Abstract
Objective
To evaluate the antimalarial and antiulcerogenic activities of leaf extract and fractions of Melanthera scandens (M. scandens).
Methods
The crude leaf extract (37–111 mg/kg) and fractions (chloroform, ethylacetate and methanol; 78 mg/kg) of M. scadens were investigated for antiplasmodial activity against chloroquine-sensitive Plasmodium berghei infections in mice and for antiulcer activity against experimentally-induced ulcers. The antimalarial activity during early and established infections as well as prophylactic was investigated. Artesunate (5 mg/kg) and pyrimethamine (1.2 mg/kg) were used as positive controls. Thin films made from tail blood of each mouse were used to assess the level of parasitaemia of the mice. Antiulcer activity of the crude extract was also evaluated against indomethacin, ethanol and histamine induced ulcers.
Results
The extract and its fractions dose-dependently reduced parasitaemia induced by chloroquine-sensitive Plasmodium berghei infection in prophylactic, suppressive and curative models in mice. These reductions were statistically significant (P<0.001). They also improved the mean survival time (MST) from 9.28 to 17.73 days as compared with the control (P<0.01–0.001). The activities of extract/fractions were incomparable to that of the standard drugs i.e. artesunate and pyrimethamine. On experimentally-induced ulcers, the extract inhibited indomethacin, ethanol and histamine induced ulcers. These inhibitions were statistically significant (P<0.001) and in a dose-dependent fashion.
Conclusions
The antiplasmodial and antiulcerogenic effects of this plant may in part be mediated through the chemical constituents of the plant.
Keywords: Melanthera scadens, Antiplasmodial activity, Antiulcer activity, Antimalarial activity, Antiulcerogenic activity, Chemical constituent, Parasitaemia, Plasmodium berghei, Artesunate, Indomethacin, Pyrimethamine
1. Introduction
Melanthera scandens (M. scandens) (Schumach. & Thonn.) Roberty (Asteraceae) is a perennial herb up to 1 m to 4 m high, with branches quadrangular and scabrid. It distributes geographically in East, West and South Africa[1]. It is known as ‘ayara edemerong’ by the Ibibios of Akwa Ibom State of Nigeria. The leaves are traditionally used to treat various ailments such as stomach ulcer and sores in Gosomtwi-Atwimakwanwoma district of Ghana[2]. In Nigeria, the leaves are used to treat dysmenorrhoea, diabetes and malaria[3]. It is also used by the Bete people of Issia district of Cote d'Ivoire to treat malaria[4],[5]. The antioxidant[6], in vitro antiplasmodial[4],[5] and antidiabetic[7] activities have been reported. Triterpenoid saponins have been reported in the leaves[8]. In this study, we reported the in vivo antiplasmodial and antiulcer activities of M. scadens as the plant is traditionally used as remedy for malaria and ulcer.
2. Materials and Methods
2.1. Plant materials
The leaves of M. scadens were collected from a bush in Ukap in Ikono Local Government area of Akwa Ibom State. The leaves were identified and authenticated by Dr. Magaret Bassey, a taxonomist in the Department of Botany and Ecological Studies, University of Uyo, Uyo. A voucher specimen of the plant was deposited in the Hebarium of Department of Botany and Ecological Studies, University of Uyo, Uyo.
2.2. Extraction
The plant parts (leaves) were washed and shade-dried for two weeks. The dried leaves were further chopped into small pieces and reduced to powder. The powdered leaf was divided into two parts, one part (1.5 kg) was macerated in 97% ethanol for 72 h to give the crude ethanolic extract while the other part (1.5 kg) was successively and gradiently macerated for 72 h in each of these solvents i.e. chloroform, ethyl acetate and methanol to give the corresponding fractions of these solvents. The liquid filtrates were concentrated and evaporated to dryness in vacuo at 4 °C using rotary evaporator. The yield of each extract was determined; crude ethanolic extract (3.98%), chloroform fraction (0.50%), ethyl acetate fraction (0.30%) and methanolic fraction (0.78%). The dried crude extract and fractions were stored in a refrigerator at 4 °C until use for the proposed experiment.
