Abstract
Objective
To compare the protein patterns from the extracts of the mutant clone T9/94-M1-1(b3) induced by pyrimethamine, and the original parent clone T9/94 following separation of parasite extracts by two-dimensional electrophoresis (2-DE).
Methods
Proteins were solubilized and separated according to their charges and sizes. The separated protein spots were then detected by silver staining and analyzed for protein density by the powerful image analysis software.
Results
Differentially expressed protein patterns (up- or down-regulation) were separated from the extracts from the two clones. A total of 223 and 134 protein spots were detected from the extracts of T9/94 and T9/94-M1-1(b3) clones, respectively. Marked reduction in density of protein expression was observed with the extract from the mutant (resistant) clone compared with the parent (sensitive) clone. A total of 25 protein spots showed at least two-fold difference in density, some of which exhibited as high as ten-fold difference.
Conclusions
These proteins may be the molecular targets of resistance of Plasmodium falciparum to pyrimethamine. Further study to identify the chemical structures of these proteins by mass spectrometry is required.
Keywords: Plasmodium falciparum, Proteomics, Pyrimethamine, Drug resistance, Protein, Molecular target
1. Introduction
Malaria is still a major public health problem affecting the populations in tropical and subtropical countries[1]. The major cause contributing to failure to eliminate malaria is the emergence and spread of resistance of malaria parasites to most of the available antimalarial drugs. Research and development of new antimalarial agents are therefore urgently needed to overcome the situation. Among the four species of human malaria, Plasmodium falciparum (P. falciparum) is the most virulent and is responsible for the vast majority of deaths worldwide. The complete genome sequence of the P. falciparum clone 3D7 provides valuable information for proteomics investigation of the parasite in order to identify new potential drug and vaccine targets[2].
Folate metabolism of malaria parasite has been well established as one of the most valuable targets for antimalarial drugs. Antifolate agents such as pyrimethamine, proguanil and chlorproguanil act by inhibiting dihydrofolate reductase (DHFR) enzyme in folate biosynthesis pathway[3]. Resistance to antifolates occurs through stepwise mutations of this enzyme, which eventually causes the change in parasite's sensitivity to the drugs[4],[5]. To understand the underlying mechanism of antifolate resistance, Thaithong et al[6] obtained the mutant clone T9/94-M1-1(b3) with increased resistance to pyrimethamine through selective drug pressure. The mutant clone T9/94-M1-1(b3) exhibited a 100 fold increase in minimal inhibitory concentration (MIC) of pyrimethamine (5×10−6 M) as compared with the original parent clone (5×10−8 M). However, no change in the coding sequence of neither DHFR nor gene amplification was observed in both clones. Since the decrease in sensitivity of the mutant clone T9/94-M1-1(b3) could not be explained on the basis of the mutation in the DHFR gene, it is imperative to ascertain whether the antifolate drugs induce differential changes in the expression levels of other proteins apart from DHFR enzyme. The aim of the present study was to explore other protein targets other than the target enzyme DHFR, which may be involved in resistance of P. falciparum to antifolates. Comparative protein patterns of both the sensitive and resistant clones were analyzed after separation by 2-dimensional electrophoresis (2-DE).
2. Materials and methods
2.1. Parasite culture
The P. falciparum clone T9/94 used in this study was cultured in vitro in group O human red blood cells according to the previously described method[6]. Briefly, parasites were grown in RPMI1640 medium (supplemented with 37.5 mM HEPES, 7 mM D-glucose, 6 mM NaOH, 25 µg/mL gentamicin sulphate, 2 mM L-glutamine and 10% human serum) under an atmosphere of 96% nitrogen, 3% carbon dioxide, and 1% oxygen. Synchronization of the parasite to early ring stage was performed using 5% sorbitol. The mutant clone T9/94M1-1(b3) with up to 1 000-fold increase in resistance to pyrimethamine was selected by exposing the parent T9/94 clone with stepwise increased concentrations of pyrimethamine in culture medium. The synchronized culture of both the parent and mutant clones were further maintained until approximately 5% parasitemia of schizont were obtained.
