Abstract
Objective
To determine the antimicrobial effects of grape seed on peri-implantitis microflora.
Methods
The grape seed extract was tested against peri-implantitis microflora most commonly found in craniofacial implants including reference strains of Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Candida albicans (C. albicans) and clinical strains of S. aureus, Klebsiella pneumonia (K. pneumonia) and Candida parapsilosis (C. parapsilosis) by disk diffusion test. Minimum inhibitory concentrations (MIC) and minimum cidal concentrations (MCC) were determined using modified agar dilution millpore method. The extract was further combined with polyethylene glycol and propylene glycol, and was tested for antimicrobial effects.
Results
Grape seed extract showed positive inhibitory effects with S. aureus at MIC of 0.625 mg/mL and MCC of 1.25 mg/mL respectively. However the extracts showed minimal or no reactivity against strains of E. coli, K. pneumonia, C. parapsilosis and C. albicans. The use of grape seed extract in combination with polyethylene glycol and propylene glycol also showed dose dependent inhibitory effect on S. aureus.
Conclusions
The results of the study showed that grape seed has potential antimicrobial effects which can be further studied and developed to be used in the treatment of infected skin-abutment interface of craniofacial implants.
Keywords: Peri-implantitis, Grape seed extract, Craniofacial implants, Staphylococcus aureus
1. Introduction
Maxillofacial defects may be a resultant of treatment consequence of neoplasm, trauma and or congenital defects. Previously the rehabilitation of facial defects had been limited or poorly accepted due to inadequacy of materials and retention. With the development of newer materials such as silicone elastomers and medical adhesives the prosthetic rehabilitation of the lost body parts has been considered as an acceptable treatment option for patients with craniofacial defects and may be preferable to complex surgical reconstruction. Furthermore, the use of craniofacial implants offers patients with the state of the art treatment for rehabilitation of facial defects. The greater retention that these implant retained facial prosthesis offer provide a great boost to the patient's psychology and overcomes the limitations of adhesives such as adhesive related skin irritation, breakdown and discoloration of the margins of the prosthesis, lack of retention for large prosthesis, and variable positioning of the prosthesis. In light to these benefits there are related complications to implant retained prosthesis, importantly tissue reactions of the implant skin interface[1].
Histological studies of the peri-implant abutment skin interface have shown that there is a lack of strong physical barrier between the soft tissue and the implants. It has been noted that the seal is more dynamic in nature comprising of inflammatory cells[2],[3] and dependent on the load of the implant site from the host response to the implant material, surface, stability, exogenous agents, and mechanical factors. A lack of proper hygiene maintenance along with the compromised skin interface can lead to change in the resident skin microflora. Many microbiological studies have shown Staphylococcus aureus to be a common isolate from infected craniofacial implant-abutment interface[4]. Few isolated strains of Escherichia coli (E.coli), Klebsiella pneumonia (K. pneumonia), Candida albicans (C. albicans), Candida krusei (C. krusei) and Candida parapsilosis (C. parapsilosis) have also been found from these sites[5].
Recently there have been many research focused towards the antimicrobial properties of herbal products. Grape (Vitis viniferea) is one of the most palatably edible fruits, grown all over the world and is considered to have many nutritional and medicinal properties. It has been reported that grape contains a large amount of phenolic compounds, especially the seed which is considered to contain 60%-70% of the total phenolic content of the fruit, comprising of monomeric phenolic compounds such as (+)-cathechins, (-)-epicatechin and (-)-epicatechin-3-o-gallate, and dimeric, trimeric and tetrameric procyanidins.
Grape seed extract obtained from grapes grown in Hasandede, Emir and Kalecik Karasi wine cultivars in Turkey showed concentrations of 2.5%- 5% exhibited the most inhibitory effect against a wide variety of microorganisms including E. coli, K. pneumoniae, and S. aureus[6]. A similar grape seed extract product IH636 was tested against 21 strains of gram positive and gram negative cocci which showed gram positive cocci to be more susceptible, especially S. aureus. In presence of 1 mg/mL, 99% inhibition was reported with no further bacterial recovery[7]. Complete inhibition of 43 clinical strains of Methicillin resistant S. aureus was noted at concentration of 3 mg/mL crude grape seed proanthrocyanide extract[8].
More researches based on grape seed extract can be beneficial to gain more knowledge towards its potential in medicinal science. This study deals with the antimicrobial effects of “Breko exGrape seed OPC 30” grape seed extract on the most commonly found microflora that exists around infected skin-abutment interface of craniofacial implants and its future prospects.
