Abstract
Objective
To identify more effective and less toxic drugs to treat animal toxoplasmosis.
Methods
Efficacy of seven kinds of sulfonamides against Toxoplasma gondii (T. gondii) in an acute murine model was evaluated. The mice used throughout the study were randomly assigned to many groups (10 mice each), which either remained uninfected or were infected intraperitoneally with tachyzoites of T. gondii (strains RH and CN). All groups were then treated with different sulfonamides and the optimal treatment protocol was determined candidates. Sulfadiazine-sodium (SD) was used for comparison.
Results
The optimal therapy involved gavaging mice twice per day with 250 mg/kg bw of sulfachloropyrazine-sodium (SPZ) for five days. Using this protocol, the average survival time and the time-point of 50% fatalities were prolonged significantly compared with SD treatment. Treatment with SPZ protected 40% of mice from death, and the heart and kidney tissue of these animals was parasite-free, as determined by nested-PCR. SPZ showed excellent therapeutic effects in the treatment of T. gondii in an acute murine model and is therefore a promising drug candidate for the treatment and prevention of T. gondii in animals.
Conclusions
It can be concluded that the effective drug sulfachloropyrazine may be the new therapeutic options against animal toxoplasmosis.
Keywords: Toxoplasmosis, Sulfonamides, Sulfachloropyrazine-sodium, Efficacy, Therapy, Toxoplasma gondii, Murine, Therapeutic effect
1. Introduction
Toxoplasma gondii (T. gondii) has been identified as a major opportunistic pathogen in immunocompromised individuals (e.g., patients with AIDS or organ transplant recipients) and neonates. Infection can result in encephalitis, chorioretinitis or congenital transmission if a seronegative pregnant women is infected[1],[2]. T. gondii is an obligate intracellular protozoan pathogen with worldwide distribution that can infect almost all warmed-blooded animals.
Other members of the same phylum, Apicomplexa, include the human pathogens Plasmodium and Cryptosporidium, and the animal pathogens Eimeria and Sarcocystis[3]–[5]. T. gondii has become a model organism for the study of the Apicomplexa, as it is the most experimentally tractable organism in this important group of intracellular parasites that includes Plasmodium, Eimeria, Cryptosporidium, Neospora, and Theileria. Felids are the key animal species in the life cycle of this parasite because they are the definitive hosts that can excrete the environmentally-resistant stage, the oocyst[6]. According to the serological investigations, about 30% of the human population is infected with this parasite[5]. A recent serosurvey using samples from the population-based National Health and Examination Nutrition Study found a decrease in the age-adjusted T. gondii prevalence in USA-born persons, of 12–49 years of age, from 14.1% in 1988–1994 to 9% in 1999–2004[6],[7]. In the mainland of China, the rate of human infection with T. gondii has been reported to be 7.88% nationwide, as detected by ELISA[8].
At present, there are no non-infective commercial vaccines to prevent clinical toxoplasmosis in humans and animals[9],[10]. Research into potential drug treatments have excluded many antibiotics, synthetic drugs and traditional Chinese medicines as being ineffective against the T. gondii. Sulfonamides are currently considered as the best therapeutic option for T. gondii infection. Sulfonamides are a class of broad spectrum synthetic antibiotics that are effective against many kinds of microorganisms and have been used clinically for over 60 years[10]. The sulfonamides that have been used against T. gondii include the sulfonamides, sulfamethoxazole, sulfanilanilides, sulfathiazole, sulfisoxazine and sulfadiazine[11]–[13]. The synergistic combination of sulfadiazine-sodium (SD) and pyrimethamine is currently considered to be the most effective first-line antitoxoplasma therapy, and is also used as a standard control therapy. This type of therapy has been used after the diagnosis of prenatal infection[2]. However, these drugs are not always efficacious and often cannot be tolerated due to severe toxic side effects[14]. Therapy is hampered by hematologic toxicity and allergic reactions in 5%–15% of patients, and some patients require continuous treatment[15],[16]. Furthermore, some patients are intolerant of one of these regimens and require alternative therapies[17].
