrations of each essential oil were prepared with Triton X-100 as surfactant at a rate of 0.1%.
2.4. Treatment of eggs
Leaf discs (3 cm in diameter) of beef steak leaves were used as substrate to ovipositor. Four leaf discs were used for each treatment and five mite females were transferred to each disc and left 24 h to lay eggs, then females were removed. Thereafter, forty eggs, on four discs, were treated with one of the five concentrations (0.5%, 1.0%, 2.0%, 3.0% and 4.0%). Eggs were sprayed by a glass atomizer, with a serial of concentrations for each essential oil. 1 mL/200 cm of the solution was used. Eggs were incubated at (25±2) °C for seven days till hatching. The numbers of hatching and non hatching eggs were recorded.
2.5. Treatment of adult females
T. urticae females, 3 days old, were obtained by placing 100 nymphs onto the culture, and wet cotton pads in Petri dishes were placed on excised beef steak leaves. The emerged females and males were transferred to new beef steak leaves for 2–3 days and allowed to mate. Afterwards, forty females were transferred equally to four discs (3 cm in diameter), and then treated with one of the previous treatments. Control treatment was operated by Triton X-100 at a rate of 0.1%. Mortality was estimated for the adult females after 24 h of spraying and estimated by Abbot's formula (1925) and LC50, LC90 and slope values were estimated according to Finney[15].
2.6. Biochemical assay
Blood of adult females (10 mg) was homogenized in 1 mL distilled water in ice for 3 min using Teflon Homogenizer. The homogenates were centrifuged at 3 500 rpm for 10 min at 4 °C and the supernatants were used directly to determine the activity of alpha and beta esterase, glutathione-S-transferaes and alkaline phosphatase and protease.
Nonspecific esterase alpha esterases (α-esterases) and beta esterases (β-esterase) were determined according to the method of Asperen[16] using α-naphthyl acetate and β-naphthyl acetate as substrates, respectively.
Glutathione-S-transferase was measured according to the method described by Villanueva et al[17] using 1-chloro-2, 4 dinitrobenzene (CDNB).
Acid phosphatase and alkaline phosphatase were determined according to the method described by Manns et al[18].
Protease activity was determined according to El-Sharabasy[19] by casein digestion methods.
2.7. Statistical analysis
Experimental data were statistically analyzed by using Costa software (cohort software, Berkeley). Significance of results was obtained by randomized one way ANOVA, and the means were separated using the Duncan's multiple range test[20] at P<0.01.
3. Results
Essential oils extracts of plants are promising alternative natural products for the control of T. urticae Koch. These extracts facilitate the handling and its application, besides they can be an option of lower cost in relation to the studies of chemical control.
Data presented in Table 1 demonstrated that chamomile essential oil extract was the most potent effective acaricidal agent against T. urticae, which enhanced the highest adult female mortality and lowest egg hatchability. Adult mortality percentages after 24 h were 42.50%, 75.00%, 90.00%, 95.00% and 100.00% for chamomile by spraying the different concentrations of 0.5%, 1.0%, 2.0%, 3.0% and 4.0%, respectively. The percentages of corresponding mortalities for marjoram were 20.00%, 30.00%, 42.50%, 72.50%, and 85.00%, while 17.50%, 27.50%, 40.00%, 70.00%, and 80.00% for Eucalyptus, respectively. Hatchability percentages after six days were 75.00%, 55.00%, 30.00%, 16.00% and 10.00% for chamomile; 95.00%, 87.50%, 80.00%, 72.50%, 57.50% for marjoram and 95.00%, 92.50%, 82.50%, 77.50% and 67.50% for Eucalyptus, respectively, for control treatment (Triton X-100 at a rate of 0.1%), adult mortality was 10.00% and egg hatchability was 95.00%.
