Abstract
Objective
To investigate the cytotoxic activity of actinomycete isolated from marine sediment.
Methods
In the present study the DNA was isolated and the ITS region of 16s rRNA was amplified by polymerase chain reaction, using two universal bacterial primers, 1492R (5′-GGTTACCTTGTTAC GACTT-3′) and Eubac27F (5′-AGAGTTTGATCCTGGCTC AG-3′). The amplified products were purified using TIANgel mini purification kit, ligated to MD18-T simple vector (TaKaRa), and transformed into competent cells of Escherichia coli DH5α. 16S rRNA gene fragment was sequenced using forward primer M13F (-47) and reverse primer M13R (-48). Blast search sequence similarity was found against the existing non-redundant nucleotide sequence database thus, identified as Streptomyces sp SU, Streptomyces rubralavandulae strain SU1, Streptomyces cacaoi strain SU2, Streptomyces cavourensis strain SU3, Streptomyces avidinii strain SU4, Streptomyces globisporus strain SU5, Streptomyces variabilis strain SU6, Streptomyces coelicolor strain SU 7. Among the eight identified isolates, one actinomycete Streptomyces avidinii strain SU4 was selected for further study.
Results
Crude extract of the actinomycete isolate exhibited IC50 in 64.5 µg against Hep-2 cell line, 250 µg in VERO cell line. This value is very close to the criteria of cytotoxicity activity for the crude extracts, as established by the American National Cancer Institute (NCI) is in IC50 < 30 µg/mL. The GC MS analysis showed that the active principle might be 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (12.17%), isooctyl phthalate (15.29%) with the retention time 15.642 and 21.612, respectively.
Conclusions
This study clearly proves that the marine sediment derived actinomycetes with bioactive metabolites can be expected to provide high quality biological material for high throughout biochemical and anticancer screening programs. These results help us to conclude that the potential of using metabolic engineering and post genomic approaches to isolate more bioactive compounds and make their possible commercial application is not far off.
Keywords: Actinomycetes, Cytotoxicity, ITS sequencing, GC MS, Streptomyces avidinii, Characterization, Cytotoxic compound, Extra cellular metabolite
1. Introduction
Actinomycetes, characterized by a complex life cycle, are filamentous Gram-positive bacteria belonging to the phylum Actinobateria that represents one of the largest taxonomic units among the 18 major lineages currently recognized within the domain bacteria[1]. Around 23 000 bioactive secondary metabolites produced by microorganisms have been reported and over 10 000 of these compounds are produced by actinomycetes, representing 45% of all bioactive microbial metabolites discovered[2]. Among actinomycetes, around 7 600 compounds are produced by Streptomyces species[2]. Many of these secondary metabolites are potent antibiotics, which has made streptomycetes the primary antibiotic-producing organisms exploited by the pharmaceutical industry[2]. Members of this group are producers, in addition, of clinically useful antitumor drugs such as anthracyclines (aclarubicin, daunomycin and doxorubicin), peptides (bleomycin and actinomycin D), aureolic acids (mithramycin), enediynes (neocarzinostatin), antimetabolites (pentostatin), carzinophilin, mitomycins and others[3],[4].
In addition, the high toxicity usually associated with cancer chemotherapy drugs and their undesirable side effects increase the demand for novel antitumor drugs active against untreatable tumors, with fewer side effects and/or with greater therapeutic efficiency[5]. Progress has been made recently on drug discovery from actinomycetes by using high-throughput screening and fermentation, mining genomes for cryptic pathways, and combinatorial biosynthesis to generate new secondary metabolites related to existing pharmacophores[6]. However, the search for novel drugs is still a priority goal for cancer therapy, due to the rapid development of resistance to multiple chemotherapeutic drugs. In addition, the high toxicity usually associated with cancer chemotherapy drugs and their undesirable side effects increase the demand for novel antitumor drugs active against untreatable tumors, with fewer side effects and/or with greater therapeutic efficiency[7]. These organisms have evolved with greatest genomic and metabolic diversity and hence efforts have been directed towards exploring marine actinomycetes as a source for the discovery of novel secondary metabolites[8].
Their study is expected to become an important component in the production of new natural bioactive products. The current study was undertaken to investigate this biodiversity and to isolate and screen marine sediment derived actinomycetes for cytotoxic activity.
