Abstract
Objective
To investigate and compare the inhibitory properties of free and bound phenolic extracts of clove bud against carbohydrate hydrolyzing enzymes (alpha-amylase & alpha-glucosidase) and Fe2+-induced lipid peroxidation in rat pancreas in vitro.
Methods
The free phenolics were extracted with 80% (v/v) acetone, while bound phenolics were extracted from the alkaline and acid hydrolyzed residue with ethyl acetate. Then, the interaction of the extracts with alpha-amylase and alpha-glucosidase was subsequently assessed. Thereafter, the total phenolic contents and antioxidant activities of the extracts were determined.
Results
The result revealed that both extracts inhibited alpha-amylase and alpha-glucosidase in a dose-dependent manner. However, the alpha-glucosidase inhibitory activity of the extracts were significantly (P<0.05) higher than their alpha-amylase inhibitory activity. The free phenolics (31.67 mg/g) and flavonoid (17.28 mg/g) contents were significantly (P<0.05) higher than bound phenolic (23.52 mg/g) and flavonoid (13.70 mg/g) contents. Both extracts also exhibited high antioxidant activities as typified by their high reducing power, 1,1 diphenyl-2- picrylhydrazyl (DPPH) and 2, 2-azinobis-3-ethylbenzo-thiazoline-6-sulfonate (ABTS) radical scavenging abilities, as well as inhibition of Fe2+-induced lipid peroxidation in rat pancreas in vitro.
Conclusions
This study provides a biochemical rationale by which clove elicits therapeutic effect on type 2 diabetes.
Keywords: Alpha-amylase, Alpha-glucosidase, Fe2+-induced lipid peroxidation Pancrea, Phenol, Antioxidant
1. Introduction
About 346 million people worldwide suffer from diabetes and this number has been estimated to be doubled in 2030[1]. The term diabetes mellitus (DM) is used to refer to a metabolic disorder of multiple etiologies in which chronic hyperglycemia is caused by defects or alterations in either the secretion or action of insulin. This results in disturbances in carbohydrate, fat and protein metabolism. Oxidative stress is known to play a significant role in the development and progression of DM[2]. Excessive generation of free radicals and depleted levels of free radical scavenging enzymes have been demonstrated in animal models of diabetes[3],[4]. Free radicals are formed disproportionately in diabetes by glucose oxidation, non-enzymatic glycation of proteins, and the subsequent oxidative degradation of glycated proteins. Abnormally high levels of free radicals and the simultaneous decline of antioxidant defense mechanisms can lead to damage of cellular organelles and enzymes and also increase lipid peroxidation and beta-cell apoptotic pathways activation[2],[5].
Type 2 diabetes is caused either predominantly by insulin resistance with a relative deficiency of insulin or by impaired insulin secretion that may or may not be accompanied by insulin resistance and accounts for about 90%-95% of all diagnosed cases of diabetes in adults[6]. Recent reports suggest that one of the therapeutic approaches for decreasing post-prandial hyperglycaemia is to prevent absorption of glucose by the inhibition of carbohydrate-hydrolysing enzymes, such as alpha-glucosidase and alpha-amylase[7]. Thus, the retardation of the action of alpha-glucosidase and alpha-amylase by inhibitors might be one of the most effective approaches to control type 2 DM[8]. Oral hypoglycemic agents/drugs may be effective for glycemic control, but they come with their attendant side effects such as liver disorders, flatulence, abdominal pain, renal tumours, hepatic injury, acute hepatitis, abdominal fullness and diarrhoea[9]. Therefore, there is an increasing need for the development of a natural and safe product without side effects.
Plant foods rich in polyphenolic fractions have been reported to cause insulin-like effects in glucose utilization[10], act as good inhibitors of key enzymes linked to type 2 diabetes[11],[12] and lipid peroxidation in tissues[13],[14]. It is well-known that phenolic compounds also contribute to quality of food in terms of modifying color, taste, aroma and flavour[15],[16]. Studies have also shown that polyphenols in plants have bioactivities and these could be attributed to their antioxidant properties[13],[17]. Abo et al[18] reported that plants used in ethno medicine possess hypoglycaemic properties.
Clove [Syzygium aromaticum (L.) Merr.& Perry (Family: Myrtaceae)] is an aromatic flower bud, commonly used in Africa, Asia and other parts of the world in preparation of various spicy rich dishes. It has deep brown colour, intense fragrance and burning taste. In addition to its culinary uses, the clove bud and its oil have an abundance of medicinal and recreational uses. It possesses antioxidant, anti-fungal, anti-viral, anti-microbial, anti-diabetic, anti-inflammatory, antithrombotic, anesthetic, pain reliving and insect repellent properties[19]. Prasad et al. reported the insulin-like actions of clove buds in hepatocytes and hepatoma cells by reducing phosphoenolpyruvate carboxykinase and glucose 6-phosphatase gene expression[20]. Recently, Atawodi et al. reported the positive correlation of polyphenol constituents of clove bud, its antioxidant/radical scavenging properties, as well as its ability to prevent diseases associated with oxidative stress such as diabetes, cancer and cardiovascular disorders[21]. Some polyphenols such as gallic acid, ellagic acid, quercetin glucoside, ellagic acid derivative, and some other unidentified phenolic compounds have been characterized and reported to be present in clove buds[21]. Moreover, there are dearths of information on the possible mechanism of action of clove bud extracts with regards to its antihyperglycemic effects and ability of clove buds to protect the pancreas against Fe2+-induced lipid peroxidation.
Therefore, this present study was designed to investigate the inhibitory potentials of polyphenol-rich extracts (free soluble and bound phenols) from the flower buds of clove on key enzymes responsible for carbohydrate-hydrolysis (alpha-glucosidase & alpha-amylase), coupled with their antioxidant properties.