2.3. Phytochemical screening
Phytochemical screening of the crude extract was carried out employing standard procedures[9], to reveal the presence of chemical constituents such as alkaloids, flavonoids, tannins, terpenes, saponins, anthraquinones, reducing sugars, cardiac glycosides and others.
2.4. Animals
Swiss albino mice (17–24 g) and rats (120–145 g) of both sexes were used for these experiments. They were obtained from University of Uyo Animal House. The animals were housed in standard cages and were maintained on a standard pelleted feed (Guinea feed) and water was given ad libitum.
2.5. Determination of median lethal dose (LD50)
LD50 of the extract was determined using albino mice. The extract was administered intraperitoneally (i.p.) and the method of Miller and Tainter[10] was adopted. This involved the administration of different doses of the extract (100–1 000 mg/kg) to groups of six mice each. The animals were observed for physical manifestation of signs of toxicity. The number of deaths in each group within 24 h was recorded.
2.6. Parasite inoculation
Each mouse used in the experiment was inoculated intraperitoneally with 0.2 mL of infected blood containing about 1×107 Plasmodium berghei (P. berghei) parasitized erythrocytes. The inoculum consisted of 5×107 P. berghei erythrocytes per mL. This was prepared by determining both the percentage parasitaemia and the erythrocytes count of the donor mouse and the blood was diluted with isotonic saline in proportions according to both determinations[11].
2.7. Drug administration
The drugs (artesunate and pyrimethamine), extract and fractions used in the antiplasmodial study were orally administered with the aid of a stainless metallic feeding cannula.
2.8. Evaluation of antiplasmodial activity of the extract and fractions
2.8.1. Evaluation of suppressive activity of the extract and fractions (4-day test)
This test was used to evaluate the schizontocidal activity of the extract, fractions and artesunate against early P. berghei infection in mice. This was done as described by Antia et al[12]. 48 mice were randomly divided into eight groups of six mice each. On the first day (DO), 42 mice were infected with the parasite and randomly divided into various groups. They were administered with the extract, fractions and artesunate. The mice in group 1 were administered with 37 mg/kg, the group 2 (74 mg/kg) and group 3 (111 mg/kg) of crude extract, groups 4, 5 and 6 were administered with 74 mg/kg of the chloroform, ethyl acetate and methanol fractions, respectively, while group 7 was administered with 5 mg/kg of artesunate (positive control), and 10 mL/kg of distilled water was given to group 8 (negative control) for four consecutive days (D0–D3) between 8 am and 9 am. On the fifth day (D4), thin blood film was made from tail blood. The film was then stained with Leishman's stain to reveal parasitized erythocytes out of 500 in a random field of the microscope. The average percentage suppression of parasitaemia was calculated in comparison with the controls as follows:
 |
2.8.2. Evaluation of prophylactic or repository activities of extract and fractions
The repository activity of the extract, fractions and pyrimethamine (daraprim) was assessed by using the method described by Okokon and Nwafor[11]. The mice were randomly divided into seven groups of six mice each. Groups 1–3 were administered with 37, 74 and 111 mg/kg/day of the extract, respectively, while groups 4–7 were respectively given 74 mg/kg/day of the acqueous and chloroform fractions, 1.2 mg/kg/day of pyrimethamine (positive control) and 10 mL/kg of distilled water (negative control). Administration of the extract/fraction/drug continued for three consecutive days (D0–D2). On the fourth day (D3) the mice were inoculated with P. berghei. The parasitaemia level was assessed by blood smears 72 h later.