Few medications are currently available to treat animal toxoplasmosis and those that are available are limited due to poor efficacy. There is therefore an urgent need for new, effective drugs to treat T. gondii, particularly in livestock and other warm-blooded animals. In this study, a variety of candidate sulfonamides, some of which are widely used as human and animal antibiotics[18] were used to treat T. gondii in an acute murine infection model and the potency and safety of these sulfonamides against infection were evaluated.
2. Materials and methods
2.1. Animals
Outbred, strain Kunming (KM), female mice (Slack Shanghai Laboratory Animal Co., Ltd., China), weighing between 20 and 24 g, were used in each experiment. The animals were acclimatized for two days, then maintained in a suitable rearing environment with free access to water and rodent food throughout the experiment[19].
2.2. Parasites
Tachyzoites of T. gondii of the swine strain, Changning (CN), and the human strain RH, were propagated in our laboratory (Shanghai Veterinary Research Institute, CAAS, China). Highly virulent tachyzoites were maintained in the peritoneal cavities by propagating every three or four days in KM mice, three times. The harvested tachyzoites were resuspended in physiological saline and each experimental mouse was inoculated intraperitoneally with 5×103 organisms (in 0.2 mL).
2.3. Drugs
Sulfadiazine-sodium (SD, minimum purity 99.53%), sulfadimidine-sodium (SDD, 99.55%), suflamathoxydiazine-sodium (SMD, 99.23%) and trimethoprimlactate (TMP, 99.55%) were obtained from Yanshi Shuoda Pharmaceutical & Co., Ltd. China. Sulfamathoxypyridazine-sodium (SMP, 99.50%), sulfachlorpryridazine-sodium (SPDZ, 99.81%) and sulfachloropyrazine-sodium (SPZ, 99.97%) were produced by Shanghai Baoshan Zhenzong Biochemistry Engineering Factory, Henan, China.
All agents were provided in the powder form of the pharmaceutical raw materials (containing minimum purity), and were dissolved in physiological saline. Then the mixtures were sonicated to produce a smooth dispersion and the desired concentration was prepared daily in physiological saline and administered to mice at a dosage of 250 mg/kg/day, according to the animal's body weight, for 5 or 10 days, 24 h after infection.
2.4. Evaluating the efficacy of sulfonamides
Ninety mice were randomly allocated into nine groups (10 mice each) designated G1–G9. Eight of these groups (G1–G8) were infected with RH strains, the other group (G9) remained uninfected. Each drug was dissolved in physiological saline and prepared daily as a liquid suspension. 24 h after intraperitoneal infection, the G1–G7 mice were administered each drug intragastrically daily for five days using a 16-gauge blunt feeding needle. Mice from the untreated group (G8) and the uninfected group (G9) were administered physiological saline intragastrically daily. Over the course of the experiment (30 days), mice were observed daily and the mortality rate was recorded.
2.5. Different methods of drug administration
Ninety mice were randomized into nine groups (G1–G9). All mice were intraperitoneally infected with RH strains, except for group G5 and G8 mice who were infected with the CN strain as a control, and G9 mice remained uninfected. After 24 h, the mice were administered orally the specified drugs. G1–G5 mice were administered the SPZ at the same dosage via different methods: G1 mice received SPZ by drinking water (100 mL) daily for five days, G2 mice by gavage once per day for ten days, G3 mice by gavage twice a day (morning and night) for five days, G4 mice by gavage once a day for five days, G5 mice by gavage once a day for 10 days. G6 mice received SD by gavage once a day for 10 days as a control. G7 and G8 mice received physiological saline solution as infected untreated controls. G9 mice remained uninfected and received physiological saline solution. All mice were observed and their condition was recorded twice daily. The experiment continued for 65 days.