2. Materials and methods
2.1. Isolation of actinomycetes
Marine sediment samples were collected from Pulicat, Ennore, Muttukadu, and Veerampattinam costal area and were immediately transferred to the laboratory condition. Approximately one gram of soil sample was aseptically transferred into 99 mL of pre sterilized 50% seawater and serially diluted. 100 µL of diluted samples were transferred to molten starch casein agar medium (10 g/L soluble starch, 1 g/L of casein and 18 g/L of agar made up with 50% of aged sea water) and incubated at (27±2)°C for 7 days[9]. After incubation, colonies appeared on the agar medium were re-streaked in the same agar medium.
2.2. Preparation of extracts
The pure culture isolated by the above method was grown in international Streptomyces project (ISP) 2 medium (4 g/L of glucose, 10 g/L of malt extract and 4 g/L of yeast extract, (7.2±2)°C made up with 50% sea water) for 10 days under continuous shaking (100 rpm). After that, cell free broth was adjusted to pH 5.0 with 1N hydrochloric acid and equal volume (1:1) of ethyl acetate was added and mixed by vigorous shaking and kept without disturbance. The organic phase was collected and evaporated in incubator at 60-70°C and the residue was stored at-20°C for further use.
2.3. Cytotoxic activity [(3,4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide (MTT) assay]
The human laryngeal cancer cell line (Hep-2) and VERO cell line were obtained from Verinary College, Chennai, India. Cells were grown as monolayer culture in MEM medium and incubated at 37°C in a 5% of CO2 atmosphere. Hep-2 and VERO cells (100 µL) were seeded in 96 well plates at a concentration of 5×103 cells/mL for 24 h. After the incubation the culture medium was replaced with 100 mL serum free medium containing various concentrations (3.87, 7.75, 15.5, 31.25, 62.5, 125, 250, 500, 1 000 and 2 000 µg/mL) of actinomycete extracts at 24 h and 48 h. After that, the medium was refreshed with 100 µL of serum free medium (MEM) and 20 µL of MTT [5 mg/mL of (3, 4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide] was added. The micro-titer plates were incubated for three hours in dark. The developed colour was measured with ELISA reader at 570 nm. Triplicates were maintained for each treatment. IC50 values were determined by calculating the % of viability:
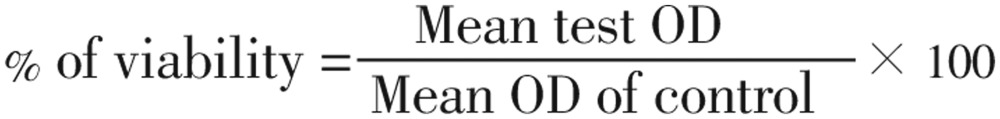 |
2.4. GC Ms analysis
The crude extract exhibiting activity was subjected to GC-MS (SHIMADZU QP2010) instrument at GC column oven temperature 70°C, injector temperature 200°C at spilt mode ratio 40 with a flow rate of 1.51 mL/min. The MS with ion source temp 200°C, interface temp: 240°C, scan range: 40-1 000 m/z, event time 0.5 sec, solvent cut time: 5 min, MS start time: 5 min, MS end time: 35 min, ionization: EI (-70ev).
2.5. Actinomycetes identification
Genomic DNA was isolated from actinomycete as described by Nathan[10]. The 16S rRNA gene of strain PCL-1 was amplified by polymerase chain reaction, using two universal bacterial primers, 1492R (5′-GGTTACCTTGTTAC GACTT-3′) and Eubac27F (5′-AGAGTTTGATCCTGGCTC AG-3′)[11]. The amplified products were purified using TIANgel mini purification kit, ligated to MD18-T simple vector (TaKaRa), and transformed into competent cells of Escherichia coli DH5α.16S rRNA gene fragment was sequenced using forward primer M13F (-47) and reverse primer M13R (-48). The derived 16S rRNA gene sequence was compared to the GENBANK database (NCBI), to search for similar sequences using the basic local alignment search tool algorithm.
3. Results
In our screening for actinomycetes showing cytotoxic activities, eight samples were collected from Pulicat, Ennore, Muttukadu, and Veerampattinam costal habitats, 52 isolates were isolated using starch casein agar medium. In the present investigation, among the 52 isolates the DNA of a fastly growing 8 isolates was isolated and the ITS region of 16s rRNA was amplified by polymerase chain reaction, using two universal bacterial primers, 1492R (5′-GGTTACCTTGTTAC GACTT-3′) and Eubac27F (5′-AGAGTTTGATCCTGGCTC AG-3′). The amplified products were purified using TIANgel mini purification kit, ligated to MD18-T simple vector (TaKaRa), and transformed into competent cells of Escherichia coli DH5α.16S rRNA gene fragment was sequenced using forward primer M13F (-47) and reverse primer M13R (-48). Blast search sequence similarity was found against the existing non-redundant nucleotide sequence database thus, identified as Streptomyces sp SU, Streptomyces rubralavandulae strain SU1, Streptomyces cacaoi strain SU2, Streptomyces cavourensis strain SU3, Streptomyces avidinii strain SU4, Streptomyces globisporus strain SU5, Streptomyces variabilis strain SU6, Streptomyces coelicolor strain SU7. Among the eight isolates, one actinomycetes Streptomyces avidinii strain SU4 was selected for further studies. The actinomycete was grown in ISP2 medium for 10 days and filtrate was subjected to ethyl acetate solvent extraction for further studies.