2. Material and methods
2.1. Materials
Commercially available grape seed extract “Breko exGrape seed OPC 30” was obtained from East Asiatic Public Company Ltd, Thailand. It is a natural procyanidin extract made from white grape seeds without the inclusion of skins and stems, grown in the south of France. The test organisms used in the study were as follows: S. aureus ATCC 6538, E. coli ATCC 25922, C. albicans ATCC 10231 and clinical strains of S. aureus, K. pneumonia and C. parapsilosis. These strains were obtained from the Dept. of Oral Microbiology, Mahidol University, Thailand.
2.2. Methods
2.2.1. Disc diffusion method
The stock solutions of the grape seed extract was prepared in water. The microorganisms were incubated in enriched blood agar at 37 °C for 24-48 hours. Fresh isolates were then transferred to distilled water and a microbial suspension was prepared and the turbidity adjusted to 0.5 McFarland's solution to yield approximately 108 CFU/mL. The inoculum was evenly spread in Mueller Hinton Agar (MHA). Two-fold dilution of the stock solution was performed to obtain grape seed extract concentrations ranging from 20 mg/mL to 1.25 mg/mL. 6 mm sterile paper discs were prepared and 20 µL of each of the concentration was transported onto each disc. These discs were placed in MHA and incubated at 37 °C for 24-48 hours, following which the inhibition zones were measured.
2.2.2. Measurement of Minimum inhibitory concentration (MIC) and minimum cidal concentration (MCC)
The minimum inhibitory concentrate of grape seed extract was determined by modified agar dilution method[9]. Various concentrations of grape seed extract were mixed along with Muller Hinton agar to obtain extract concentration ranging from 10 mg/mL to 0.078 mg/mL in the agar. 0.45 µm porosity Millipore membranes were placed into the prepared MHA and were inoculated with 20 µL of microbial suspension which approximately contained 104 CFU/spot. The agar plates were incubated at 37 °C for 24-48 hours and observed for subsequent microbial growth on the Millipore membranes.
After determination of MIC, the membranes were transported from the agar to DifcoTM Brain Heart Infusion broth solution in test tubes. The broth media were then incubated at 37 °C for 24-48 hours to observe for microbial growth and the MCC was determined.
2.2.3. Preparation of anti-microbial paste
The MCC extracts were mixed with propylene glycol and polyethylene glycol which served as a base for drug delivery. Brain heart infusion agar was prepared and was inoculated with fresh strains of the microorganism, which were prior adjusted to 0.5 McFarland's solution to yield approximately 108 CFU/mL before inoculation. A 4 mm diameter copper coil was used to create wells in the agar which were subsequently filled with the antimicrobial paste. After an incubation time of 24-48 hours at 37 °C, the inhibition zones so formed around the wells were observed and measured to determine the antimicrobial properties of the paste.
2.3. Statistical analysis
All tests were done in duplicates for verification of the results. The values were expressed in mean. To determine the correlation between concentration of extract and inhibition zone, Spearman rank correlation coefficient was used. Statistical significance was set at P-value <0.05. All data analyses were performed using SPSS (Version 17).
3. Results
3.1. Disk diffusion test
Both strains of S. aurues showed sensitivity to the effects of grape seed extract with inhibition zones ranging from 12.5 mm to 7 mm. C. albicans showed minimum growth inhibitory effects to grape seed concentration with inhibition zones measuring from 8 mm to 7.5 mm. However strains of E. coli, K. pneumoniae, C. parapsilosis showed no sensitivity to the effects of the extract, even at a concentration of 20 mg/mL.
3.2. MIC and MCC results
The MIC and MCC was carried out based on the results of the disk diffusion test. The test results showed potent effects of the grape seed extract with MIC at 0.625 mg/mL and MBC at 1.250 mg/ml for both strains of S. aureus. However even at a high concentration of 20 mg/mL extract in agar, MIC for C. albicans and C. parapsilosis could not be established (Table 1).
Table 1. MIC and MCC results of grape seed extract (mg/mL).