2.6. Nested-PCR detection
At the end of the 65-day experiment described above, live mice were euthanized and the viscera organs and brain tissue were excised. These tissues were then digested in trypsin overnight at 37 °C, and genomic DNA was extracted using the Resin-based™ Genomic DNA Extraction Kit (Shanghai SBS Genetech Co., Ltd., China). The presence of parasites was then detected by nested-PCR, using the extracted genomic DNA as a template, to estimate the effectiveness of the treatment.
For specific T. gondii detection, nested-PCR was performed by two rounds of PCR with two pairs of primers targeting a 433 bp fragment of the ITS1-5.8S rRNA-ITS2 gene. The primers included: an external forward primer: TOXO1 (5′-ACC TTT GAA TCC CAA GCA-3′), an external reverse primer: TOXO2 (5′-TAA ATC GGA CAA ACG CCC-3′), an inner forward primer: TOXO3 (5′-TTT GCA TTC AAG AAG CGT G-3′) and an inner reverse primer: TOXO4 (5′-AAG GTG CCA TTT GCG TTC-3′).
2.7. Statistical analysis
Statistical analysis was done with the Statistical Package for the Social Sciences (SPSS, version 13.0.) (SPSS, SL, Madrid, Spain).
3. Results
3.1. Efficacy of sulfonamides against toxoplasmosis
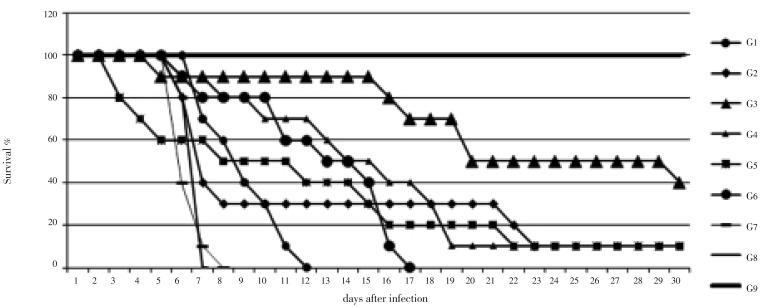
Seven kinds of sulfonamides were selected to treat acute murine toxoplasmosis. Infected mice were treated daily with drugs, as shown in Table 1. The results demonstrated that SD and SPZ administered orally have significant anti-toxoplasmosis activities. Mice treated with these drugs in groups G1 and G6, respectively, became more active and healthy than the untreated mice which appeared ill with ruffled fur and a hunched posture. There was no significant difference between the mice in groups G1 and G6 regarding the average number of days of survival following treatment, and the time of death was significantly delayed in these two groups. A significant difference was detected between these two groups and the other groups regarding the time-point of 50% fatalities and the average number of survival days (P<0.05).
Table 1. Activities of seven kinds of sulfonamides against T. gondii (mean±SD).
| Groups | Drugs* | Average survival days | Time-point of 50% fatalities (d) | Time-point of 100% fatalities (d) |
| 1 | SD | 19.00±5.92c | 20.0 | >30 |
| 2 | SDD | 10.25±4.43b | 12.0 | 15.0 |
| 3 | SMD | 7.30±1.67ab | 7.0 | 9.0 |
| 4 | SMP | 7.60±1.10ab | 8.0 | 9.0 |
| 5 | SPDZ | 5.85±0.85a | 5.5 | 7.0 |
| 6 | SPZ | 16.05±4.58c | 17.0 | >30 |
| 7 | TMP | 6.95±1.57ab | 6.0 | 9.0 |
| 8 | Untreated | 5.40±0.21a | 5.5 | 5.5 |
| 9 | Uninfected | >30 | >30 | >30 |
*: A dosage of 250 mg/kg/day b.w. was used for each drug; a, b, c indicates drugs for which the average number of survival days was significantly different from the other groups (P<0.05).