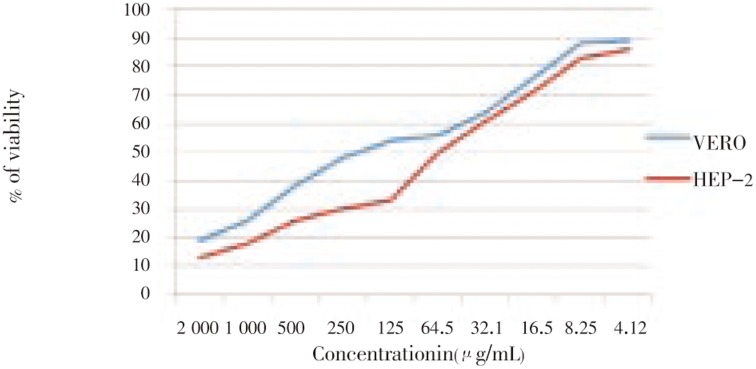
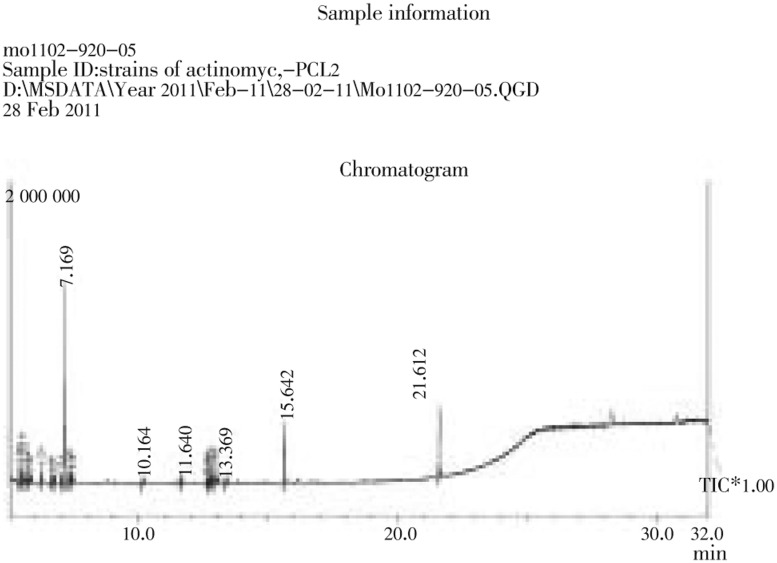
Cytotoxicity of the extracts against Hep2 cell lines and normal cell lines are shown in Table 1. Generally, the extracts were found to be more effective against cancer cell line than normal cell line. The extracts showed activity [IC50 of 64.5 µg/mL against Hep-2 cell line, 250 µg/mL in VERO cell line) g/mL] (Figure 1). These values are very close to the criteria of cytotoxicity activity for the crude extracts, as established by the American National Cancer Institute is in IC50 < 30 µg/mL (Figure 2). The GC MS analysis shows that the active principle might be tributylamine (34.34%), 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester (12.17%), isooctyl phthalate (15.29%) with the retention time 7.169, 15.642 and 21.612, respectively (Figure 3).
Table 1. Anticancer activity against HEP-2 cell line and VERO cell line.
| Concentration (µg/mL) | Dilution | VERO | HEP-2 |
| 2 000 | Neat | 19 | 13 |
| 1 000 | 1:1 | 26 | 18 |
| 500 | 1:2 | 38 | 26 |
| 250 | 1:4 | 48 | 30 |
| 125 | 1:8 | 54 | 33 |
| 64.5 | 1:16 | 56 | 50 |
| 32.1 | 1:32 | 64 | 61 |
| 16.5 | 1:64 | 76 | 71 |
| 8.25 | 1:128 | 88 | 83 |
| 4.12 | 1:256 | 89 | 86 |
Figure 1. MTT cytotoxicity for the crude extract on Hep-2 cell line and VERO cell lines.