2.2. Extraction of parasite protein
Synchronized parasite culture was harvested and cell pellet was resuspended in 0.15% saponin in PBS and incubated on ice for 1 h in order to lyse red cells. The lysate was collected through centrifugation at 13 000 × g for 5 min (4 °C) and washed three times with 1 mL of PBS until the supernatant was clear. Red blood cell pellet was washed in 1 mL of 10 mM Tris-HCl (pH 7.4) containing 1 × protease inhibitor Cocktail (Roche Co. Ltd.) until red cell ghost was colorless. Pellet was then re-suspended in 500 µL of lysis buffer (8 M urea, 2 M thiourea, 1% CHAPS, 65 mM DTT, 0.5% ampholyte pH 3–10) and sample was vortexed and sonicated on ice four times, eight seconds each (21% amplitude, 8 sec, interspersed with 9 sec), followed by centrifugation at 13 000 × g for 1 h (4 °C), and the supernatant was subjected to 2-DE. Quantification of protein concentrations of the extracts was performed by using Bradford reagent (BioRad Co. Ltd.).
2.3. Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis (2-DE) was carried out using a 2D electrophoresis system (BioRad Co. Ltd., USD) according to the manufacturer's recommendation with modifications. The extract of parasite protein (100 µg) was mixed with rehydration buffer (8 M urea, 1% CHAPS, 15 mM dithiothretol, 0.001% bromophenol blue). Protein mixture (125 µL) was applied onto 7 cm IPG strips in an isoelectric focusing (IEF) system (BioRad Co. Ltd., USA), and was subjected to pH gradient (3–10) with an electrical charge using ampholytes as carrier. IEF was performed initially at 250 v for 15 min, followed by 4 000 v for 1 h, and terminated with 4 000–20 000 v-h. The focused strips were equilibrated in 5 mL equilibration solution I (37.5 mM Tris-HCl, pH 6.8, 6 M urea, 20% glycerol, 2% SDS) containing the reducing agent DTT (130 mM) for 10 min, followed by 5 mL equilibration solution II containing iodoacetamide (135 mM) for additional 10 min. The strips were then briefly washed with 1 × gel running buffer and loaded onto 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for second dimension separation. The gels were run on 1 × electrode buffer pH 8.3. After electrophoresis, gels were fixed and stained with silver stain (BioRad Co. Ltd., USA) according to the manufacturer's recommendation. The 2-DE gel images were scanned and analyzed by PDQuestTM software (BioRad Co. Ltd., USA). At least four independent gels were analyzed for each sample. The density of protein spots separated from the extracts of pyrimethamine-sensitive and pyrimethamine-resistant clones were compared as the ratio between the spot density of T9/94 and T9/94M1-1(b3).
3. Results
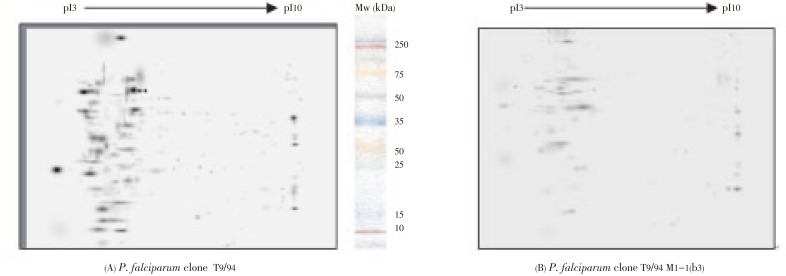
Proteins extracted from pyrimethanine-sensitive (T9/94) and pyrimethamine-resistant [T9/94M1-1(b3)] P. falciparum clones were analyzed by 2-DE. Protein spots were separated from the extracts of both clones using 7 cm immobiline IPG strip at the pH range of 3–10, and were visualized by silver staining gels. The differentially expressed proteins separated by 2-DE gel for each sample were shown in Figure 1. The 2-DE gel images were scanned and analyzed using PDQuestTM software (BioRad Co. Ltd.). The extracts of both clones showed similar basic protein patterns (64% similarity), but with a number of some different spots. A total of 223 and 134 protein spots were detected from the extracts of T9/94 and T9/94-M1-1(b3) clones, respectively. Marked reduction in density of protein expression was observed with the extract from the mutant (resistant) clone compared with the parent (sensitive) clone. A total of 25 protein spots showed at least two-fold difference in density, some of which exhibited as high as ten-fold difference (Table 1).