3.2. Effects of different administration methods
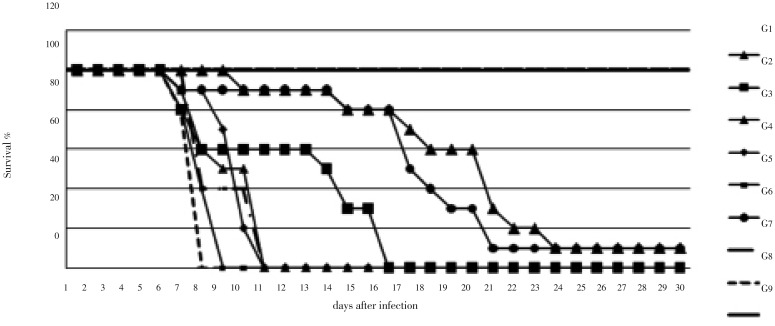
Based on the results presented in Table 1, the anti-toxoplasmosis potency of SD and SPZ was validated in mice via different therapeutic approaches, as shown in Table 2. The results demonstrated that administration via gavage was more effective than free drinking, and oral administration of SPZ twice a day for five days was more effective than once a day. The average number of survival days for mice in the G3 group, who were administered SPZ orally twice a day for five days, was significantly different from that of other groups (P<0.05). At the end of this study (after 65 days), four mice survived in the G3 group, compared with only one mouse in the G2, G4 and G5 groups. The virulence of T. gondii strains RH and CN showed no significant differences. An increased survival time was observed in all of the mice treated with drugs.
Table 2. Efficacy of different administration methods with SPZ and SD (mean±SD).
| Groups | Drugs* | T. gondii strains | Treatment method and duration | Number of surviving mice | Mean survivaldays | Time-point of 50% fatalities (d) |
| 1 | SPZ | RH | Watering 5 d (all day) | 0/10a | 8.95±1.78a | 9.0 |
| 2 | SPZ | RH | Gavage 10 d (once) | 1/10b | 16.00±18.37a | 7.5 |
| 3 | SPZ | RH | Gavage 5 d (twice) | 4/10c | 41.25±25.76b | 19.5 |
| 4 | SPZ | RH | Gavage 5 d (once) | 1/10b | 19.10±16.76a | 14.0 |
| 5 | SPZ | CN | Gavage 10 d (once) | 1/10b | 15.25±18.70a | 8.0 |
| 6 | SD | RH | Gavage 10 d (once) | 0/10a | 13.05±3.85a | 13.0 |
| 7 | Untreated | RH | Physiological saline | 0/10a | 6.25±0.72a | 6.0 |
| 8 | Untreated | CN | Physiological saline | 0/10a | 6.60±0.61a | 6.5 |
| 9 | Uninfected | – | Physiological saline | 10/10d | >65 | >65 |
*: A dosage of 250 mg/kg/day b.w. was used for each drug; a, b, c, d indicates drugs for which the average number of survival days was significantly different from the other groups (P<0.05).
3.3. Detection of T. gondii by nested PCR
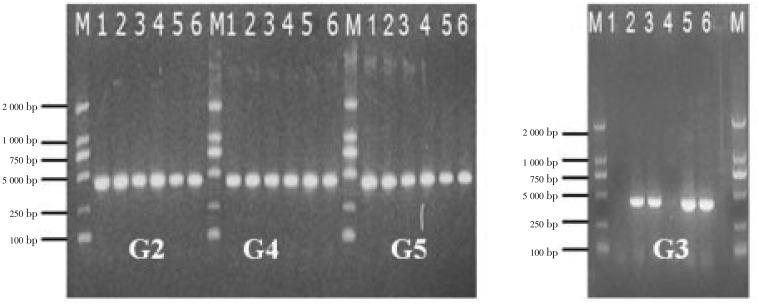
At the end of the experiment (after 2 months), surviving mice (as indicated in Table 2 and Figure 2) were euthanized and the specified internal organs (heart, liver, lung, kidney), muscle and brain were removed and used in the detection of T. gondii by nested PCR. As shown in Figure 3, in G3 mice, who were administered SPZ orally twice a day for five days, no T. gondii could be detected in the heart and kidney tissues. In G2, G4, and G5 mice, in which SPZ was administered intragastrically once a day for five or 10 days, T. gondii was detected in all of the visceral organs and muscles, as well as the brain tissue.