2.8.3. Evaluation of curative activities of extract and fractions (Rane's test)
This was used to evaluate the schizontocidal activity of the extract, fractions and artesunate in established infection. This was done as described by Okokon and Nwafor[11]. P. berghei was injected intraperitoneally into another 48 mice on the first day (DO). 72 h later (D3), the mice were divided randomly into eight groups of six mice each. Different doses of the extract (37, 74 and 111 mg/kg) were orally administered respectively to mice in groups 1–3. 74 mg/kg of chloroform, ethyl acetate and methanol fractions were administered to groups 4, 5 and 6, respectively, 5 mg/kg/day of artesuante to the group 7 (positive control) and group 8 was given 10 mL/kg of distilled water (negative control). The extract, fractions and drugs were administered once daily for 5 days. Leishman's stained thin smears were prepared from tail blood samples collected on each day of treatment to monitor parasitaemia level. The mean survival time (MST) of the mice in each treatment group was determined over a period of 29 days (D0–D28).
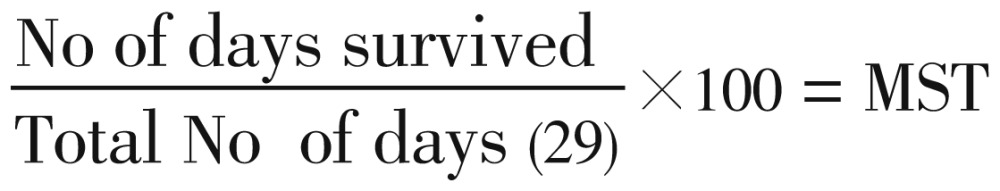 |
2.9. Evaluation of antiulcer activity
2.9.1. Indomethacin-induced ulcer
Male adult albino rats (150–170 g) were randomly divided into five groups of six rats each. The animals were starved from food and water for 24 h and 2 h, respectively before the commencement of experiment[13]. They were treated as follows: group 1 (control) received only indomethacin (Sigma, 60 mg/kg p.o. dissolved in 5% Na2CO3); groups 2–4 were pretreated with M. scandens extract (37, 74 and 111 mg/kg p.o., respectively); group 5 received cimetidine (100 mg/kg p.o. dissolved in 50% Tween 80). 1 h later, groups 2–5 were administered with indomethacin. Animals were killed by cervical dislocation, 4 h after indomethacin administration. The stomaches were removed and opened along the greater curvature. The tissues were fixed with 10% formaldehyde in saline. Macroscopic examination was carried out with a hand lens and the presence of ulcer lesion was scored[13]. Ulcer index (UI) and preventive ratio (PR) of each of the groups pretreated with extract were calculated using standard methods[13],[14].
2.9.2. Ethanol-induced gastric ulceration
The procedures were similar to that used in indomethacin-induced ulceration except that the negative control group (group 1) received only ethanol (2.5 mL/kg p.o.); groups 2–4 were pretreated with M. scandens extract (37, 74 and 111 mg/kg p.o., respectively); and positive control group (group 5) received propranolol (40 mg/kg p.o. dissolved in distilled water). Time of administration of ulcerogen and sacrifice, stomach processing and examination as well as ulcer scoring were similar to that used in indomethacin-induced ulceration.
2.9.3. Histamine-induced gastric ulceration in rats
The procedures were similar to that used in indomethacin-induced ulceration except that the negative control group (group 1) received only histamine acid phosphate (Sigma, 100 mg/kg i.p. dissolved in distilled water)[15]; and positive control group (group 5) received cimetidine (100 mg/kg p.o. dissolved in 50% Tween 80); groups 2–4 were pretreated with M. scandens extract (37, 74 and 111 mg/kg p.o., respectively); group 5 received cimetidine (100 mg/kg p.o. dissolved in 50% Tween 80). Time of administration of ulcerogen (histamine acid phosphate, 100 mg/kg, i.p.), stomach processing and examination as well as ulcer scoring were similar to that used in indomethacin-induced ulceration except that the animals were killed by cervical dislocation 18 h after histamine administration.
2.10. Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM) and were analyzed statistically using one way ANOVA followed by Tukey-kramer multiple comparison test and values of P<0.01 were considered significant.
3. Results
3.1. Phytochemical screening
The phytochemical screening of the ethanolic extract of the leaves of M. scandens revealed the presence of cardiac glycosides, tannins, saponins, terpenes and flavonoids.