Figure 2. Hep 2 cell line and VERO cell line treated with crude extract.
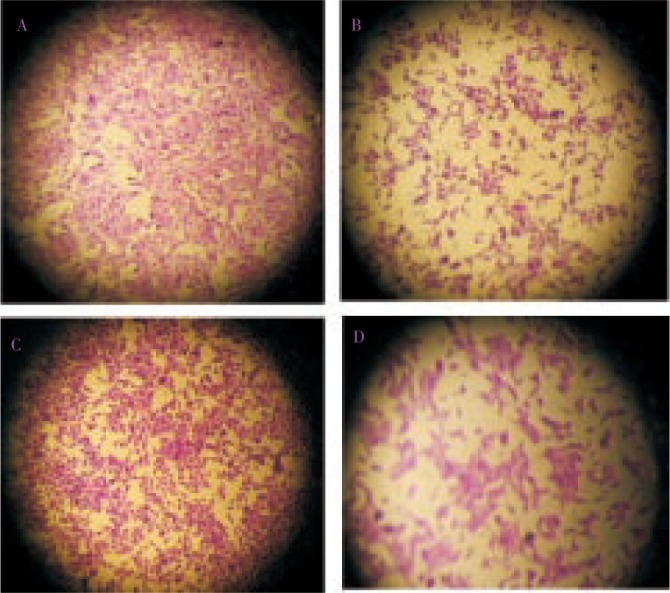
2 A: Hep-2 control, 2 B: Hep-2 treated, 2 C: VERO control, 2 D: VERO treated.
Figure 3. GC MS analysis spectrum.
The percentage of similarity between the actinomycetes and database suggests it as novel strain. Thus, the novel strain was named as Streptomyces avidinii strain SU4 and made publically available in GenBank with an assigned accession number JF730120.
4. Discussion
Marine actinomycetes are a prolific source of secondary metabolites and the vast majority of these compounds are derived from the single genus Streptomyces. Streptomyces species are distributed widely in marine and terrestrial habitats[12] and are of commercial interest due to their unique capacity to produce novel metabolites.
Marine actinomycetes have been reported to harbour potential antitumor value. As an evidence to the previous statement, the crude extract of a marine actinomycete Saccharopolyspora salina VITSDK4 inhibited the growth of cancer HeLa cells with an IC50 of 26.2 mg/mL, which is significant with control. The IC50 against mouse erythrocytes was found to be 266 mg/mL which shows that the cytotoxicity on HeLa cells was not related to membrane integrity[13].In the present study, the crude extracts of actinomycete was made with ethyl acetate suggest that the crude extract is toxic to Hep-2 cell line and less cytotoxic activity on normal cell line Previously we have reported a Streptomyces strain with antitumor activity against cancer cell line and less cytotoxicity activity against normal cell line[14].Thus, it should be useful if future cytotoxic tests of these marine derived actinomycetes are carried out on other cancer cell lines.
Bis (ethyl hexyl) phthalate reported from Streptomyces bangladeshiensis show antimicrobial activity against gram positive bacteria and some pathogenic fungi[15],[16]. Di (2-ethyl hexyl) phthalate isolated from Alchornea cordifolia reported to lower anti-inflammatory activity[17]-[20]. Di-isooctyl phthalate isolated from Nigella glandulifera Freyn. was identified as inhibiting melanogenesis[21]. The extract of Gongronema latifolium decne contains Phthalic acid, monoterpenes, and several compounds to be responsible for the activity against bacterial isolates from HIV infected patients[22]. The essential oil of Leea indica (Burm. F) Merr flowers showed phthalic acid esters (95.6%) as major constituents, had good antibacterial and antifungal activity[23]. In the present study GC MS analysis of crude extract shows the presence of 1,2-benzenedicarboxylic acid, bis (2-methylpropyl) ester and isooctyl phthalate in dominant proportion that confirms it must be responsible to the anticancer activity.
In conclusion, it is believed that a rich source of anticancer drug candidates could be obtained from marine organisms or their metabolites. This preliminary screening of actinomycetes revealed their potential to yield potent bioactive compounds for drug discovery programmes.