Figure 2. The efficacy of different treatment methods using the same drug SPZ.
G1: watering 5 d (all day); G2: gavage 10 d (once); G3: gavage 5 d (twice); G4: gavage 5 d (once); G5: gavage 10 d (once); G6: gavage 10 d (once); G7, G8 and G9: physiological saline.
Figure 3. Detection of T. gondii in the tissues of the internal organs and brains of mice from the various treatment groups by nested PCR.
The PCR products amplified from the internal organs, muscles and brains of mice treated with SPZ once a day for five or ten days (groups G2, G3, G4 and G5), run on a 2.0% agarose gel. Line M is the molecular weight marker (DL 2 000 DNA marker). PCR products amplified from the heart, liver, lung, kidney, muscle and brain were loaded in lanes 1, 2, 3, 4, 5 and 6, respectively.
Figure 1. The activities of seven types of sulfonamides against T. gondii.
G1: SD; G2: SDD; G3: SMD; G4: SMP; G5: SPDZ; G6: SPZ; G7: TMP; G8 and G9: physiological saline.
4. Discussion
T. gondii causes serious disease and death in humans and animals, as well as significant socioeconomic and public health problems, and there are considerable economic losses in the meat industry[20]. Infections by the protozoan parasite T. gondii are widely prevalent in humans and other animals on all continents[21].
Sulfonamides are chemosynthesis drugs that are well-known inhibitors of dihydropteroate synthase, an important enzyme in the folate pathway, in many microorganisms[10]. Many types of sulfonamides have been applied clinically (e.g., sulfathiazole, sulfamethoxazole, sulfamethazine, SD, sulfabenzamide, sulfamethizole, sulfisoxazole, sulfapyridine, sulfamerazine, sulfacetamide, sulfanilamide, sulfadoxine, sulfinpyrazone, sulfadimethoxine), but little effectiveness has been reported against parasites[11],[22]. Pyrimethamine plus SD are routinely used for drug screening[13],[14],[23]–[25]. The seven sulfonamides selected in this study had not previously been applied to treat T. gondii infection, but had been used against other protozoan infections, such as poultry coccidiosis for many years. As T. gondii belongs to the phylum Apicomplexa, it is similar to the animal pathogens Eimeria[26]. We therefore chose therapeutic agents widely used in treating poultry coccidiosis (such as SPZ) for testing in this study.
Our results indicated that SPZ has great efficacy against T. gondii. SPZ was first issued a veterinary certificate in China by the Ciba-Geigy Animal Health Co., Ltd. in 1995. It is now made and widely used in China, in particular for treating poultry coccidiosis, but its activity against T. gondii has not been reported. During our experiment (one month), the average survival time of mice in the groups being treated with SPZ and SD orally at a dosage of 250 mg/kg/day increased significantly, compared with the mice in the infected untreated group who appeared ill with ruffled fur and a hunched posture and died after 5.5 days. A significant difference was detected in the average survival time of mice between the drug treated groups and the untreated group (P<0.05). A significant difference was also detected between the drug treated groups and the untreated group regarding the time-points for 50% or 100% fatalities. Only one mouse of these two groups survived until the end of the experiment. These findings demonstrated that SPZ was as effective as SD at treating T. gondii infection and protected the infected mice for over one month. Taken together, our results suggested that SPZ has significant anti-toxiplasmosis activity and has the ability to potentially replace SD or other second line drugs in the treatment of toxoplasmosis.
The virulence of RH (human) and CN (swine) strains of T. gondii showed no significant difference regarding the average number of survival days and the time-point of 50% fatalities. CN strains were isolated from swine in Changning (Shanghai, China) and were maintained in our laboratory (Shanghai Veterinary Research Institute, CAAS, China). The RH strain is a virulent strain recognized internationally as a reference strain. Comparison with the pathogenicity of CN and RH strains indicated that local strains of T. gondii, isolated by our laboratory, were highly virulent. This result was consistent with a previous study that found that RH and CN strains belonged to clonal type I, as determined by the typing of 10 genetic markers, including nine nuclear loci and an apicoplast locus[27]. The population structure of T. gondii is highly clonal, consisting of three distinct lineages (type I, II, III) that differ dramatically in virulence. Clonal type I strains typically display an LD100 of a single infectious organism and the RH strain is characteristic of clonal type I strains[28].