3.2. Acute toxicty
The median lethal dose (LD50) was calculated to be (370.00±23.33 mg/kg). The physical signs of toxicity included excitation, paw licking, increased respiratory rate, decreased motor activity, gasping and coma which was followed by death.
3.3. Suppressive activity of ethanolic leaves extract and fractions of M. scandens
The crude extract demonstrated a dose-dependent chemosuppressive effect on the parasitaemia. These effects were statistically significant relative to the control (P<0.001). The chloroform fraction exerted the highest activity that was comparable to that of the standard drug, artesunate. The chemoinhibitory percentages ranged from 37.60 to 68.83 (Table 1).
Table 1. Suppressive activity of ethanolic leaves extract and fractions of M. scadens on P. berghei infection in mice (mean±SEM) (n=6).
| Treatment | Dose (mg/kg) | Parasitaemia | % Chemosuppression |
| Control (normal saline) | 10 mL/kg | 25.66±2.84 | – |
| M. scadens crude extract | 37 | 16.00±1.40* | 37.60 |
| 74 | 13.00±2.53* | 49.34 | |
| 111 | 10.00±2.10* | 61.00 | |
| Methanol fraction | 74 | 16.33±1.02* | 62.46 |
| Ethyl acetate fraction | 74 | 11.33±2.30* | 55.80 |
| Chloroform fraction | 74 | 8.00±2.10* | 68.83 |
| Artesunate | 5 | 7.00±1.86* | 72.75 |
*P<0.001 as compared with the control.
3.4. Repository activity of ethanolic leaves extract and fractions of M. scandens
The ethanolic crude leaves extract showed a dose-dependent chemosuppressive effect on the parasitaemia. These effects were statistically significant relative to the control (P<0.001) with the chloroform fraction exerting the highest activity (Table 2).
Table 2. Repository/Prophylactic activity of ethanolic leaves extract and fractions of M. scadens on P. berghei infection in mice (mean±SEM) (n=6).
| Treatment | Dose (mg/kg) | Parasitaemia | % Chemosuppression |
| Control (normal saline) | 10 mL/kg | 21.56±1.01 | – |
| M. scadens crude extract | 37 | 14.41±0.32* | 33.16 |
| 74 | 12.88±0.40* | 40.25 | |
| 111 | 10.40±1.01* | 51.76 | |
| Methanol fraction | 74 | 12.15±1.04 | 43.64 |
| Ethyl acetate fraction | 74 | 13.54±1.04 | 37.19 |
| Chloroform fraction | 74 | 11.20±0.06* | 48.05 |
| Pyrimethamine | 1.2 | 8.00±0.42* | 62.89 |
*P<0.001 as compared with the control.
3.5. Antiplasmodial effect of ethanolic leaf extract and fractions of M. scandens on established infection
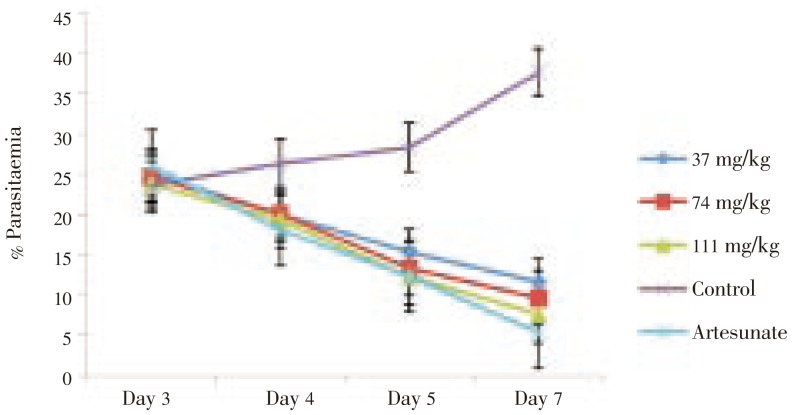
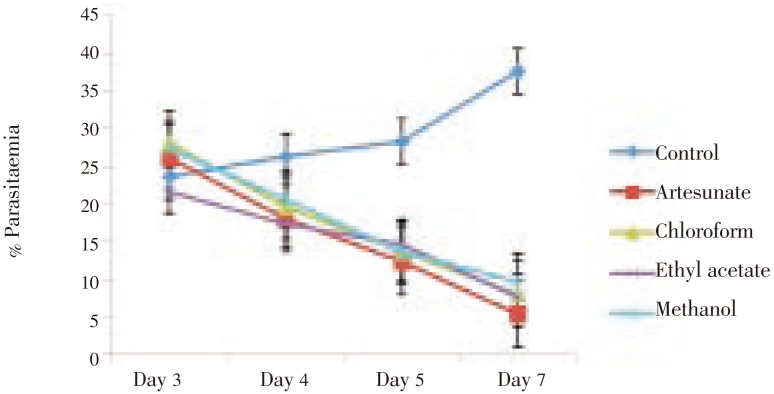