2. Materials and methods
2.1. Materials
Clove (Syzygium aromaticum) buds were purchased from the Akure main market, Akure Nigeria and authenticated in the Department of Crop, Soil and Pest Management, Federal University of Technology, Akure, Nigeria. The buds were dried in the sun and ground to fine powder. Porcine pancreatic alpha-amylase and rat intestinal alpha-glucosidase were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Unless stated otherwise, all chemicals were from Sigma Chemical Co., while the water used was glass-distilled.
2.2. Handling and use of animal
The handling and the use of the animals were in accordance with NIH Guide for the care and use of laboratory animals. In the experiment, Wister strain albino rats weighing 200-230 g were purchased from the breeding colony of Department of Veterinary Medicine, University of Ibadan, Nigeria. Rats were maintained at 25 °C, on a 12 h light/12 h dark cycle, with free access to food and water. They were acclimatized under these conditions for 2 weeks before the commencement of the experiment.
2.3. Extraction of free soluble phenols
The extraction of free soluble phenolics was conducted according to the method reported by Chu et al[22]. About 10 g of the ground leaves was extracted with 80% acetone (1:5, v/v) and filtered (filter paper Whatman no. 2) under vacuum. The filtrate was then evaporated using a rotary evaporator under vacuum at 45 °C until about 90% of the filtrate had been evaporated. The extract was frozen at -4 °C, while the residue was kept for the extraction of bound phenols.
2.4. Extraction of bound phenols
The residue from free soluble extraction above was flushed with nitrogen and hydrolyzed with about 20 mL of 4 M/L sodium hydrate solution at room temperature for 1 h with shaking. Then, the pH value of the mixture was adjusted to 2 with concentrated hydrochloric acid and the bound phytochemicals were extracted with ethylacetate (6 times). The ethyl acetate fraction was then evaporated at 45 °C[22].
2.5. Alpha-amylase inhibition assay
Briefly, appropriate dilutions (0-200 µL) of the extracts and 500 µL of 0.02 M/L sodium phosphate buffer (pH 6.9 with 0.006 M/L sodium chloride) containing porcine pancreatic alpha-amylase (EC 3.2.1.1, 0.5 mg/mL) were incubated at 25 °C for 10 min. Then, 500 µL of 10 g/L starch solution in 0.02 M/L sodium phosphate buffer (pH 6.9 with 0.006 M/L sodium chloride) was added to each tube. The reaction mixtures was incubated at 25 °C for 10 min and stopped with 1.0 mL of dinitrosalicylic acid colour reagent. Thereafter, the mixture was incubated in a boiling water bath for 5 min and cooled to room temperature. The reaction mixture was then diluted by adding 10 mL of distilled water, and absorbance measured at 540 nm. The alpha-amylase inhibitory activity was expressed as percentage inhibition[23]. The percentage inhibition was calculated using the following formula:
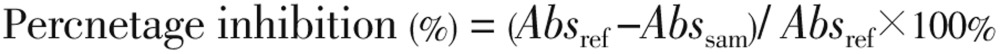 where, Absref represents absorbance of reference, while Abssam represents absorbance of sample. where, Absref represents absorbance of reference, while Abssam represents absorbance of sample.
|
(1) |
2.6. Alpha-glucosidase inhibition assay
Appropriate dilutions of the extracts (0-200 µL) and 100 µL of alpha-glucosidase (EC 3.2.1.20) solution (1.0 U/mL) in 0.1 M/L phosphate buffer (pH 6.9) were incubated at 25 °C for 10 min. Then, 50 µL of 5 mM/L p-nitrophenyl-alpha-D-glucopyranoside solution in 0.1 M/L phosphate buffer (pH 6.9) was added. The mixtures were incubated at 25 °C for 5 min, before reading the absorbance at 405 nm in the spectrophotometer. The alpha-glucosidase inhibitory activity was expressed as percentage inhibition[24]. The percentage inhibition was calculated using the formula (1) in section 2.5.
2.7. Lipid peroxidation assay
2.7.1. Preparation of tissue homogenates
The rats were decapitated under mild diethyl ether anaesthesia and the pancreas were rapidly excised, placed on ice and weighed. This tissue was subsequently homogenized in 10 g/L cold saline with about 10 up and down strokes at approximately 1 200 rpm in a Teflon glass homogenizer. The homogenate was centrifuged for 10 min at 5 180.1 r/min to yield a pellet that was discarded, and a low-speed supernatant (S1) containing mainly water, proteins and lipids (cholesterol, galactolipid, individual phospholipids, and gangliosides) was kept for lipid peroxidation assay[25].
2.7.2. Lipid peroxidation and thiobarbibutric acid reactions
The lipid peroxidation assay was performed by the modified method of Ohkawa et al[26]. Briefly, 100 µL of S1 fraction was mixed with a reaction mixture containing 30 µL of 0.1 M/L Tris-HCl buffer (pH 7.4), extract (0-100 µL) and 30 µL of the pro-oxidant (25µM/L freshly prepared ferric sulfate). The volume was made up to 300 µL by water before incubation at 37 °C for 2 h. The color reaction was developed by adding 300 µL of 81 g/L SDS (Sodium duodecyl sulphate) to the reaction mixture containing S1, followed by the addition of 600 µL of acetic acid/HCl (pH 3.4) and 600 µL of 0.8% (v/v) TBA (Thiobarbituric acid). This mixture was incubated at 100 °C for 1 h. The absorbance of TBARS (Thiobarbituric acid reactive species) produced were measured at 532 nm in an UV-Visible spectrophotometer (Model 6305; Jenway, Barloworld Scientific, Dunmow, United Kingdom). Malondialdehyde (MDA) produced was expressed as percentage of the control.