Figure 1. Differential expression of protein spots from the extracts of (A) T9/94, and (B) T9/94 M1-1(b3), separated by 2-DE according to their charges (first dimension by isoelectric focusing IPG strips pH range 3–10), followed by molecular weights (second dimension using 12% SDS-PAGE).
Table 1. Protein spots separated from the extracts of T9/94 (pyrimethamine-sensitive) and T9/94 M1-1(b3) mutant (pyrimethamine-resistant) clones with at least two-fold difference in protein density.
| Spot ID | Protein spot density |
Spot ID | Protein spot density |
||||
| T9/94 | T9/94 M1-1(b3) | Ratio of spot density [T9/94: T9/94 M1-1(b3)] | T9/94 | T9/94 M1-1 b3 | Ratio of spot density [T9/94: T9/94 M1-1(b3)] | ||
| SSP0214 | 9 078.00 | 2 598.20 | 3.49 | SSP6615 | 8 567.38 | 411.97 | 20.79 |
| SSP2003 | 15 301.15 | 1 588.42 | 9.63 | SSP6616 | 3 501.46 | 344.86 | 10.15 |
| SSP2119 | 15 411.90 | 205.78 | 74.89 | SSP7111 | 4 100.69 | 772.46 | 5.30 |
| SSP2208 | 24 868.95 | 770.26 | 32.28 | SSP7112 | 1 601.11 | 135.01 | 11.85 |
| SSP2313 | 18 998.45 | 2 144.37 | 8.85 | SSP7307 | 1 422.20 | 216.88 | 6.55 |
| SSP2614 | 13 781.72 | 302.75 | 45.52 | SSP7308 | 10 054.00 | 373.10 | 26.94 |
| SSP2817 | 4 245.18 | 674.55 | 6.29 | SSP8507 | 1 079.04 | 143.98 | 7.49 |
| SSP3111 | 21 031.61 | 662.08 | 31.76 | SSP9205 | 5 932.10 | 2 260.99 | 2.62 |
| SSP3214 | 7 075.11 | 2 539.02 | 2.78 | SSP9206 | 11 978.64 | 2 081.70 | 5.75 |
| SSP3306 | 9 143.13 | 604.13 | 15.13 | SSP9405 | 15 157.39 | 2 442.15 | 6.20 |
| SSP4707 | 16 223.82 | 3 894.43 | 4.16 | SSP9406 | 19 770.03 | 6 221.96 | 3.17 |
| SSP5713 | 32 681.61 | 3 332.01 | 9.80 | SSP9507 | 19 310.89 | 4 090.94 | 4.72 |
| SSP6106 | 4 944.89 | 475.05 | 10.40 | ||||
4. Discussion
Proteomics offers a new approach for exploring potential targets for drug and vaccine development. Traditional methods for characterization and identification of large numbers of proteins from a complex protein mixture have relied mainly on 2-DE combined with mass spectrometry. Numerous proteomics studies of bacterial pathogen as well as other organisms signify the value of these approaches[7],[8]. Nevertheless, the application of 2-DE in the investigation of malaria parasite proteomes is still limited. The extraction and solubilization of all components are the critical steps that are essential for the success of the technique. The inefficient extraction procedure, including the large size of some proteins, the contamination of haemoglobin-derived products and thiourea result in low reproducibility of the separated proteins.
Few proteomic research have been undertaken to elucidate the mechanisms of action or resistance of antimalarial drugs in P. falciparum[9]–[11]. In the present study, the proteomic approach was applied to analyze the pattern of protein expression in the schizont stage of P. falciparum following being subjected to selective pressure by the antifolate drug pyrimethamine. Protein expression of entire P. falciparum proteome of the pyrimethamine-sensitive and induced resistant clones were compared in order to elucidate potential target(s) of pyrimethamine resistance apart form DHFR enzyme. Although analysis of malaria proteins by 2-DE gel has widely been established, major problem encountered is the insolubility of membrane proteins which leads to reduced efficiency in discriminating the separated protein spots on 2-DE. In addition, contamination by human host proteins with a wide range of molecular weights and isoelectric points (pI) remains problematic for improvement of proteomic profiles. Modification of the extraction protocol based on types of samples, or utilization of a variety of chaotropic mixtures with detergents, has been applied to solve these problems[12]–[25]. Results from a previous study indicated that approximately 52% of the proteins identified from P. falciparum blood stage samples were host red cell proteins[26]. The contamination by haemoglobin-derived products during extraction steps result in smearing and high background after 2-DE separation[27].