The extract showed a dose-dependent schizonticidal effect on the parasitaemia. These effects were statistically significant relative to the control (P<0.001) (Figure 1 and 2). Though both the extract and its fractions showed a significant dose-dependent MST on established infection (P<0.001), the ethyl acetate fraction showed greater protective effect with a much longer MST than other fractions and crude extract but lower than that of the standard drug, artesunate (Table 3).
Figure 1. Curative effect of crude leaf extract of M. scadens on established infection in mice.
Figure 2. Curative effect of leaf fractions of M. scadens on established infection in mice.
Table 3. MST of mice receiving the various doses of ethanolic leaves extract and fractions of M. scadens during established P. berghei infections in mice (mean±SEM) (n=6).
| Treatment | Dose (mg/kg) | MST (days) |
| Control (normal saline) | 10 mL/kg | 9.28±0.80 |
| M. scadens crude extract | 37 | 10.68±0.40 |
| 74 | 12.70±0.25* | |
| 111 | 17.01±0.40* | |
| Chloroform fraction | 74 | 12.38±0.44* |
| Ethyl acetate fraction | 74 | 17.73±2.33* |
| Methanol fraction | 74 | 12.00±0.73* |
| Artesunate | 5 | 29.83±0.12* |
*P<0.001 as compared with the control.
3.6. Indomethacin-induced gastric ulceration
The extract pretreatment on indomethacin induced gastric ulceration showed a dose-dependent reduction in ulcer indices in pretreated groups relative to control. The reduction was statistically significant (P<0.001) compared with control (Table 4). The effect was incomparable to that of the standard drug, cimetidine.
Table 4. Effect of M. scadens extract on indomethacin-induced ulcer (mean±SEM) (n=6).
| Treatment | Dose (mg/kg) | Ulcer indices | Preventive ratio |
| Control (indomethacin) | 60 | 18.78±1.22 | – |
| M. scadens extract | 37 | 7.46±1. 42* | 60.27 |
| 74 | 5.20±0.34* | 72.31 | |
| 111 | 4.08±1.38* | 78.27 | |
| Cimetidine | 100 | 1.25±0.40* | 93.34 |
*P<0.001 as compared with the control.
3.7. Ethanol-induced gastric ulceration
The extract significantly protected rats from ethanol-induced ulcer (Table 5). There was a significant (P<0.001) dose-dependent reduction in the ulcer indices relative to control. The effect was comparable to that of control.
Table 5. Effect of M. scadens extract on ethanol-induced ulcer (mean±SEM) (n=6).
| Treatment | Dose (mg/kg) | Ulcer indices | Preventive ratio |
| Control (ethanol) | – | 5.26±0.55 | – |
| M. scadens extract | 37 | 2.45±0.25* | 53.42 |
| 74 | 2.45±0.17* | 53.42 | |
| 111 | 1.32±0.86* | 74.90 | |
| Propranolol | 40 | 1.41±0.42* | 73.19 |
*P<0.001 as compared with the control.
3.8. Histamine-induced ulceration
Administration of the extract significantly (P<0.001) reduced histamine-induced gastric ulceration in a dose-dependent fashion compared with control (Table 6).