Table 1. Effect of three essential oil plant extracts against T. urticae egg hatchability and adult mortality (mean±SD) (%).
| Concentration (%) |
C. recutita |
M. hortensis |
Eucalyptus sp. |
|||
| Adult mortality | Egg hatchability | Adult mortality | Egg hatchability | Adult mortality | Egg hatchability | |
| Control | 10.00±1.29 | 95.00±0.58 | 10.00±1.14 | 95.00±0.58 | 10.00±1.29 | 95.00±0.58 |
| 0.5 | 42.50±1.71 | 75.00±1.29 | 20.00±1.29 | 95.00±0.58 | 17.50±0.96 | 95.00±0.58 |
| 1.0 | 75.00±1.70 | 55.00±2.38 | 30.00±0.82 | 87.50±0.50 | 27.50±1.71 | 92.50±0.96 |
| 2.0 | 90.00±0.82 | 30.00±0.58 | 42.50±2.06 | 80.00±1.15 | 40.00±1.41 | 82.50±1.26 |
| 3.0 | 95.00±0.58 | 16.00±0.10 | 72.50±1.50 | 72.50±1.50 | 70.00±2.24 | 77.50±1.71 |
| 4.0 | 100.00±0.00 | 10.00±1.41 | 85.00±0.82 | 57.50±2.36 | 80.00±0.82 | 67.50±0.58 |
Table 2 proved that chamomile essential oil extract represented the most potent acaricidal activities followed by marjoram and Eucalyptus. The LC50 values after 24 h for adults were 0.65%, 1.84% and 2.18%, respectively, while for eggs 1.17%, 6.26% and 7.33% were recorded after seven days. The slope values of the regression line were 2.41, 2.53 and 2.49 for adults and 2.28, 1.89 and 2.15 for eggs, respectively. LC90 values were 2.27%, 5.91% and 7.13% for adults and 4.34%, 9.81% and 28.95% for eggs, respectively.
Table 2. Toxicity of three essential oil plant extracts against T. urticae adult females and eggs.
| Toxicity parameters |
C. recutita |
M. hortensis |
Eucalyptus sp. |
|||
| Adults | Eggs | Adults | Eggs | Adults | Eggs | |
| LC50 (%) | 0.65 | 1.17 | 1.84 | 6.26 | 2.18 | 7.33 |
| Lower limit | 0.46 | 0.94 | 1.53 | 4.18 | 1.82 | 4.74 |
| Upper limit | 0.82 | 1.45 | 2.21 | 25.40 | 2.67 | 39.05 |
| Index | 100.00 | 100.00 | 35.44 | 19.11 | 29.82 | 16.31 |
| Slope | 2.41 | 2.28 | 2.53 | 1.89 | 2.49 | 2.15 |
| LC90 (%) | 2.27 | 4.34 | 5.91 | 9.81 | 7.13 | 28.95 |
3.1. Enzyme activities
Table 3 showed that the activity of glutathione-S-transferase significantly increased after treatment with LC50 of the essential oils and arranged as (1 003.00±15.30), (881.30±8.50), and (771.70±10.10) nmole/min/mg for chamomile, marjoram and positive control (Triton X-100), respectively. Regarding α, β esterase, they were significantly increased and reached (19.90±1.34) mg-α-naphthol released/min/gb wt and (7.85±0.14) mg-β-naphthol released/min/gb wt with LC50 of marjoram compared with other treatments. While alkaline phosphatase was significantly decreased to (4.03±0.15) and (4.66±0.06) U/gb wt treated with LC50 of marjoram and chamomile compared with positive control (5.70±0.11) U/gb wt.
Table 3. Effect of 24 h treatment by LC50 of essential oils on non specific esterases, phosphatase (alkaline, acid), glutathione-S-transferase and protease activities of adult T. urticae (mean ±SD).
| Treatment | α-Esterase (A) | Fold | β-Esterase (B) | Fold | Alkaline phosphatase (C) | Fold | Acid phosphatase (C) | Fold | Glutathione-S-transferase (D) | Fold | Protease (E) | Fold |
| Negative control | 2.79±0.21c | 0.90±0.01c | 7.15±0.17a | 2.26±0.06b | 771.00±38.50c | 129.00±4.00a | ||||||
| Positive control (Triton X-100) | 8.62±0.13b | 2.72±0.05b | 5.70±0.11b | 2.22±0.11c | 771.70±10.10c | 112.20±1.52b | ||||||
| M. hortensis | 19.90±1.34a | 2.3 | 7.85±0.14a | 2.8 | 4.03±0.15d | 0.7 | 2.21±0.04b | 0.9 | 881.30±8.50b | 1.1 | 80.33±2.52d | 0.7 |
| C. recutita | 8.99±0.12b | 2.65±0.05b | 4.66±0.06c | 0.8 | 2.50±0.10a | 1.1 | 1 003.00±15.30a | 1.2 | 65.33±1.15c | 0.5 |
Mean bearing different superscript are significantly different at P<0.01; A: mg-α-naphthol released/min/gb wt; B: mg-β-naphthol released/min/gb wt; C: U/gb wt; D: nmole/min/mg; E: OD unit×103/min/gb wt.