The current results of the 2-DE profiles revealed differently expressed proteins separated from the extracts of P. falciparum original clone T9/94 and mutant clone T9/94-M1-1(b3). This indicates that the up- or down-regulated proteins may be involved in sensitivity of P. falciparum to pyrimethamine. A total of 223 and 134 protein spots, respectively, were detected in T9/94 and T9/94-M1-1(b3) clones. Abundant malaria proteins were mainly distributed in the 2-DE in the pH gradient ranging from 3 to 7, and molecular weights ranging from low to high (10–100 kDa). The low abundant proteins were located at the pH of greater than 7. The protein densities of a total of 25 spots from both clones were found to exhibit at least two-fold difference. Of these protein spots, a 10-fold reduction in protein density was observed in the mutant (resistant) clone compared with the parent (sensitive) clone. The ability to measure the levels of protein expression following drug challenge is one of the main goals in malaria proteomics research. Identification and quantification of proteins from the parasite extracts could be performed in these differentially expressed proteins. Prediction of the chemical structures of these protein spots according to molecular weights and isoelectric points can be obtained by comparison with those reported in SWISS-PROT database. Nevertheless, in order to clearly elucidate the mechanism of resistance of P. falciparum to pyrimethamine, further identification of these proteins by either peptide mass fingerprinting using MALDI-TOF or direct peptide sequencing by tandem mass spectrometry is required. In addition, the optimized 2-DE method with high reproducibility and sensitivity is essential to allow for the highly expedient analysis of differentially expressed malaria proteins at low amounts.
Acknowledgments
The study was supported by the Thailand Research Fund (TRF), Rachadaphisek Sompok Research Fund, Chulalongkorn University, the National Research University (NRU) Project of Thailand, CHE-RES Project, Office of Higher Education Commission and Ministry of Education of Thailand.
Footnotes
Foundation Project: Supported by the Thailand Research Fund (TRF), Rachadaphisek Sompok Research Fund, Chulalongkorn University, the National Research University (NRU) Project of Thailand, CHE-RES Project, Office of Higher Education Commission and Ministry of Education of Thailand.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Guerra CA, Snow RW, Hay SI. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22:353–358. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 3.Bzik DJ, Li WB, Horii T, Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci USA. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregson AL, Plowe CV. Mechanism of resistance of malaria parasite to antifolates. Pharmacol Rev. 2005;57:117–145. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 6.Thaithong S, Chan SW, Songsomboob S. Pyrimethamine resistance mutations in Plasmodium falciparum. Mol Biochem Parasitol. 1992;52:149–158. doi: 10.1016/0166-6851(92)90047-n. [DOI] [PubMed] [Google Scholar]
- 7.Cash P. Proteomics of bacterial pathogens. Adv Biochem Eng Biotechnol. 2003;83:93–115. doi: 10.1007/3-540-36459-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Washburn MP, Wolters D, Yates JR. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 9.Cooper RA, Carucci DJ. Proteomic approaches to studying drug targets and resistance in Plasmodium. Curr Drug Targets Infect Disord. 2004;4:41–51. doi: 10.2174/1568005043480989. [DOI] [PubMed] [Google Scholar]
- 10.Makanga M, Bray PG, Horrocks P, Ward SA. Towards a proteomic definition of CoArtem action in Plasmodium falciparum malaria. Proteomics. 2005;5:1849–1858. doi: 10.1002/pmic.200401076. [DOI] [PubMed] [Google Scholar]