2.8. Determination of total phenolic content
The total phenolic content was determined according to the method described by Singleton et al[27]. Briefly, appropriate dilution of the extracts was oxidized with 2.5 mL 10% Folin-Ciocalteau's reagent (v/v) and neutralized by 2.0 mL of 75 g/L sodium carbonate. The reaction mixture was incubated for 40 min at 45 °C and the absorbance was measured at 765 nm in the spectrophotometer. Gallic acid was used as standard phenol and the total phenolic content was subsequently calculated as gallic acid equivalent (GAE).
2.9. Determination of total flavonoid content
The total flavonoid content of the extracts was determined using a slightly modified method reported by Meda et al[28]. Briefly, 0.5 mL of appropriately diluted sample was mixed with 0.5 mL methanol, 50 µL of 100 g/L aluminium chloride, 50 µL of 1 M/L potassium acetate and 1.4 mL of water, and allowed to incubation at room temperature for 30 min. Thereafter, the absorbance of the reaction mixture was subsequently measured at 415 nm. Quercetin was used as standard flavonoid and the total flavonoid content was calculated as quercetin equivalent antioxidant (QEAC).
2.10. DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical-scavenging ability
The free radical-scavenging ability of the spice extracts against DPPH free radical was evaluated as described by Gyamfi et al[29]. Briefly, an appropriate dilution of the spice extracts (1 mL) was mixed with 1 mL of 0.4 mM/L methanol solution containing DPPH radicals. The mixture was left in the dark for 30 min and the absorbance was measured at 516 nm in the spectrophotometer. The DPPH free radical scavenging ability was subsequently calculated with respect to the reference, which contained all the reagents without the test sample. The DPPH radical scavenging ability was calculated using the following formula:
| (2) |
where, Absref represents absorbance of reference, while Abssam represents absorbance of sample.
2.11. Total antioxidant capacity
The total antioxidant capacity of the vegetable extracts was determined as their scavenging ability on ABTS [2,2-azinobis(3-ethylbenzo-thiazoline-6-sulfonate)] as described by Re et al[30]. ABTS radical (ABTS.+) was generated by reacting an ABTS aqueous solution (7 mM/L) with potassium peroxydisulfate (2.45 mM, final concentration) in the dark for 16 h and adjusting the absorbance of 734 nm to 0.700 with ethanol. Then, 0.2 mL of appropriate dilution of the extracts was added to 2.0 mL ABTS.+ cation solution and the absorbance were measured at 734 nm after 15 min. The trolox equivalent antioxidant capacity (TEAC) was subsequently calculated.
2.12. Reducing power determination
The reducing power of the extracts was determined by assessing the ability of the extracts to reduce ferric trichloride solution as described by Pulido et al[31]. A 2.5 mL aliquot was mixed with 2.5 mL of 200 mM/L sodium phosphate buffer (pH 6.6) and 2.5 mL of 10 g/L potassium ferricyanide. The mixture was incubated at 50 °C for 20 min, and then 2.5 mL of 10% trichloroacetic acid was added. This was then centrifuged at 2 411.2 r/min for 10 min. Subsequently, 5 mL of the supernatant was mixed with an equal volume of water and 1 mL of 1 g/L ferric chloride. The absorbance was measured at 700 nm and reducing power was calculated as ascorbic acid equivalent (AAE).
2.13. Calculation
2.13.1. Determination of IC50 values
IC50 (extract concentration causing 50% enzyme inhibition or concentration of sample required to scavenge 50% DPPH radicals) values were calculated using non-linear regression analysis.
2.13.2. Data analysis
The results of triplicate experiments were pooled and expressed as mean ± standard deviation (SD)[32]. Means were compared by one way analysis of variance (ANOVA) followed by Duncan's multiple range test. Significant difference was accepted at P≤0.05. Origin 6.1 version software was used for the calculation of IC50 values.