Table 6. Effect of M. scadens extract on histamine-induced ulceration in rats (mean±SEM) (n=6).
| Treatment | Dose (mg/kg) | Ulcer index | Preventive ratio |
| Control (histamine) | 100 | 15.33±0.45 | – |
| M. scadens extract | 37 | 4.50±0.15* | 70.64 |
| 74 | 3.64±0.46* | 76.25 | |
| 111 | 2.78±1.37* | 81.86 | |
| Cimetidine | 100 | 1.21±0.24* | 92.10 |
*P<0.001 as compared with the control.
4. Discussion
M. scandens leaves are traditionally used to treat various ailments such as stomach ulcer and sores[2], dysmenorrhoea[3] and malaria[4],[5]. These prompted the need to evaluate the antiplasmodial and antiulcer potentials of the crude extract, fractions of the leaves of M. scandens.
In this work, LD50 was determined to be (370.00±23.33 mg/kg) and the extract was relatively safe[16].
The antiplasmodial properties of the extract and its fractions were investigated using standard models. It was found that both the extract and its fractions significantly reduced the parasitaemia in prophylactic, suppressive and curative models in a dose-dependent fashion. Some secondary metabolites of plants have been reported to have antiplasmodial activity. Among these metabolites are alkaloids, flavonoids and triterpenoids such as limonoids and quassinoids[16]. These compounds (alkaloids, flavonoids and triterpenoids) present in this plant extract may in part have contributed to the plasmocidal activity of this extract and therefore explained the mechanism of antiplasmodial effect of the extract and its fractions.
The antiulcer activity of the leaf extract was evaluated using indomethacin, ethanol and histamine-induced ulcer models. Indomethacin is known to cause ulcer especially in an empty stomach and mostly on the glandular (mucosal) part of the stomach by inhibiting prostaglandin synthetase through the cycloxygenase pathway[13]. Prostaglandins function to protect the stomach from injury by stimulating the secretion of bicarbonate and mucus, maintaining mucosal blood flow and regulating mucosal turn over and repair[17]. Suppression of prostaglandins synthesis by indomethacin results in increased susceptibility of the stomach to mucosal injury and gastroduodenal ulceration. The extract was observed to significantly reduce mucosal damage in the indomethacin-induced ulcer model, suggesting the possible extract mobilization and involvement of prostaglandin in the anti-ulcer effect of the extract. Administration of ethanol has been reported to cause disturbances in gastric secretion, damage to the mucosa, alterations in the permeability, gastric mucus depletion and free radical production[13]. It was observed in this study that the extract significantly reduced ethanol-induced ulcer. This may be due to cytoprotective effect of the extract via antioxidant effects. Ethanol is also reported to cause gastric mucosal damage by stimulating the formation of leukotriene C4 (LTC4)[18]. The gastroprotective effect of the extract may in part be due to the suppression, by the extract of lipoxygenase activity. Histamine is known to enhance gastric acid secretion thereby leading to ulceration. Inhibition of histamine-induced ulcer by the extract may be due to its suppression of histamine-induced vasospastic effect and gastric secretion. The leaf extract has been found to contain flavonoids, terpenes, saponins, alkaloids and cardiac glycosides among others. Flavonoids have been reported to protect the gastric mucosa from damage by increasing the mucosal prostaglandin content, inhibiting histamine secretion from mast cells, inhibition of histidine decarboxylase and free radical scavenging ability[13],[19]. This activity corroborates the antioxidant activity of the leaf of M. scandens earlier reported by Adeseguna[6] and maybe attributable to its antiulcer potential.
The results of this study demonstrated that M. scandens possesses considerable antiplasmodial and antiulcer activities. These confirm its use to treat malaria and ulcer in folkloric medicine. Therefore, it would be interesting if the active principle is isolated, identified and characterised.