- 11.Prieto JH, Koncarevic S, Park SK, Yates J, Becker K. Large-scale differential proteome analysis in Plasmodium falciparum under drug treatment. PLoS One. 2008;3:e4098. doi: 10.1371/journal.pone.0004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantumthong P, Vattanaviboon P. Improvement of proteomic profile of Plasmodium falciparum by two step protein extraction in two dimensional gel electrophoresis. Thammasat Int J Sci Technol. 2006;11:61–68. [Google Scholar]
- 13.Gbotosho GO, Okuboyejo TM, Happi CT, Sowunmi A. Plasmodium falciparum hyperparasitaemia in Nigerian children: epidemiology, clinical characteristics, and therapeutic responses to oral artemisinin-based combination treatments. Asian Pac J Trop Dis. 2011;1(2):85–93. [Google Scholar]
- 14.Jombo GTA, Araoye MA, Damen JG. Malaria self medications and choices of drugs for its treatment among residents of a malaria endemic community in West Africa. Asian Pac J Trop Dis. 2011;1(1):10–16. [Google Scholar]
- 15.George P, Alexander LM, Shetty A. Study comparing the clinical profile of complicated cases of Plasmodium falciparum malaria among adults and children. Asian Pac J Trop Dis. 2011;1(1):35–37. [Google Scholar]
- 16.Jombo GTA, Alao OO, Araoye MO, Damen JG. Impact of a decade-long anti-malaria crusade in a West African community. Asian Pac J Trop Dis. 2011;1(2):100–105. [Google Scholar]
- 17.Alaya-Bouafif NB, Chahed MK, Bez HE, Bellali H, Ayari L, Achour N. Completeness of malaria notification in Tunisia assessed by capture recapture method. Asian Pac J Trop Dis. 2011;1(3):187–191. [Google Scholar]
- 18.Osonuga OA, Osonuga AA, Osonuga IO, Osonuga A, Kwarteng DL. Prevalence of hypoglycemia among severe malaria children in a rural African population. Asian Pac J Trop Dis. 2011;1(3):192–194. [Google Scholar]
- 19.Gbotosho GO, Okuboyejo TM, Happi CT, Sowunmi A. Recrudescent Plasmodium falciparum infections in children in an endemic area following artemisinin-based combination treatments: Implications for disease control. Asian Pac J Trop Dis. 2011;1(3):195–202. [Google Scholar]
- 20.Fan Z, Zhang L, Yan G, Wu Q, Gan X, Zhong S, et al. Bioinformatics analysis for structure and function of CPR of Plasmodium falciparum. Asian Pac J Trop Med. 2011;4(2):85–87. doi: 10.1016/S1995-7645(11)60042-4. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim EA, Kheir MM, Elhardello OA, Almahi WA, Ali NI, Elbashir MI, et al. Cortisol and uncomplicated Plasmodium falciparum malaria in an area of unstable malaria transmission in eastern Sudan. Asian Pac J Trop Med. 2011;4(2):146–147. doi: 10.1016/S1995-7645(11)60056-4. [DOI] [PubMed] [Google Scholar]
- 22.Khan HM, Shujatullah F, Ashfaq M, Raza A. Changing trends in prevalence of different Plasmodium species with dominance of Plasmodium falciparum malaria infection in Aligarh (India) Asian Pac J Trop Med. 2011;4(1):64–66. doi: 10.1016/S1995-7645(11)60035-7. [DOI] [PubMed] [Google Scholar]
- 23.Tangpukdee N, Wai KM, Muangnoicharoen S, Kano S, Phophak N, Tiemprasert J, et al. Indicators of fatal outcome in severe Plasmodium falciparum malaria: a study in a tertiary–care hospital in Thailand. Asian Pac J Trop Med. 2010;3(11):855–859. [Google Scholar]
- 24.Raewadee W, Wanna C, Poonuch M, Prapichaya P, Na-Bangchang K. Polymorphisms of the oxidant enzymes glutathione S–transferase and glutathione reductase and their association with resistance of Plasmodium falciparum isolates to antimalarial drugs. Asian Pac J Trop Med. 2010;3(9):673–677. [Google Scholar]
- 25.Nmorsi OPG, Isaac C, Ohaneme BA, Obiazi HAK. Pro–inflammatory cytokines profiles in Nigerian pregnant women infected with Plasmodium falciparum malaria. Asian Pac J Trop Med. 2010;3(9):731–733. [Google Scholar]
- 26.Johnson JR, Florens L, Carucci DJ, Yates JR. Proteomics in malaria. J Proteome Res. 2004;3:296–306. doi: 10.1021/pr0340781. [DOI] [PubMed] [Google Scholar]
- 27.Sims PFG, Hyde JE. Proteomics of the human malaria parasite Plasmodium falciparum. Expert Rev Proteomics. 2006;3:87–95. doi: 10.1586/14789450.3.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]