Protease enzymes were significantly decreased at LC50 of both chamomile and marjoram compared with positive control, 65.33, 80.33, 112.20, respectively which help mite for recovery and survival and defense against degradation of protein (Table 3).
3.2. GC-MS analysis of essential oil of chamomile
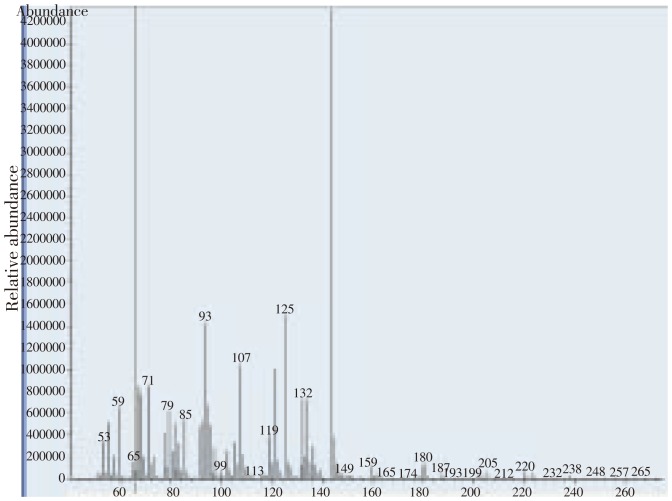
The two essential oil extracts of chamomile and marjoram had the most potent acaricidal activities against T. urticae. The detailed chemical compositions of the two essential oils were analyzed by GC/MS as shown in Table 4. GC-MS analysis of C. recutita (Table 4, Table 5 and Figure 1) proved the presence of thirteen components. The major essential oil contents of C. recutitae are α-bisabolol oxide A (35.251%), and trans β-farersene (7.758%).
Table 4. Composition of chamomile (C. recutita L) and marjoram (M. hortensis L) essential oil.
| Compound | Retention time (min) | Percentage (%) | Molecular formula | Molecular weight | |
| Chamomile | β-Ocimene | 13.597 | 1.435 | C10H16 | 136.23 |
| γ-Terpinene | 13.963 | 0.678 | C10H16 | 136.23 | |
| Artemisia ketone | 14.255 | 1.305 | C10H16O | 152.23 | |
| Bicycloe lemene | 26.603 | 0.739 | C15H24 | 204.00 | |
| Trans-β-farnesene | 34.379 | 7.758 | C15H24 | 204.19 | |
| Germacrene-D | 34.819 | 0.122 | C15H24 | 204.19 | |
| α-Farnesene | 36.319 | 1.399 | C15H24 | 204.19 | |
| α-Calacorene | 36.702 | 1.534 | C15H24 | 204.35 | |
| 6 α-Cadina-4,9-diene | 43.843 | 0.893 | C15H24 | 204.35 | |
| Bisabolol oxide A | 52.128 | 35.251 | C15H26O | 238.54 | |
| Hexahydrofarnesyl acetone | 53.690 | 1.249 | C18H36O | 268.00 | |
| Tricosane | 67.160 | 0.839 | C23H48 | 324.63 | |
| Heptacosane | 70.856 | 1.636 | C27H56 | 380.00 | |
| Marjoram | α-Pinene | 8.476 | 1.757 | C10H16 | 136.23 |
| Sabinene | 10.473 | 10.904 | C10H16 | 136.24 | |
| β-Myrcene | 11.125 | 1.386 | C10H16 | 136.24 | |
| p-Cymene | 13.093 | 23.404 | C10H14 | 134.22 | |
| γ-Terpinene | 14.512 | 9.034 | C10H16 | 136.23 | |
| Cis-β-terpineol | 15.039 | 1.152 | C10H18O | 154.24 | |
| α-Terpinolene | 15.645 | 2.678 | C10H16 | 136.23 | |
| Cis-sabinehydrate | 16.927 | 1.685 | C16H18O | 154.24 | |
| Trans-4-thujanol | 16.990 | 0.164 | C16H18O | 154.25 | |
| Terpinene-4-ol | 21.287 | 23.860 | C16H18O | 154.25 | |
| α-Terpineol | 21.768 | 6.421 | C12H20O | 196.29 | |
| Linalyl acetate | 23.707 | 3.693 | C16H18O | 154.28 | |
| β-Caryphollene | 30.974 | 4.820 | C15H24 | 204.35 | |
| Spathulenol | 39.197 | 2.876 | C15H24O | 220.00 | |
Chamomile: Non identified peaks=45.872%; Identified peaks=54.238%.