3. Results
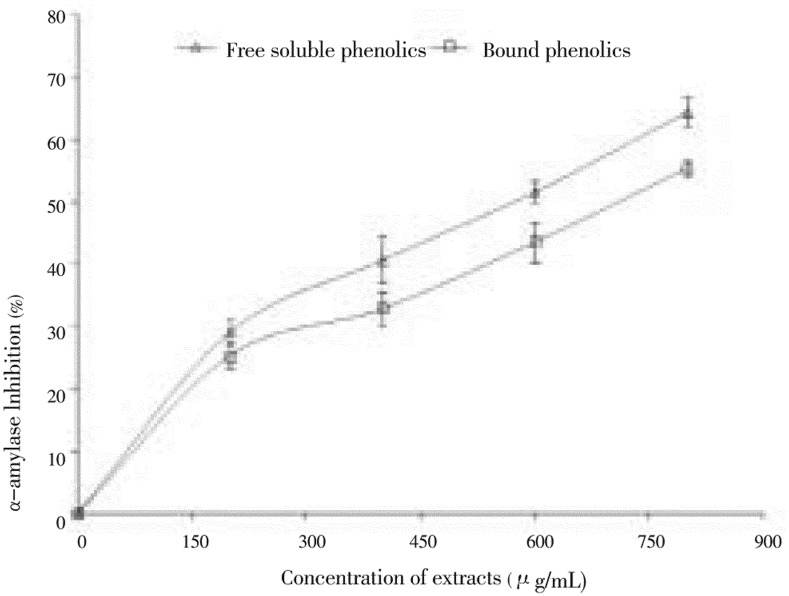
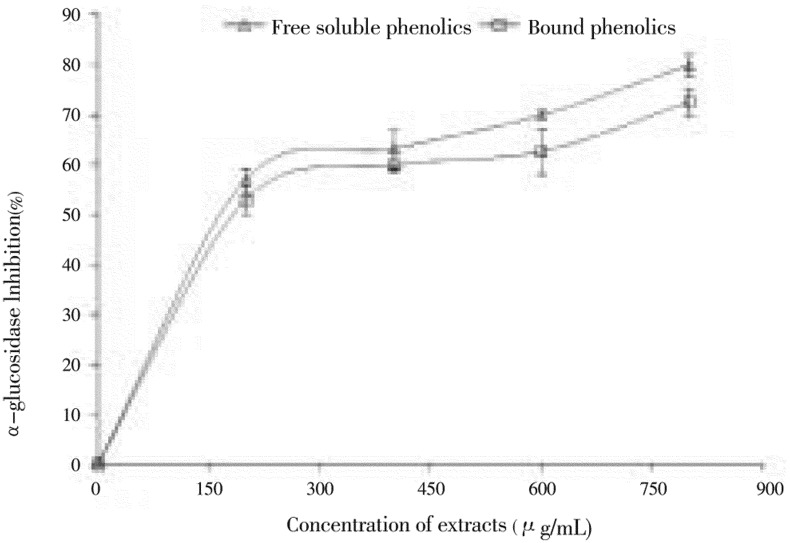
The ability of polyphenol-rich (free and bound) extracts from clove buds to inhibit alpha-amylase and alpha-glucosidase activity was investigated in vitro and the results are presented in Figures 1 & 2. The result revealed that both extracts inhibited alpha-amylase in a dose-dependent manner (200-800 µg/mL). However, judging by the IC50 as shown in Table 1, the free soluble phenolic extract (497.27 µg/mL) had significantly (P<0.05) higher inhibitory activity than the bound phenolic extract (553.77 µg/mL). In the same way, the ability of the free and bound phenolic extracts to inhibit alpha-glucosidase was also assessed in Figure 2. Both extracts inhibited alpha-glucosidase in a dose dependent (200-800 µg/mL) manner. However, the bound phenolic extract (127.31 µg/mL) had higher inhibitory activity than the free soluble phenolic extract (145.07 µg/mL) as shown in Table 1, although there was no significant difference in their ability to inhibit alpha-glucosidase activity.
Figure 1. Inhibitory activities of phenolic extracts from clove (Syzygium aromaticum [L.] Merr. & Perry) buds toward alpha-amylase.
Values are represented as mean of triplicate experiments ± standard deviation.
Figure 2. Inhibitory activities of phenolic extracts from clove (Syzygium aromaticum [L.] Merr. & Perry) buds toward alpha-glucosidase.
Values are represented as mean of triplicate experiments ± standard deviation.
Table 1. The extract concentration causing 50% enzyme inhibition (IC50) values for the α-amylase and α-glucosidase inhibitory activities and concentration of extract required to scavenge 50% DPPH radicals values for DPPH radical scavenging ability of phenolic extracts from clove (Syzygium aromaticum [L.] Merr. & Perry) buds.
| IC50 (µg/mL) |
DPPH free radical scavenging ability | ||
| α-amylase inhibition | α-glucosidase inhibition | ||
| Free phenol extract | 497.27a | 145.07b | 212.53b |
| Bound phenol extract | 553.77a | 127.31c | 256.14c |
*Values with the same superscript letter across the same row are significantly different (P<0.05).
Furthermore, the result of the phenolic content of clove bud extracts was reported as the GAE and that of total flavonoid content reported as the QEAC. The phenolic contents in clove bud as presented in Table 2 showed that the free soluble phenolic content (31.67 mg GAE/g) was significantly (P<0.05) higher than the bound phenolic content (23.52 mg GAE/g) and the total flavonoid content was significantly (P<0.05) higher in free soluble phenolic extract (17.28 mg QEAC/g) than in bound phenolic extract (13.70 mg QEAC/g).
Table 2. Total phenol and total flavonoid contents of phenolic extracts of clove (Syzygium aromaticum[L.] Merr. & Perry) buds (mg/g).
| Total phenol | Total flavonoid | |
| Free phenol extract | 31.67±1.54a | 17.28±0.07b |
| Bound phenol extract | 23.52±1.05a | 13.70±0.79b |
Values represent mean ± standard deviation of triplicate readings, n = 3. Values with the same superscript letter across the same row are significantly (P<0.05) different.
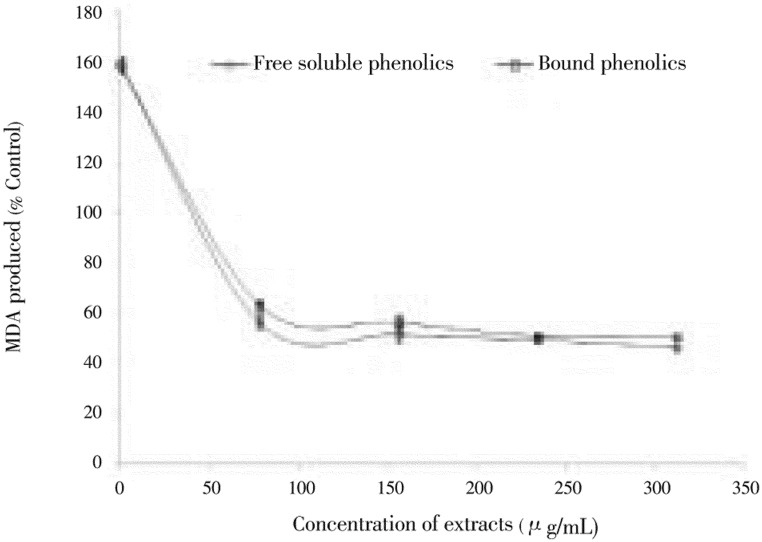
Subsequently, the ability of free and bound phenolic extracts of clove buds to inhibit Fe2+-induced lipid peroxidation in rat pancreas (in vitro) is presented in Figure 3. The incubation of rat pancreas in presence of Fe2+ caused a significant increase (P<0.05) in MDA content. However, the free soluble phenolic and bound phenolic extracts of clove buds (78.15-312.60 µg/mL) caused a significant (P<0.05) decrease in MDA content of the pancreas [free soluble phenolic (56.65%-46.56%) and bound phenol (63.54%-50.69%)] in a dose-dependent pattern. Nevertheless, there was no significant difference in the inhibition of Fe2+-induced lipid peroxidation between the free soluble and bound phenolics extracts of clove buds.