Acknowledgments
The authors are grateful to the authorities of Sathyabama University for providing necessary facilities and we also thankful to Dr. M. Bavanilatha, Associate professor of Sathyabama University for helps us to do MTT assay.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, et al. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berdy J. Bioactive microbial metabolites. J Antibio. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 4.Olano C, Méndez C, Salas JA. Antitumor compounds from actinomycetes: from gene clusters to new derivatives by combinatorial biosynthesis. Nat Prod Rep. 2009;26:628–660. doi: 10.1039/b822528a. [DOI] [PubMed] [Google Scholar]
- 5.Demain AL, Sánchez S. Microbial drug discovery: 80 years of progress. J Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltz RH. Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol. 2008;8:557–563. doi: 10.1016/j.coph.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Demain AL, Sánchez S. Microbial drug discovery: 80 years of progress. J Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udwary DW, Zeigler L, Asolkar RN, Singan V, Lapidus A, Fenical W, et al. Genome sequencing reveals complex secondary metabolome in the marine actinomycete Salinispora tropica. Proc Natl Acad Sci. 2007;104(25):10376. doi: 10.1073/pnas.0700962104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravikumar S, Suganthi P, Moses F. Crude bioactive compounds of actinomycetes from manakkudy mangrove sediment. J Pharm Res. 2011;4(3):877–887. [Google Scholar]
- 10.Nathan A, Magarvey, Jessica M, Keller, Bernan V, Dworkin M, Sherman DH. Isolation and characterization of novel marine-derived actinomycete taxa rich in bioactive metabolites. Appl Environ Microbiol. 2004;70(12):7520–7529. doi: 10.1128/AEM.70.12.7520-7529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Dong H, Zhang G, Yu B, Chapman LR, Fields MW. Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl Environ Microbiol. 2006;72:3832–3845. doi: 10.1128/AEM.02869-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pathom-aree W, Stach JEM, Ward AC, Horikoshi K, Bull AT, Goodfellow M. Diversity of actinomycetes isolated from Challenger Deep sediment (10 898 m) from the Mariana Trench. Extremophiles. 2006;10:181–189. doi: 10.1007/s00792-005-0482-z. [DOI] [PubMed] [Google Scholar]
- 13.Kannabiran S. Cytotoxic and antimicrobial potential of actinomycete species Saccharopolyspora salina VITSDK4 isolated from the bay of Bengal Coast of India. Am J Inf Dis. 2009;5(2):90–98. [Google Scholar]
- 14.Sudha S, Masilamani Selvam M. Streptomyces cavourensis sp. SU 3 Nov., a novel marine Streptomyces isolated from a sea shore sediment in Chennai. Advanced Biotech. 2011:32–36. [Google Scholar]
- 15.Al-Bari MAA, Sayeed MA, Rahman MS, Mossadik MA. Characterization and antimicrobial activities of a phthalic acid derivative produced by Streptomyces bangladeshiensis- A novel species in Bangladesh. Res J Med & Medical Sci. 2006;1:77–81. [Google Scholar]
- 16.Chairman K, Ranjit Singh AJA, Alagumuthu G. Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac J Trop Dis. 2012;2(3):234–238. [Google Scholar]
- 17.Mavar MH, Haddad Pieters M, Bacceli C, Penge A, Quetin LJ. Anti-inflammatory compounds from leaves and root bark of Alchornea cordifolia (Schum and Thonn.) Muell. Arg J Ethnopharmacol. 2008;115:25–29. doi: 10.1016/j.jep.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Hussain T, Fareed S, Siddiqui HH, Vijaykumar M, Rao CV. Acute and subacute oral toxicity evaluation of Tephrosia purpurea extract in rodents. Asian Pac J Trop Dis. 2012;2(2):129–132. [Google Scholar]
- 19.Kumbhare MR, Guleha V, Sivakumar T. Estimation of total phenolic content, cytotoxicity and in-vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac J Trop Dis. 2012;2(2):144–150. [Google Scholar]
- 20.Chairman K, Ranjit Singh AJA, Alagumuthu G. Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac J Trop Dis. 2012;2(2):234–238. [Google Scholar]
- 21.Nguyen DT, Nyugen DH, Lyun HL, Lee HB, Shin JH, Kim EK. Inhibition of melanogenesis by diocyl phthalate isolated from Nigella glandulifera Freyn. J Microbio Biotechnol. 2007;17:1585–1590. [PubMed] [Google Scholar]
- 22.Adeleye IA, Omadime ME, Daniels FV. Antimicrobial activity of essential oils and extracts of Gongronema latifolium Decne on bacterial isolates from blood stream of HIV infected patients. J Pharm & Toxicol. 2011;3:312–320. [Google Scholar]
- 23.Srinivasan GV, Sharanappa P, Leela NK, Sadashiva CT, Vijayan KK. Chemical composition and antimicrobial activity of Leea indica (Burm. F) Merr flowers. Natl Prod Rad. 2009;8:5. [Google Scholar]