Marjoram: Non identified peaks=10.218%; Identified peaks=89.892%.
Table 5. Major compounds of chamomile and marjoram essential oil and degradation products.
| Compound | Molecular weight | Mass to charge ratio | |
| Chamomile | Bisabolol oxide A | 238.54 | 143-132-125-119-107-99-93-85-79-71-65-59-55-53 |
| Trans-β-farnesene | 204.19 | 228-216-202-185-173-159-143-129-107-93-71-55 | |
| Marjoram | Terpinene-4-ol | 154.25 | 148-140-136-132-125-121-117-111-105-101-97-93-86-81-77-71-67-63-59-55-51 |
| p-cymene | 134.22 | 134-119-115-107-103-91-87-81-77-65-51 | |
| Sabinene | 136.24 | 136-121-105-93-77-69-63-53 | |
| γ-Terpinene | 136.23 | 136-121-115-105-102-98-80-77-68-65-62-55-51 | |
| α-Terpineol | 196.29 | 136-121-107-97-93-85-81-77-71-67-59-55-51 | |
| β-Caryphllene | 204.35 | 204-189-175-161-147-133-125-125-120-115-110-105-98-93-84-79-69-55-50 | |
Figure 1. Chromatogram of bisabolol oxid A in chamomile.
3.3. GC-MS analysis of essential oil of marjoram
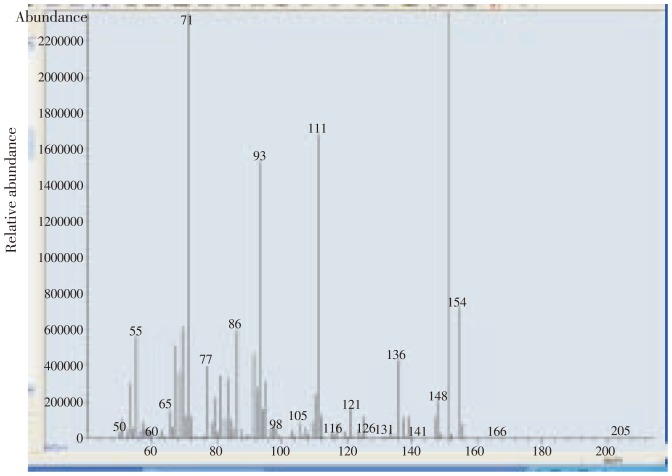
The major essential oil of marjoram was terpinen-4-ol (23.860%) (Figure 2 and Table 5) and in chamomile the major component was α-bisabolol oxide A (35.251%) (Figure 1 and Table 5) which may be responsible for controlling T. urticae.
Figure 2. Chromatogram of terpinene-4-ol in marjoram.
4. Discussion
No resistance was noted to essential oils in mites while the difference in the potency of the type of essential oils was reported. The highly effective essential oils were used at an early stage to control mite populations at isolated loci, conserve natural enemies and maximize their role in natural pest control. The rotation of different highly effective extracts for controlling acaricides was an effective method.