| Tested strains | MIC | MCC |
| S. aureus ATCC 6538 | 0.625 | 1.250 |
| S. aurues clinical strain | 0.625 | 1.250 |
| C. albicans ATCC 10231 | < 20 | - |
| C. parapsilosis clinical strain | < 20 | - |
3.3. Antimicrobial paste
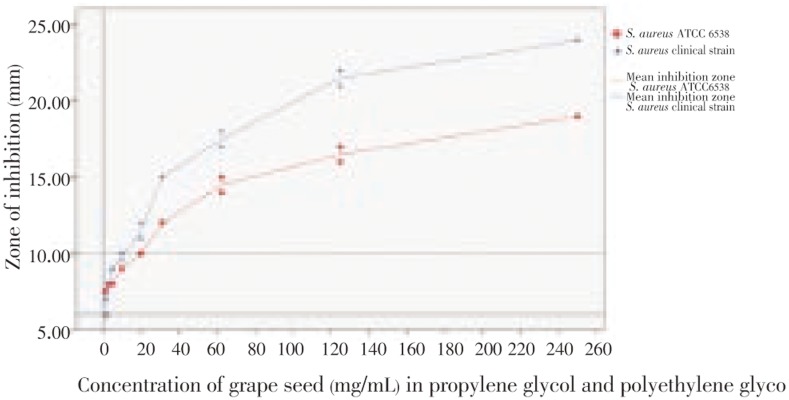
Overall the grape seed extracts mixed with propylene glycol and polyethylene glycol showed a dose dependent inhibitory effects on S. aureus where increasing the concentration of the grape seed extract in the base increased the zone of inhibition (S. aureus ATCC 6538 Spearman corr =0.995 and S. aureus clinical strain Spearman corr = 0.987 at P<0.05; Table 2, Figure 1). The positive control was used as 4% Erythromycin which showed a mean inhibition zone of 43 mm and negative control was 1:1 mixture of propylene glycol and polyethylene glycol which did not exhibit any inhibition zone.
Table 2. Mean inhibition zone (mm) measured with grape seed extract paste.
| Concentration of grape seed (mg/mL) | S. aureus ATCC 6538 strain | S. aureus clinical strain |
| 1.25 | 6.5 | 7.5 |
| 2.50 | 7.0 | 8.0 |
| 5.00 | 9.0 | 8.0 |
| 10.00 | 9.75 | 9.0 |
| 20.00 | 11.5 | 10.0 |
| 31.25 | 15.0 | 12.0 |
| 62.50 | 17.5 | 14.5 |
| 125.00 | 21.5 | 16.5 |
| 250.00 | 24.0 | 19.0 |
Figure 1. Comparison of the zone of inhibition with the concentration of grape seed extract in the antimicrobial paste.
4. Discussion
The disk agar diffusion method is considered to be a standard screening test to check the antimicrobial activity of a reagent. The disk diffusion test revealed that grape seed extracts showed moderate activity against both strains of S. aureus with inhibition zones of 12.5 mm to 7 mm. However the reagent showed minimal or no reactivity against E. coli, K. pneumonia, C. albicans and C. parapsilosis.
MIC and MCC tests were performed on the micro-organisms which were susceptible to the effects of the grape seed extract, namely S. aureus and C. albicans. Different quantitative tests, such as broth dilution, agar dilution, and gradient dilution, are available to measure the lowest concentrate of a reagent that inhibits the visible growth of test microbes, ie. MIC. In this study the determination of MIC and MCC was based on the modified Millipore membrane agar dilution method. This method has been used to measure the antimicrobial properties of essential oils extracted from various herbs[9]. The grape seed extract was effective against both strains of S. aureus at MIC values of 0.625 mg/mL and MCC at 1.25 mg/mL. However the agar dilution test had limitations and it failed to obtain a homogenous set when the concentration of the extract was increased more than 20 mg/mL in the agar. The test for MIC for Candida species which required concentration of more than 20 mg/mL could not be established.
Phenolic contents found in grape seed are partially hydrophobic, and are considered to interact with the bacterial cell wall and lipopolysaccharide interfaces by decreasing membrane stability. The amount of phenolic content in grape seed extract, measured in gallic acid equivalent, has been directly correlated to the antibacterial properties[6],[10]. A study on the bacteriostatic and bactericidal effects of grape seed specially dealt with the pharmacodynamics of gallic acid on E. coli, S. enteritidis, and S. aureus. Structure-activity correlation assays showed that three hydroxyl groups of the compound are effective against E. coli and S. enteritidis and all of the substituents of the benzene ring were effective against S. aureus. The study conducted on the effects of muscadine grape seed extract on H. pylori suggested that damage to the bacteria occurs on multiple levels including the inhibition of basic energy production and/or virulence factors[11]. Further supportive researches have shown that grape seed extract, resembled THF structurally and interfered with folate mediated one-carbon metabolic pathway of S. aureus[7].
Propylene glycol and polyethylene glycol, polymers with dihydric alcohol, are considered to be non-toxic, water-soluble which resists recognition by the immune system. They have been used as solvents and moisturizers in many pharmaceutical preparations. Propylene glycol and polyethylene glycol have also been used as vehicles in the preparation of 3 Mix, which is a mixture of 3 types of antibiotics and has been used to treat pulpally involved primary molars. Propylene glycol and polyethylene glycol serves as good drug delivery system because of its biocompatibility, ease in manipulation, and handling[12]. In the study, propylene glycol and polyethylene glycol combination did not exhibit any microbial inhibitory effects of its own. However the combination of grape seed extract with propylene glycol and polyethyelene glycol showed a dose dependent inhibitory effect on both strains of S. aureus.