In this study, optimization of the treatment protocol indicated that administering the drug via drinking water was not as effective as oral administration, according to the data on protection mortality. This is likely due to the fact that administering the drug via drinking water cannot guarantee that the required dosage is received by the animal, potentially leading to lower efficacy of the drug. However, oral administration ensured that the prescribed amount was received allowing for the full therapeutic effects of the medication. The treatment protocol can therefore determine the dosage of drug intake, affecting the efficacy of therapeutic drugs.
In addition, to determine whether SPZ could protect against the formation of T. gondii tissues cysts, a dynamic tracking test (i.e., nested-PCR) of parasite infection was performed on the tissues of the brain, muscles and internal organs of surviving mice from four of the groups[15]. The results showed that treating mice twice a day with SPZ could eradicate the tissues cysts in the heart and kidneys observed in the other three groups. This demonstrated the importance of selecting the correct treatment protocol.
SPZ is widely used in veterinary medicine[26] in the control of systemic infections due to its low cost and relatively high efficiency. The results of this study indicate the effectiveness of SPZ in the control of T. gondii. It can protect 40% of mice from death and prevent the formation of tissues cysts. In future studies, further optimization of the treatment protocol will be performed and the effects of combining SPZ with other drugs, such as pyrimethamine, will be investigated. SPZ displays equal efficacy to SD but with other favorable characteristics and therefore potentially constitutes the next generation of candidate drug to treat T. gondii.
Acknowledgments
This work was supported in part by a grant from the National Special Research Program for Non-Profit Trades (Agriculture, China) (grant No. 200803017).
Footnotes
Foundation Project: Supported by a grant from The National Special Research Program for Non-Profit Trades (Agriculture, China) ( grant No. 200803017).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Lorraine JB, John DA, Posner GH, Yolken R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother. 2006;50:4206–4208. doi: 10.1128/AAC.00793-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valentini P, Annunziata ML, Angelone DF, Masini L, Santis MD, Testa A, et al. Role of spiramycin/cotrimoxazole association in the mother-to-child transmission of toxoplas- mosis infection in pregnancy. Eur J Clin Microbiol Infect Dis. 2009;28:297–300. doi: 10.1007/s10096-008-0612-5. [DOI] [PubMed] [Google Scholar]
- 3.Kellogg JS, James JM. Development of a PCR-enzyme immunoassay oligoprobe detection method for Toxoplasma gondii oocysts, incorporating PCR controls. Appl Environ Microbiol. 2003;69:5819–5825. doi: 10.1128/AEM.69.10.5819-5825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunay IR, Heimesaat MM, Bushrab FN, Müller R, Stocker HH, Arasteh K, et al. Atovaquone maintenance therapy prevents reactivation of Toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis. Antimicrob Agents Chemother. 2004;48:4848–4854. doi: 10.1128/AAC.48.12.4848-4854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lass A, Pietkiewicz H, Modzelewska E, Dumètre A, Szostakowska B, Myjak P. Detection of Toxoplasma gondii oocysts in environmental soil samples using molecular methods. Eur J Clin Microbiol Infect Dis. 2009;28:599–605. doi: 10.1007/s10096-008-0681-5. [DOI] [PubMed] [Google Scholar]
- 6.Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. 2008;38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Jones JL, Deanna KM, Kolby SL, Wilson M. Toxoplasma gondii infection in the United States, 1999-2004, decline from the prior decade. Am J Trop Med Hyg. 2007;77:405–410. [PubMed] [Google Scholar]
- 8.Coordinating Office of the National Survey on the Important Human Parasitic Diseases A national survey on current status of the important parasitic diseases in human population. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005;23:332–340. [PubMed] [Google Scholar]