The present results of chamomile are in agreement with those documented by Ma et al[21] who found that the highest effect of terpinene-4-ol on esterase activity was noted during recover stage of housefly adult (Musca domestica). The activities of both acid phosphatase and alkaline phosphatase in insects were induced by terpinen-4-ol. The activities of glutathione-S-transferase were inhibited at exciting, convulsing and paralysis stages, but gradually recovered at recovering stage. The activities of glutathione-S-transferase probably had relations with toxicity of terpinen-4-ol against larvae of the Mythimna separata[21]. This point will be taken in our consideration in the near future to clarify inhibition of both phosphatase or its individual one. The activity of glutathione-S-transferase was inhibited in exciting, convulsing and paralysis stages of the 5th star larvae of Mythimna separata, but it gradually recovered in the recovery stage. This affected the metabolism and activity of phosphatase and esterase enzymes. On the other hand, the inhibited insect glutathione-S-transferase will inhibit normal metabolism. The activity of glutathione-S-transferase at LC50 of the essential oil indicates that the activities of this enzyme were recovered and could defend against free radical and it showed more activity when it could be detected at specific LC50 of essential oils extract in recovered mite.
Acaricidal activities of three essential oil extracts (chamomile, marjoram and Eucalyptus) against T. urticae Koch have been approved that chamomile is the most efficient one[22]. Chamomile and marjoram essential oils showed relationship between essential oil contents and activity of enzyme glutathione-S-transferase, non specific esterase and alkaline phosphatase as well as inhibition of protease enzyme in T. urticae. The major essential oil contents of chamomile are α-bisabolol oxide A (35.251%), and trans-α-farersene (7.758%), while the main components of marjoram are terpinen-4-ol (23.860%), p-cymene (23.404%) and sabinene (10.904%). The major components of both plant extracts may be responsible for the changes in enzyme activities of T. urticae. The present results are in agreement with the data cited by Kawka[23] who studied the effect of chamomile extracts from fresh and dry flowers on T. urticae. Leaves extract showed greater mortality. It has been claimed that increased activities of detoxifying and antioxidant enzyme systems in acaricides had been responsible for the resistance[5].
The decrease in proteinase enzyme which is involved in the biological system of defense proves the presence of proteinase inhibitor in the extracts as cited by Manns, Merijn and Azzouz et al[18],[24],[25] who suggest that the extracts can induce defense gene expression of proteinase inhibitor activity. Proteinase inhibitors are proteins that inhibit digestive enzymes in the gut of arthropod herbivores, which can reduce their growth, reproduction. Glutathione-S-transferases are major enzymes involved in metabolic resistance to insecticides, as well as in the detoxification mechanisms of many molecules and, probably, in the transport of physiologically important lipophilic compounds. Glutathione-S-transferases play an important role in protecting tissues from oxidative damage and stress[11],[26].
The changes in the activity of α, β esterase, glutathione-S-transferase and alkaline phosphatase and protease enzymes in target site susceptibility are key biochemical mechanisms of development of active component of essential oils which show more potency against Tetranychidae. These studies laid a solid foundation for further studies on the biochemical mechanisms of resistance in Tetranychus cinnabarinus and other spider mites. These points need further investigations in the future to prove our suggestions by using individual component and its effect on the enzyme activities of T. urticae. Even this suggestion was approved by Ma et al[21] who determined the bioactivity and effect of terpinen-4-ol on activities of some enzymes in adult housefly (Musca domestica). The results showed that the LD50 of terpinen-4-ol was 23.91 µg/insect. The poisoning symptom of terpinen-4-ol could be divided into four stages i.e. excitation, convulsion, paralysis and recover stages. The highest effect of terpinen-4-ol on esterase activity was measured during recover stage (0.216 8±0.009 1 µmol/20 min). Glutathione-S-transferase, monooxygenase (P450) and esterases activity were detected in resistance in T. urtica[1]. In contrast, no sesquiterpenes were detected in the fresh resin oil and it was constituted basically by monoterpenes hydrocarbons (42.4%) and oxygen-containing monoterpenes (27.7%), of which α-phellandrene (13.9%) and terpinen-4-ol (7.4%) were the major components, respectively[27]. Conceivably, such challenge has forced the development of mechanisms for survival and adaptation throughout evolution and insecticides activity of these essential oils against Anopheles stephensi[28]. Furthermore, and in the above context, induction of detoxifying enzymes by a large number of toxicants has been observed in arthropods[29]. The present results are in agreement with that of Wendel et al[27] who studied the evaluation of the acaricidal activity of some essential oils against T. urticae, such as fresh and aged resin (Protium bahianum) showed higher oil yield 4.6% and 3.2%, respectively. About 22 and 13 components were identified in the oils from the fresh and aged resins, comprising 95.8% and 98.6%, respectively. In the fresh resin oil, monoterpenes (70%) were the major group of constituents, mainly p-cymene (18.3%) followed by hydrocarbons, such as α-phellandrene (14.0%), tricyclene (11.4%) and b-phellandrene (9.1%), while the aged resin oil contained sesquiterpenes as the major group with santalol acetate (83.1%) as the principal component.