Infections of the soft tissue surrounding extra-oral implants can be managed by proper treatment planning and patient selection, adequate ventilation of the skin, meticulous maintenance of skin hygiene, administration of antimicrobial agents, and, occasionally, surgical revision and debridement[13]. Although antibiotic preparations may help to decrease the peri-implantitis flora, resistant strains may arise if used for a long duration. Plant extracts can be used as alternatives but like other naturally occurring products the quality control of the batches should be well maintained to obtain uniform results.
The current study showed that grape seed extract “Breko exGrape seed OPC 30” showed moderate effect on S. aureus but showed minimal or no inhibitory effects on gram negative and yeast microorganisms. Grape seed extract also exhibited a dose dependent inhibitory effect on S. aureus when combined with propylene glycol and polyethylene glycol. These extracts can be further researched and developed to be used as potential anti-microbial agents to treat skin infections around the implant abutment interface of craniofacial implants.
Acknowledgments
This research was supported by Maxillofacial Prosthetic Service Research Fund (Grant No. 194/2010), Faculty of Dentistry, Mahidol University, Bangkok, Thailand.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Gumieiro EH, Dib LL, Jahn RS, Santos Junior JF, Nannmark U, Granström G, et al. Bone-anchored titanium implants for auricular rehabilitation: case report and review of literature. Sao Paulo Med J. 2009;127:160–165. doi: 10.1590/S1516-31802009000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgers KM, Thomsen P, Tjellstrom A, Bjursten LM. Immunohistochemical study of the soft tissue around long-term skin-penetrating titanium implants. Biomaterials. 1995;16:611–616. doi: 10.1016/0142-9612(95)93858-b. [DOI] [PubMed] [Google Scholar]
- 3.Holgers KM, Thomsen P, Tjellstrom A, Ericson LE. Electronic microscopic observation on the soft tissue around clinical long-term percutaneous titanium implants. Biomaterials. 1995;16:83–90. doi: 10.1016/0142-9612(95)98267-i. [DOI] [PubMed] [Google Scholar]
- 4.Klein M, Weisz I, Camerer C, Menneking H, Kim DM. Therapy of percutaneous infection around craniofacial implants. Int J Prosthodont. 2009;22:594–596. [PubMed] [Google Scholar]
- 5.Visuttiwattanakorn S, Srithavaj T, Thaweeboon S. Evaluation of microflora around extraoral per-implant percutnaeous tissues in a group of Thai patients. Mahidol Dental J. 2006;26:281–288. [Google Scholar]
- 6.Baydar NG, Sagdic O, Ozkan G, Cetin S. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L. ) seed extracts. Int J Food Sci Technol. 2006;41:799–804. [Google Scholar]
- 7.Kao TT, Tu HC, Chang WN, Chen BH, Shi YY, Chang TC, et al. Grape seed extract inhibits the growth and pathogenicity of Staphylococcus aureus by interfering with dihydrofolate reductase activity and folate-mediated one-carbon metabolism. Int J Food Microbiol. 2010;141:17–27. doi: 10.1016/j.ijfoodmicro.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Al-Habib A, Al-Saleh E, Safer AM, Afzal M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA) J Toxicol Sci. 2010;35:357–364. doi: 10.2131/jts.35.357. [DOI] [PubMed] [Google Scholar]
- 9.Tantaoui-Elaraki A, Beraoud L. Inhibition of growth and aflatoxin production in Aspergillus parasiticus by essential oils of selected plant materials. J Environ Pathol Toxicol Oncol. 1994;1:67–72. [PubMed] [Google Scholar]
- 10.Jayaprakasha GK, Selvi T, Sakariah KK. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Research Int. 2003;36:117–122. [Google Scholar]
- 11.Brown JC, Huang G, Haley-Zitlin V, Jiang X. Antibacterial effects of grape extracts on Helicobacter pylori. Appl Environ Microbiol. 2009;48:848–852. doi: 10.1128/AEM.01595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakornchai S, Banditsing P, Visetratana N. Clinical evaluation of 3Mix and Vitapex as treatment options for pulpally involved primary molars. Int J Paediatr Dent. 2010;20:214–221. doi: 10.1111/j.1365-263X.2010.01044.x. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Serriah MM, McGowan DA, Moos KF, Bagg J. Extra-oral endosseous craniofacial implants: current status and future developments. Int J Oral Maxillofac Surg. 2003;32:452–458. [PubMed] [Google Scholar]