Acknowledgments
The authors are grateful to Mr Nsikan Malachy and Ms Sifon Akpan of Pharmacology and Toxicology Department for their technical assistance.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Burkill HM. The useful plants of west tropical Africa. 2nd ed. London: Royal Botanic Garden; 1985. p. 127. [Google Scholar]
- 2.Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensi A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Gosomtwi-Atwima-Kwanwoma area, Ghana. J Ethnopharmacol. 2009;125:393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol. 2008;115:387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Guede ZN, Mambu L, Guede GF, Bodo B, Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol. 2005;98:281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Guede NZ, N'guessan K, Dibie TE, Grellier P. Ethnopharmacological study of plants used to treat malaria in traditional medicine by Bete populations of Issia (Cote d'voire) J Pharm Sci Res. 2010;2(4):216–227. [Google Scholar]
- 6.Adeseguna SA, Alabia SA, Olabanja PA, Alexander H, Coker B. Evaluation of antioxidant potential of Melanthera scandens. J Acupunct Meridian Stud. 2010;3(4):267–271. doi: 10.1016/S2005-2901(10)60047-7. [DOI] [PubMed] [Google Scholar]
- 7.Offong E. Antidiabetic and hypolipidemic activities of Melanthera scadens (M.Sc. dissertation) Uyo, Nigeria: University of Uyo; 2011. [Google Scholar]
- 8.Penders A, Delaude C. Triterpenoids saponins from Melanthera scadens. Phytochemistry. 1994;37(3):821–825. doi: 10.1016/s0031-9422(00)90364-9. [DOI] [PubMed] [Google Scholar]
- 9.Trease GE, Evans WC. Pharmacognosy. 13th ed. London: Bailliere Tindal; 1996. pp. 683–684. [Google Scholar]
- 10.Miller LC, Tainter ML. Estimation of ED50 or LD50 values and their error using logarithmic-probit graph paper. Proc Soc Exp Biol Med. 1944;57:261–264. [Google Scholar]
- 11.Okokon JE, Nwafor PA. Antiplasmodial activity of ethanolic root extract and fractions of Croton zambesicus. J Ethnopharmacol. 2009;121:74–78. doi: 10.1016/j.jep.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Antia BS, Okokon JE, Etim EI, Umoh UF, Bassey EO. Evaluation of the in vivo antimalarial activity of ethanolic leaf and stembark extracts of Anthocleista djalonensis. Indian J Pharmacol. 2009;41(6):258–261. doi: 10.4103/0253-7613.59924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nwafor PA, Bassey AL. Evaluation of antidiarrhoeal and antiulcerogenic potential of ethanolic extract of Carpolobia lutea leaves in rodents. J Ethnopharmacol. 2007;111:619–624. doi: 10.1016/j.jep.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Okokon JE, Nwafor PA. Antiulcer and anticonvulsant activities of root extract of Croton zambesicus. Pak J Pharm Sci. 2009;22(4):384–390. [PubMed] [Google Scholar]
- 15.Okokon JE, Umoh UF, Udobang JA, Etim EI. Antiulcerogenic activity of ethanolic leaf extract of Croton zambesicus in rats. Afr J Biomed Res. 2010;13:119–123. [Google Scholar]
- 16.Christensen SB, Kharazmi A. Antimalarial natural products: isolation, characterization and biological properties. In: Tringali C, editor. Bioactive compounds from natural sources: isolation, characterization and biological properties. London: Taylor & Francis; 2001. pp. 379–432. [Google Scholar]
- 17.Hiruma-Lima CA, Calvo TR, Rodriguez CM, Andrade F, Vilegas W, Brito ARM. Antiulcerogenic activity of Alchornea castaneaefolia effects on somatostatin, gastrin and prostaglandin. J Ethnopharmacol. 2006;104:215–224. doi: 10.1016/j.jep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Salim AS. Removing oxygen derived free radicals stimulates healing of ethanol-induced erosive gastritis in the rats. Digestion. 1990;47:24–28. doi: 10.1159/000200472. [DOI] [PubMed] [Google Scholar]
- 19.Borrelli F, Izzo AA. The plant kingdom as source of anti-ulcer remedies. Phytother Res. 2000;14:581–591. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]