Figure 3. Inhibition of Fe2+-induced lipid peroxidation in rat pancreas by phenolic extracts from clove (Syzygium aromaticum [L.] Merr. & Perry) buds.
Values are represented as mean of triplicate experiments ± standard deviation.
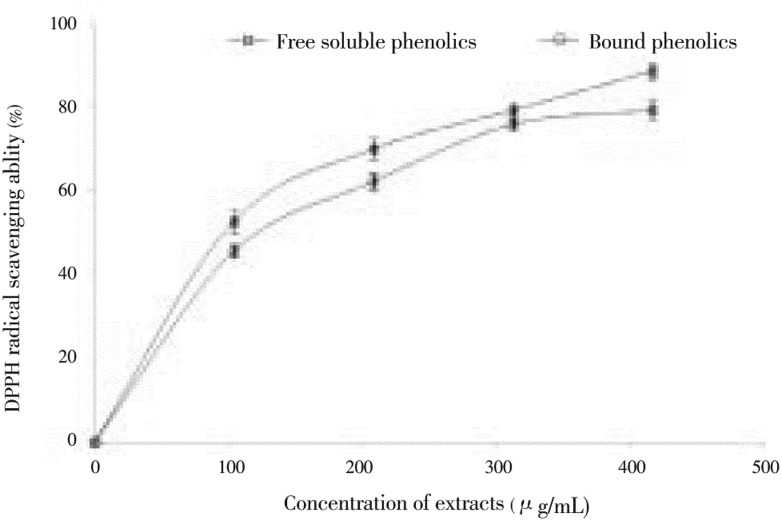
Other antioxidant indices of the polyphenol-rich extracts of clove buds are radical-scavenging abilities (DPPH & ABTS) and reducing power, as shown in Figures 4, 5 & 6) respectively. The DPPH free radical scavenging ability of the free soluble and bound phenolic extract of clove buds is presented in Figure 4. The result revealed that the polyphenol-rich extracts of clove buds scavenged DPPH radicals in a dose dependent manner (104.17-416.67 µg/mL). However, judging by the IC50 (extract concentration that will cause 50% DPPH radical scavenging activity) as shown in Table 2, there was no significant difference in DPPH radical scavenging ability of free soluble phenolic extract (212.53 µg/mL) and bound phenolic extract (256.14 µg/mL). In a similar manner, the ABTS radical (ABTS.+) scavenging ability of polyphenolic extracts of clove bud is and reported as TEAC. The result also revealed that there was no significant difference in the ABTS.+ scavenging ability of the free soluble phenolic (0.059 mM TEAC/g) and bound phenolic (0.0576 mM TEAC/g) extracts.
Figure 4. DPPH radical scavenging ability of phenolic extracts from clove (Syzygium aromaticum [L.] Merr. & Perry) buds.
Values are represented as mean of triplicate experiments ± standard deviation.
Subsequently, the reducing property of free and bound phenolics extract of clove bud was reported as AAE per gram. The results revealed that both extracts were able to reduce Fe3+ to Fe2+. However, the free soluble phenolics extract (0.4774 mM AAE/g) had a significantly (P<0.05) higher ability to reduce Fe3+ to Fe2+ than the bound extract (0.3394 mM AAE/g).
4. Discussion
Decreasing postprandial hyperglycemia peak is crucial in the treatment of diabetes[33]. We therefore hypothesize that possible inhibition of carbohydrate-hydrolyzing enzymes (Alpha-amylase & alpha-glucosidase) and lipid peroxidation in pancreas could represent part of the biochemical rationale in which plant phenols exert their therapeutic potentials in the management/treatment of DM. The ability of phenolic (free and bound) extracts from clove buds to inhibit alpha-amylase activity was investigated and the result revealed that both extracts inhibited alpha-amylase activity significantly (P<0.05) in a dose-dependent (200-800 µg/mL) pattern. However, the free soluble phenolic extract (IC50 = 497.27 µg/mL) had significantly (P<0.05) higher inhibitory activity than the bound phenol extract (IC50 = 553.77 µg/mL).The result of the alpha-amylase inhibitory properties of clove bud extracts follow a similar trend with our earlier reports on the inhibitory properties of plant phytochemicals from two ginger varieties (Zingiber spp.) on alpha-amylase[16]. It also agrees with the report that Allium spp. inhibited alpha-amylase activity[34].