Treatment with chloroform extract from Kochia scoparia enhanced SOD, POD and CAT activities during the 24 hour after treatment[30],[31] and traditional Chinese plant can cause toxicity to Tetranychus cinnabarinus[32]–[34] and even glucoside had acaricial effect[35]. Acaricidal activities of Wikstroemia chamaedaphne extracts against Tetranychus were also reported. Twenty-nine compounds were identified with potential acaricidal activity against Tetranychus cinnabarinus[36] and had effect on the activity of Tetranychus enzymes[37]. The essential oils from accessions of Lippia sidoides Cham. (Verbenaceae) were characterized by GC and GC/MS and investigated for their acaricidal activity against the two-spotted spider mite T. urticae Koch[38].
In conclusion, three essential oils of chamomile, marjoram and Eucalyptus possess the acaricidal activity against T. urticae. However, futher investigation is needed to study the components of theses plants which are responsible for inhibiting the activities of enzymes in T. urticae.
Acknowledgments
Authors would like to thank the Management of the Faculty of Agriculture, Cairo University, Department Biochemistry (Protein Lab) for ongoing cooperation to support research and providing funds and facilities necessary to achieve the desired goals of research.
Footnotes
Foundation Project: Supported by Cairo University, Faculty of Agriculture, Deptarment of Biochemistry, Cairo, Egypt.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Puinean AM, Denholm I, Millar NS, Nauen R, Williamson MS. Characterisation of imidacloprid resistance mechanisms in the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae) Pestic Biochem Physiol. 2010;97(2):129–132. [Google Scholar]
- 2.Soliman MMM. Phytochemical and toxicological studies of Artemisia L. (Compositae) essential oil against some insect pests. Arch Phytopathol Plant Prot. 2007;40(2):128–138. [Google Scholar]
- 3.Tsagkarakou A, Van Leeuwen T, Khajehali J, Ilias A, Grispou M, Williamson MS, et al. Identification of pyrethroid resistance associated mutations in the para sodium channel of the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae) Insect Mol Biol. 2009;18(5):583–593. doi: 10.1111/j.1365-2583.2009.00900.x. [DOI] [PubMed] [Google Scholar]
- 4.Sanil D, Shetty NJ. Genetic study of propoxur resistance-a carbamate insecticide in the malaria mosquito, Anopheles stephensi Liston. Malar Res Treat. 2010;2010:1–6. doi: 10.4061/2010/502824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afify AMR, El-Beltagi HS, Fayed SAS, Shalaby EA. Acaricidal activity of different extracts from Syzygium cumini L. Skeels (Pomposia) against Tetranychus urticae Koch. Asian Pac J Trop Biomed. 2011;1(5):359–364. doi: 10.1016/S2221-1691(11)60080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray MB. Botanical insecticides detrrents and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 7.Afify AMR, Mahmoud AM, El-Gammal HA, Attallah ER. Multiresidue method of analysis for determination of 150 pesticides in grapes using quick and easy method (QuEChERS) and LC-MS/MS determination. Int J Food Agric Environ. 2010;8(2):602–606. [Google Scholar]