- 9.Dubey JP, Shen SK. Rat model of congenital toxoplasmosis. Infect Immun. 1991;59:3301–3302. doi: 10.1128/iai.59.9.3301-3302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chio LC, Bolyard LA, Nasr M, Queener SF. Identification of a class of sulfonamides highly active against dihydropteroate synthase from Toxoplasma gondii, Pneumocystis carinii, and Mycobacterium avium. Antimicrob Agents Chemother. 1996;40:727–733. doi: 10.1128/aac.40.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegra CJ, Boarman D, Kovacs JA, Morrison P, Beaver J, Chabner BA, et al. Interaction of sulfonamide and sulfone compounds with Toxoplasma gondii dihydropteroate synthase. J Clin Invest. 1990;85:371–379. doi: 10.1172/JCI114448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo FG, Huskinson J, Remington JS. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416–1429. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Slifer TR, Araujo FG, Remington JS. Activity of gatifloxacin alone or in combination with pyrimethamine or gamma interferon against Toxoplasma gondii. Antimicrob Agents Chemother. 2001;45:48–51. doi: 10.1128/AAC.45.1.48-51.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romand S, Pudney M, Derouin F. In vitro and in vivo activities of the hydroxynaphthoq- uinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob Agents Chemother. 1993;37:2371–2378. doi: 10.1128/aac.37.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katlama C, Wit SD, O'Doherty E, Glabeke MV, Clumeck N. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1996;22:268–275. doi: 10.1093/clinids/22.2.268. [DOI] [PubMed] [Google Scholar]
- 17.Baggish AL, Hill DR. Antiparasitic agent atovaquone. Antimicrob Agents Chemother. 2002;46:1163–1173. doi: 10.1128/AAC.46.5.1163-1173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberkorn A. Chemotherapy of human and animal coccidioses: state and perspectives. Parasitol Res. 1996;82:193–199. doi: 10.1007/s004360050094. [DOI] [PubMed] [Google Scholar]
- 19.Xiao SH, Keiser J, Jian X, Tanner M, Morson G, Utzinger J. Effect of single-dose oral artemether and tribendimidine on the tegument of adult Clonorchis sinensis in rats. Parasitol Res. 2009;104:533–541. doi: 10.1007/s00436-008-1227-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ. Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 2008;24:190–196. doi: 10.1016/j.pt.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Wess LM, Kim K. Toxoplasma gondii: the model apicomplexan-perspectives and methords. London: Academic Press, Elsevier; 2007. pp. 185–206. [Google Scholar]
- 22.Liguoro MD, Fioretto B, Poltronieri C, Gallina G. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere. 2009;75:1519–1524. doi: 10.1016/j.chemosphere.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Derouin F, Hayat MS. Anti-toxoplasma activities of antiretroviral drugs and interactions with pyrimethamine and sulfadiazine in vitro. Antimicrob Agents Chemother. 2000;44:2575–2577. doi: 10.1128/aac.44.9.2575-2577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons-2002. Ann Intern Med. 2002;137:435–477. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]
- 25.Meneceur P, Bouldouyre MA, Aubert D, Villena I, Menotti J, Sauvage V, et al. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob Agents Chemother. 2008;52:1269–1277. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fajer-Avila EJ, Morales-Covarrubias MS, Selene AR, Roque A, Pablo MB, Crisantema HG. Effectiveness of oral ElancobanTM and Avimix-STTM against Nematopsis (Apicomplexa: Porosporidae) gametocyts infecting the shrimp Litopenaeus vannamei. Aquaculture. 2005;244:11–18. [Google Scholar]
- 27.Zhou P, Zhang H, Lin RQ, Zhang DL, Song HQ, Su CL, et al. Genetic characterization of Toxoplasma gondii isolates from China. Parasitol Int. 2009;58:193–195. doi: 10.1016/j.parint.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Sibley LD, Dana GM, Su CL, Paul MR, Dan KH. Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii. Philos Trans R Soc Lond Biol Sci. 2002;357:81–88. doi: 10.1098/rstb.2001.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]