Subsequently, the ability of the free and bound phenol extracts to inhibit alpha-glucosidase inhibitory activities was also assessed. Both extracts inhibited alpha-glucosidase in a dose dependent (200-800 µg/mL) manner. However, the bound phenol extract (IC50= 127.31 µg/mL) had higher inhibitory activity thanthe free soluble phenolic extract (IC50=145.07 µg/mL), although there was no significant difference in their ability to inhibit alpha-glucosidase activity. It is noteworthy that the phenol-rich extracts from clove buds showed significantly (P< 0.05) higher alpha-glucosidase inhibitory activities than their respective alpha-amylase inhibitory activities. This result follows similar trend with previously published works that plant phytochemicals are mild inhibitors of alpha-amylase and strong inhibitors of alpha-glucosidase activities[7],[11],[16]. Therefore, stronger inhibition of ahpha-glucosidase activity and mild inhibition of alpha-amylase activity exhibited by the extracts may be of great nutraceutical importance, in minimizing some of the side effect (such as abdominal distention, flatulence, meteorism and possibly diarrhea) associated with the drugs (Acarbose and Voglibose), presently used for the management of diabetes. This action could also be part of the possible mechanism explored by clove bud in the management/treatment of diabetes in traditional medicine. These findings could lead us to a further functional and structural association of polyphenolics from clove buds from the point of view of their biological activity as anti-diabetic agents. Therefore, we suggest that inhibition of alpha-amylase and alpha-glucosidase activities could be part of the possible mechanisms involved in the use of clove buds in therapeutic/dietary management of diabetes, by retardation of starch hydrolysis in the gastrointestinal tract.
Polyphenols such as flavonoid have potent alpha-glucosidase inhibitory activities[35]. Alpha-glucosidase inhibitors are essential in retardation of carbohydrates digestion and mitigation of postprandial hyperglycemic excursions[7]. Recent reports on the polyphenolic characterization of clove buds revealed the presence of gallic acid, ellagic acid, quercetin glucoside, ellagic acid derivative, and some other unidentified phenolic compounds[21]. Several authors have studied the relationship of phenolic contents, antioxidant properties and enzyme inhibitory activities of plants foods, in order to survey the nutraceutical significance of plant foods[7],[16],[35]. Therefore, the phenolic content of the extracts was subsequently determined. The free phenol content (31.67 mg GAE/g) was significantly (P<0.05) higher than the bound phenol content (23.52 mg GAE/g). This agrees with earlier reports by Sun et al.[36] on phenol distribution in commonly consumed fruits and vegetables[22], where free soluble phenol content was reported to be more abundant than the bound phenol content of some plant foods. It is note worthy to know that free phenols are more readily absorbed and thus, exert beneficial bioactivities in early digestion, but the importance of bound phytochemicals to human health is not clear[22],[36]. However, it is possible that different plant foods with different amounts of bound phytochemicals can be digested and absorbed at different sites of the gastrointestinal tract where they play their unique health benefits. Bound phytochemicals, mainly in beta-glycosides, cannot be digested by human enzymes and could survive stomach and small intestine digestion to reach the colon where they are digested by bacteria flora to release phytochemicals locally[22],[36]. Of all polyphenols present in plant foods, flavonoids are the most abundant and have been reported to possess antioxidant activity[37]. Therefore, the total flavonoid content of clove bud extracts was determined. The flavonoid content was significantly (P<0.05) higher in free phenol extract (17.28 mg QEAC/g) than in bound phenol extract (13.70 mg QEAC/g). However, the trend in the total flavonoid content agreed with the total phenol content. Furthermore, strong correlation existed between the enzyme inhibitory activities and the phenolic content of the extracts [alpha-amylase inhibition; free phenol extract (r = 0.981 0) & bound phenol extract (r = 0.971 7), alpha-glucosidase inhibition; free phenol extract (r = 0.906 4), bound phenol extract (r = 0.897 8)].
Lipid peroxidation in biological membranes is considered as one of the major mechanisms of cell injury in aerobic organisms subjected to oxidative stress[13]. The chain reaction of lipid peroxidation ensures continuous supply of free radicals which initiate further peroxidation[13],[47]. Recent report suggests that iron accumulation contributes to increased free radical formation and oxidative stress. The generation of reactive oxygen species by metal oxidants like iron may be involved in the cell damage that occurs in some human pathology[48]. Fe2+ could initiate lipid peroxidation and suppress the species responsible for the initiation of the peroxidation. Therefore, possible depletion of iron could decrease oxidative stress throughout the whole body[49]. In the pancreas, Fe2+ accumulates in acinar cells and in the islets of Langerhans, thereby resulting in the destruction of beta-cells associated with DM[48]. The ability of the phenolic extracts to inhibit Fe2+-induced lipid peroxidation in Rat's pancreas was investigated. The results clearly showed that incubation of the rat pancreas in the presence of 25 µM/L Fe2+ caused a significant increase (P<0.05) in the MDA contents of the rat pancreas (159.2%) when compared with the basal pancreas homogenate (100.0%). The increased lipid peroxidation in the presence of Fe2+ could be attributed to the fact that Fe2+ can catalyze one-electron transfer reactions that generate reactive oxygen species. The incubation of rat pancreas in presence of Fe2+ caused a significant increase (P<0.05) in MDA content; however, both extracts exhibited high inhibitory effects on pancreatic MDA produced in a dose-dependent pattern (78.13-312.50 µg/mL). However, there was no significant difference in the ability of the extracts to inhibit MDA production, although the free soluble phenolic extract had higher inhibitory activity than the bound phenol extract. The reason for the inhibition of the lipid peroxidation in the pancreas by the polyphenolic rich extracts cannot be ascertained; however, it could be attributed to the phenolic phytochemicals present in the extracts; which could have formed complexes with the Fe (II), thereby preventing them from catalyzing the initiation of lipid peroxidation.
At present, several reports have correlated the antioxidant properties of plant foods with phenolic contents[13],[22],[36]. This study also revealed a strong correlation between the inhibition of Fe2+-induced lipid peroxidation in rat pancreas and phenolic contents [(free phenol extract (r = 0.883 2), bound phenol extract (r = 0.906 0)]. The antioxidant properties of plant foods have been attributed mainly to their phenolic content[13],[22],[36]. Antioxidants play their protective role on cells either by preventing the production of free radicals or by neutralizing/scavenging free radicals produced in the body or by reducing/chelating the transition metal composition of food[14]. The antiradical activity of flavonoids and phenols is principally based on the structural relationship between different parts of their chemical structure[37].