- 8.Raina R, Pawan K, Verma NK, Shahid P, Prawez PS. Induction of oxidative stress and lipid peroxidation in rats chronically exposed to cypermethrin through dermal application. J Vet Sci. 2009;10(3):257–259. doi: 10.4142/jvs.2009.10.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M, Hossain A, Islam MS. Effect of neem leaf dust and a commercial formulation of neem compound on the longevity, fecundity and ovarian development of melon fly, Bactrocera cucurbitae (Coquillet) and oriental fruit by Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) Pak J Biol Sci. 2007;10(20):3656–3661. doi: 10.3923/pjbs.2007.3656.3661. [DOI] [PubMed] [Google Scholar]
- 10.Jourdie V, Alvarez N, Molina-ochoa J, Wiliams T, Bergvinson D, Benrey B, et al. Population genetic structure of two primary parasitoids of Spodoptera frugiperda (Lepidoptera), Chelonus insularis and Campoletis sonorensis (Hymenoptera): to what extent is the host plant important? Mol Ecol. 2010;19(10):2168–2179. doi: 10.1111/j.1365-294X.2010.04625.x. [DOI] [PubMed] [Google Scholar]
- 11.Gui Z, Hou C, Liu T, Qin G, Li M, Jin B. Effects of insect viruses and pesticides on glutathione-S-transferase activity and gene expression in Bombyx mori. J Econ Entomol. 2009;102(4):1591–1598. doi: 10.1603/029.102.0425. [DOI] [PubMed] [Google Scholar]
- 12.Afify AMR. Biological function of xenobiotics through protein binding and transportation in living cells. Int J Agric Res. 2010;5:562–575. [Google Scholar]
- 13.Çalmaşur Ö, Aslanand I, Şahin F. Insecticidal and acaricidal effect of three Lamiaceae plant essential oils against Tetranychus urticae Koch and Bemisia tabaci Genn. Ind Crops Prod. 2006;23(2):140–146. [Google Scholar]
- 14.Aroiee H, Mosapoor S, Karimzadeh H. Control of greenhouse whitefly (Trialeurodes vaporariorum) by thyme and peppermint. KMITL Sci J. 2005;5(2):511–514. [Google Scholar]
- 15.Finney DJ. Probit analysis. 3rd ed. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- 16.Asperen KV. A study of housefly esterase by means of sensitive colourimetric method. J Insect Physiol. 1962;8:401–416. [Google Scholar]
- 17.Villanueva RT, Walgenbach JF. Acaricidal properties of spinosad against Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae) J Econ Entomol. 2006;99(3):843–849. doi: 10.1603/0022-0493-99.3.843. [DOI] [PubMed] [Google Scholar]
- 18.Born K, Manns A, Dzeyk K, Lutz-Wahl S, Gau D, Fischer L. Evaluation of ultrasound velocity measurements for estimating protease activities using casein as substrate. Biotechnol Lett. 2009;32(2):249–253. doi: 10.1007/s10529-009-0135-x. [DOI] [PubMed] [Google Scholar]
- 19.El-Sharabasy HM. Acaricidal activities of Artemisia judaica L. extracts against Tetranychus urticae Koch and its predator Phytoseiulus persimilis Athias Henriot (Tetranychidae: Phytoseiidae) J Biopestic. 2010;3(2):514–519. [Google Scholar]
- 20.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 21.Ma ZQ, Feng J, Guo ZB, Zhang X. Effects of terpinen-4-ol on four kinds of metabolizing enzymes and polyphenol oxidase in Musca domestica. J Zhejiang Univ Agric Life Sci. 2008;34(5):509–515. [Google Scholar]
- 22.Sertkaya E, Kamuran KK, Soylu S. Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite (Tetranychus cinnabarinus Boisd.) (Acarina: Tetranychidae) Ind Crops Prod. 2010;31(1):107–112. [Google Scholar]
- 23.Kawka B. Effect of chamomile extracts on biology of Tetranychus urticae Koch feeding on Algerian ivy (Hedera canariensis L.) Ann Warsaw Agric Univ Hortic Landsc Archit. 2004;25:75–79. [Google Scholar]