DPPH is a free radical donor that accepts an electron or hydrogen to become a stable diamagnetic molecule[29]. Therefore, the ability of the polyphenolic rich extracts to scavenge DPPH radicals was investigated. However, judging by the IC50, there was no significant difference in DPPH radical scavenging ability of the free soluble phenolic extract (IC50 = 212.53 µg/mL) and bound phenolic extract (IC50 = 256.14 µg/mL). Moreover, the apparent high DPPH radical scavenging ability of extracts could have been due to high total phenolic contents.
The total antioxidant capacity of the extracts, a function of the radical scavenging ability of the extracts, was studied using a moderately stable nitrogen-centered radical species, ABTS.+[30]. The result agrees with that of the DPPH radical scavenging ability, in that there is no significant difference in the ABTS.+ scavenging ability of the free soluble phenolic (0.059 0 mM TEAC/g) and bound phenolic (0.057 6 mM TEAC/g) extracts.
Reducing power is a potent antioxidant defense mechanism which is based on either electron transfer or/and hydrogen atom transfer by the antioxidant molecule31. Ferric-to-ferrous iron reduction occurs rapidly with all reductants with half reaction reduction potentials above that of Fe3+/Fe2+, thus, the values in the reducing power assay will express the corresponding concentration of electron-donating antioxidants. However, the results revealed that the free soluble phenolic extract (0.477 4 mM AAE/g) had a significantly (P<0.05) higher ability to reduce Fe3+ to Fe2+ than the bound extract (0.339 4 mM AAE/g). The trend in the reducing properties of the phenolic extracts is in agreement with the phenol content (total phenol and flavonoid). This supports the assertion that the antioxidant activity of phenolics is mainly due to their redox properties which allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers[13],[14].
This study reports a strong correlation between the phenolic content of clove bud and the enzyme inhibitory activities. Furthermore, the inhibition of key enzymes linked to type-2 diabetes (alpha-amylase and alpha-glucosidase) coupled with strong antioxidant properties of the tropical cloves phenolics could be part or possible mechanism through which the clove bud elicits its antidiabetic potentials. However, the strong alpha-glucosidase and mild alpha-amylase inhibition of clove bud could make it an excellent functional food and fantastic nutraceutical source for the management of type-2 diabetes with minimal side effects. Although, this study revealed the in vitro antioxidant and enzyme inhibitory actions of phenolic extracts from clove buds. Further investigation is ongoing to ascertain possible effects in vivo.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.World Health Organization [cited 2011 August]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
- 2.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2010;51(5):993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin and heme oxygenase. Free Rad Biol Med. 2007;43(5):720–9. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adisa RA, Choudhary MI, Olorunsogo OO. Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in streptozotocin-induced diabetic rats and mice. Exp Toxicol Pathol. 2011;63(7-8):619–25. doi: 10.1016/j.etp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Centre for Disease Control. cited 2011 Aug. Available from: http://www.cdc.gov/chronicdisease/resources/publications/AAG/ddt.htm_2011.
- 7.Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans and pumpkin: In vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10(2):266–75. doi: 10.1089/jmf.2006.234. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Narwal S, Kumar V, Prakash O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Phcog Rev. 2011;5(9):19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: An update. Curr Diabetes Rev. 2011;7(6):392–405. doi: 10.2174/157339911797579160. [DOI] [PubMed] [Google Scholar]
- 10.Gruenwald J, Freder J, Armbruester N. Cinnamon and Health. Crit Rev Food Sci Nutr. 2010;50(9):822–34. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 11.Ranilla LG, Kwon YI, Apostolidis E, Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour Technol. 2010;101(12):4676–89. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 12.Cheplick S, Kwon Y, Bhowmik P, Shetty K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresource Technol. 2010;101(1):404–13. doi: 10.1016/j.biortech.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 13.Oboh G, Rocha JBT. New York: Nova Science Publishers Inc; 2007. Antioxidant in foods: A new challenge for food processors: Leading edge antioxidants research; pp. 35–64. [Google Scholar]
- 14.Oboh G, Akomolafe TL, Adefegha SA, Adetuyi AO. Inhibition of cyclophosphamide induced oxidative stress in brain by polar and non-polar extracts of Annatto (Bixa orellana) seeds. Exp Toxicol Pathol. 2011;63(3):257–62. doi: 10.1016/j.etp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Memnune S, Ailal Y, Neva G, Bulent C, Zaynep E, Sezal E. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharm Sci. 2009;22(1):102–6. [PubMed] [Google Scholar]
- 16.Oboh G, Akinyemi AJ, Ademiluyi AO, Adefegha SA. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes-in vitro. J Food Nutr Res. 2010b;49(1):14–20. [Google Scholar]
- 17.Sultana S, Ripa FA, Hamid K. Comparative antioxidant activity study of some commonly used spices in Bangladesh. Pak J Biol Sci. 2010;13(7):340–3. doi: 10.3923/pjbs.2010.340.343. [DOI] [PubMed] [Google Scholar]
- 18.Abo KA, Fred-Jaiyesimi AA, Jaiyesimi AE. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J Ethnopharmacol. 2008;115(1):67–71. doi: 10.1016/j.jep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Parle M, Khanna D. Clove: a champion spice. Int J Res in Ayurveda Pharm. 2011;2(1):47–54. [Google Scholar]
- 20.Prasad RC, Herzog B, Boone B, Sims L, Waltner-Law M. An extract of Syzygium aromaticum represses genes encoding hepatic gluconeogenic enzymes. J Ethnopharmacol. 2005;96(1-2):295–301. doi: 10.1016/j.jep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Atawodi SE, Atawodi JC, Pfundstein B, Spiegelhalder B, Bartsch H, Owen R. Assessment of the polyphenol components and in vitro antioxidant properties of Syzygium aromaticum (L.) Merr.& Perry. EJEAChe. 2011;10(3):1970–8. [Google Scholar]
- 22.Chu Y, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activity of common vegetables. J Agric Food Chem. 2002;50(23):6910–6. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 23.Worthington V. Worthington Enzyme Manual. Freehold: Worthington Biochemical Corp; 1993. Alpha amylase; pp. 36–41. [Google Scholar]
- 24.Oboh G, Ademiluyi AO, Faloye YM. Effect of combination on the antioxidant and inhibitory properties of tropical pepper varieties against α-amylase and α-glucosidase activities in vitro. J Med Food. 2011;14(10):1152–8. doi: 10.1089/jmf.2010.0194. [DOI] [PubMed] [Google Scholar]
- 25.Belle NAV, Dalmolin GD, Fonini G, Rubim MA, Rocha JBT. Polyamine reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008(2):245–251. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi, K Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau's reagent. Met Enzymology. 1999;299:152–78. [Google Scholar]
- 28.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and praline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 1999;91:571–7. [Google Scholar]
- 29.Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging actionof medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen Pharmacol. 1999;32(6):661–7. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 30.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Rad Biol Med. 1999;26(9-10):1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 31.Pulido R., Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48(8):3396–402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 32.Zar JH. Upper Saddle River: Prentice-Hall, Inc; 1984. Biostatistical analysis; p. 620. [Google Scholar]
- 33.Aguilar-Santamaría L, Ramírez G, Nicasio P, Alegría-Reyes C, Herrera-Arellano A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J Ethnopharmacol. 2009;124(2):284–8. doi: 10.1016/j.jep.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Nickavar B, Yousefian N. Inhibitory effects of six Allium species on α-amylase enzyme activity. Iranian J Pharm Res. 2009;8:53–57. [Google Scholar]
- 35.Oboh G, Ademosun AO. Phenolics from orange peels (Citrus sinensis) inhibit key enzymes linked to non-insulin dependent diabetes mellitus (NIDDM) and hypertension. La Rivista Italiana Delle Sostanze Grasse. 2011;88(1):16–23. [Google Scholar]
- 36.Sun J, Chu Y, Wu X, Liu R. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem. 2002;50(25):7449–54. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 37.Rong T. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–46. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adaramoye O, Amanlou M, Habibi-Rezaei M, Pasalar P, Ali MM. Methanolic extract of African mistletoe (Viscum album) improves carbohydrate metabolism and hyperlipidemia in streptozotocin-induced diabetic rats. Asian Pac J Trop Med. 2011;5(6):427–433. doi: 10.1016/S1995-7645(12)60073-X. [DOI] [PubMed] [Google Scholar]
- 39.Mi-Ja Kim, Hye Kyung Kim. Insulinotrophic and hypolipidemic effects of Ecklonia cava in streptozotocin-induced diabetic mice. Asian Pac J Trop Med. 2011;5(5):374–379. doi: 10.1016/S1995-7645(12)60062-5. [DOI] [PubMed] [Google Scholar]
- 40.Arunachalam K, Parimelazhagan T. Antidiabetic activity of aqueous root extract of Merremia tridentata (L.) Hall. f. in streptozotocin-induced diabetic rats. Asian Pac J Trop Med. 2011;5(3):175–179. doi: 10.1016/S1995-7645(12)60020-0. [DOI] [PubMed] [Google Scholar]
- 41.Poongothai K, Ponmurugan P, Ahmed KSZ, Kumar BS, Sheriff SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(10):778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 42.Mishra Shanti Bhushan, Verma Amita, Mukerjee Alok, Vijayakumar M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4(9):689–693. doi: 10.1016/S1995-7645(11)60175-2. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran S, Rajasekaran A, Manisenthil Kumar KT. Antidiabetic, antihyperlipidemic and antioxidant potential of methanol extract of Tectona grandis flowers in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4(8):624–631. doi: 10.1016/S1995-7645(11)60160-0. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, Kumar V, Prakash O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac J Trop Med. 2011;4(8):347–352. doi: 10.1016/S1995-7645(11)60101-6. [DOI] [PubMed] [Google Scholar]
- 45.Tewari Vineeta, Tewari Ajoy, Bhardwaj Nikha. Histological and histochemical changes in placenta of diabetic pregnant females and its comparision with normal placenta. Asian Pac J Trop Dis. 2011;1(1):1–4. [Google Scholar]
- 46.Kumar S, Narwal S, Kumar D, Singh G, Narwal S, Arya R. Evaluation of antihyperglycemic and antioxidant activities of Saraca asoca (Roxb.) De Wild leaves in streptozotocin induced diabetic mice. Asian Pac J Trop Dis. 2011;1(1):170–176. [Google Scholar]
- 47.Puntel RL, Nogueira CW, Rocha JBT. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem Res. 2005;30(2):225–35. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 48.Shah SV, Fonseca VA. Iron and diabetes revisited. Diabetes Care. 2011;34(7):1676–7. doi: 10.2337/dc11-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minamiyama Y, Takemura S, Kodai S, Shinkawa H, Tsukioka T, Ichikawa H, et al. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am J Physiol Endocrinol Metabol. 2010;298(6):e1140–9. doi: 10.1152/ajpendo.00620.2009. [DOI] [PubMed] [Google Scholar]