- 24.Kant MR, Sabelis MW, Haring MA, Schuurink RC. Intraspecific variation in a generalist herbivore accounts for differential induction and impact of host plant defenses. Proc R Soc Biol Sci. 2008;275:443–452. doi: 10.1098/rspb.2007.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzouz H, Campan ED, Cherqui A, Saguez J, Couty A, Jouanin L, et al. Potential effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid parasitoid Aphidius ervi Haliday (Hymenoptera, Braconidae) J Insect Physiol. 2005;51(8):941–951. doi: 10.1016/j.jinsphys.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Ugurlu S, Konus MB, Iscan M. Pyrethroid resistance and possible involvement of glutathione-S-transferases in Helicoverpa armigera from Turkey. Phytoparasitica. 2007;35(1):23–26. [Google Scholar]
- 27.Wendel JTP, Jose CSD, Claudio AG, Adelmo CHR. Composition and acaricidal activity of the Resin's essential oil of Protium bahianum daly against two spotted spider mite (Tetranychus urticae) J Essent Oil Res. 2007;19:379–383. [Google Scholar]
- 28.Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour Technol. 2005;96:1749–1757. doi: 10.1016/j.biortech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Cao H, Wang YN, Liu SQ, Li XH, Shi GL. Effects of Kochia scoparia extracts to activities of several enzymes of Tetranychus viennensis. Sci Silvae Sinicae. 2008;42(2):68–72. [Google Scholar]
- 30.Wang YN, Cheng J, Jin YS, Ren JJ, Guo HL, Zhao L, et al. Effects of chloroform extracts from Kochia scoparia on protect enzyme activity of Tetranychus viennensis; Bioinformatics and Biomedical Engineering (iCBBE), 4th International Conference; 2010. pp. 1–4. [Google Scholar]
- 31.Cao H, Wang YN, Liu SQ, Zhao LL, Ping L, Yu TQ, et al. Effects of the chloroform extracts of Kochia scoparia to several enzyme systems in Tetranychus viennensis. Sci Silvae Sinicae. 2007;43:68–72. [Google Scholar]
- 32.Ren JJ, Shi GL, Gu JC, Wang JW, Zheng Y, Wang YN. Contact toxicity of crude extracts from thirty-one acaricidal plants in Northeastern China against Tetranychus cinnabarinus. J Beijing Univ Agric. 2009;24:17–21. [Google Scholar]
- 33.Shen ZJ, Wang HX, Shi GL, Wang YN. Biological activities of extracts from 3 species of plants against Tetranychus cinnabarinus. J Beijing Univ Agric. 2008;23:22–24. [Google Scholar]
- 34.Xiao DS, Yang YM, Yu GY. The relationship among the organelles and the implication of yin-yang and wuxing in Chinese traditional medicine. J Zhejiang Univ Tradit Chin Med. 2008;32(3) [Google Scholar]
- 35.Ren XH, Du G, Zhou J, Bing-Feng Zhou BF, Zhang XH, et al. Study on the spectroscopy of two andrographolide glucoside. Chin J Anal Chem. 2007;35(2) [Google Scholar]
- 36.Wang YN, Bu CY, Jin YS, Ren JJ, Guo HL, Zhao L, et al. Acaricidal activities of Wikstroemia chamaedaphne extracts against Tetranychus urticae and Tetranychus cinnabarinus (Acari: Tetranychidae); 4th International Conference on Bioinformatics and Biomedical Engineering; 2010. pp. 1–5. [Google Scholar]
- 37.Wang YN, Jin YS, Shi GL, Bu CY, Zhao L, Du J, et al. Effects of the root extracts of Stellera chamaejasmel L. on the activity of two enzymies of Tetranychus cinnabarinus; Symposium on Photonics and Optoelectronics; 2009. pp. 1–5. [Google Scholar]
- 38.Cavalcanti SC, Niculau Edos S, Blank AF, Câmara CA, Araújo IN, Alves PB. Composition and acaricidal activity of Lippia sidoides essential oil against two-spotted spider mite (Tetranychus urticae Koch) Bioresour Technol. 2010;101(2):829–832. doi: 10.1016/j.biortech.2009.08.053. [DOI] [PubMed] [Google Scholar]