Abstract
Objective
To investigate the cytotoxic effect of Elaeis guineensis methanol extract on MCF-7 and Vero cell.
Methods
In vitro cytotoxicity was evaluated in by MTT assay. Cell morphological changes were observed by using light microscope.
Results
The MTT assay indicated that methanol extract of the plant exhibited significant cytotoxic effects on MCF-7. Morphological alteration of the cell lines after exposure with Elaeis guineensis extract were observed under phase contrast microscope in the dose dependent manner.
Conclusions
The results suggest the probable use of the Elaeis guineensis methanol extract in preparing recipes for cancer-related ailments. Further studies on isolation of metabolites and their in vivo cytotoxicity are under investigation.
Keywords: Cytotoxicity, Growth inhibitory, Elaeis guineensis, Cell morphology
1. Introduction
The crescendo of a new anticancer agent begets in company with new leading bioactive compounds being identified. Normatively, new bioactive compounds displaying anticancer activities are obtained from pharmaceutical industries and research laboratories that relativize to academic institutions. Major resources scilicet natural products and synthetic compounds analog to known agents have been disclose in possessing various bioactive compounds yearned by the drug development industries. The evaluation and the discovery of new anticancer agents is long-term process that encompasses many steps. The step broaches with the screening for anticancer properties, followed by the isolation and identification of bioactive compounds obliged to anticancer properties, toxicity estimation of the isolated compounds and finally in vivo anticancer activity testing to verify the aptitude of the compounds. The winnowing natural products particularly plant extracts after effect the breakthrough of few excellent anticancer agents. The vinca alkaloids (vincristine, vinblastine and vindesine) and the podophylotoxin derivatives (etoposide and teniposide) are examples of clinically active plant products[1]. The goal of screening medicinal plant is to search for excellent anticancer agent avertable to human malignancies. In defiance of astonishing advances in modern medicine, such as surgery, radiotherapy, chemotherapy, and hormone therapy, cancer disease remains a worldwide health problem, thus endeavoring the search for new alternate approach. The nature as a huge valuable contributor of potential source for chemotherapeutic agents has recently been reviewed[2]. Newman and Cragg[2] reported in their analysis that the sources of new drugs over the period 01/1981-06/2006 possess 974 small molecules, out of which 66% were new chemical entities which are classified synthetic, 17% correspond to synthetic molecules containing pharmacophores derived directly from natural products, and 12% are actually modeled on a natural product inhibitor of the molecular target of interest, or mimic (i.e., competitively inhibit) the endogenous substrate of the active site, such as ATP. These facts are in favor with the new call for medicinal plant identification namely local plants, in conjunction with anticancer properties. Since the methanol extract of Elaeis guineensis (E. guineensis) insinuated good biological activity earlier, considerably evaluation on the anticancer potentiality is discussed in this study. The current study was undertaken with the objective to rationalize the cytotoxicity effect of E. guineensis methanol extract on MCF-7 and Vero cell lines in accordance to the observable changes of cell morphology upon exposure to the extract.
2. Materials and methods
2.1. Plant material and extraction
The leaves of E. guineensis were collected in Semeling, Kedah, Malaysia around January 2010. Plant material was air dried in the laboratory for 5 days at room temperature followed by oven drying at 40oC then grinded to powder form using an electric mill. The powdered sample was kept in an air tight container until required. About 45 g of the powdered leaves of E. guineensis was macerated in 250 mL of methanol for 72 h. Rotary evaporator was used to filter and concentrated methanolic plant material at 40oC and the resulting extract was kept in the refrigerator.
2.2. Cytotoxicity Screening
2.2.1. Cell Lines
All cell lines used during the present study were obtained from Tissue Culture Laboratory of Institute for Research in Molecular Medicine, Universiti Sains Malaysia, Pulau Pinang, Malaysia.
The Vero cell line was initiated from kidney of a normal adult African green monkey on March 27th, 1962, by Yasummura and Kawakita at the Chiba University, Japan American Public Health Association, 1992). Vero cells were maintained in RPMI-1640 medium supplemented with 10% FBS, glutamine (2 raM), penicillin (100 units/mL) and streptomycin (100 µg/mL). The cells were cultured at 37°C in a humidified 5% CO2 incubator.
Human breast adenocarcinoma (MFC-7) cells were derived from breast cancer which was obtained from American Type Culture Collection (ATCC: Manassas, VA). MCF-7 cells were maintained in RPMI-1640 medium supplemented with 10% FBS, glutamine (2 raM), penicillin (100 units/mL) and streptomycin (100 µg/mL). The cells were cultured at 37°C in a humidified 5% CO2 incubator.
2.1.2. Cytotoxicity assay
The extract of E. guineensis leaf was tested for in vitro cytotoxicity, using Vero and MCF-7 cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay[3]. Briefly, 100 µL of media (RMPI 1640) was added into each of the 96-well plates from row B to row G (triplicate). Then, 100 µL of diluted plant extract or fractions were added in row A and row B. Starting from row B the 200 µL of solution (100 µL drug + 100 µL media) were mixed and 100 µl from row B were added into next row (row C) by using micropipette and a serial dilution was done up to row G. Finally, excessive 100 µL from row G were discarded. The final volume for each well was 100 µL. The cultured Vero/MCF-7 cells were harvested by trypsinization, pooled in a 50 mL vial. Then, the cells were plated at a density of 1×106 cells/mL cells/well (100 µL) into 96-well micro-titer plates from row B to row G. Finaly, 200 µL of cells (Vero/MCF-7) were added in row H as a control. Each sample was replicated 3 times and the cells were incubated at 37°C in a humidified 5% CO2 incubator for 24 h. After the incubation period, MTT (20 µL of 5 mg/mL) was added into each well and the cells incubated for another 2-4 h until purple precipitates were clearly visible under a microscope. Flowingly, the medium together with MTT (190 µL) were aspirated off the wells, DMSO (100 µL) was added and the plates shaken for 5 min. The absorbance for each well was measured at 540 nm in a micro-titre plate reader[3] and the percentage cell viability (CV) was calculated manually using the formula:
 |
A dose-response curve were plotted to enable the calculation of the concentrations that kill 50% of the Vero/MCF-7 cells (IC50).
2.2.3. Morphological analysis
Morphological observation of cell treated with E. guineensis extract from cytotoxicity study was done to determine the changes induced by the extracts. Changes such as shrinking of the cells, membrane blebbing, ballooning, chromatin condensation, formation of apoptotic bodies were observed in predicting the apoptotic mechanism for cell death. Meanwhile, vacuolations of the cytoplasm and formation of double membrane vesicle containing organelles were assessed for authophagic cell death.
3. Results
3.1. Proliferative effects of MCF-7 and Vero cells
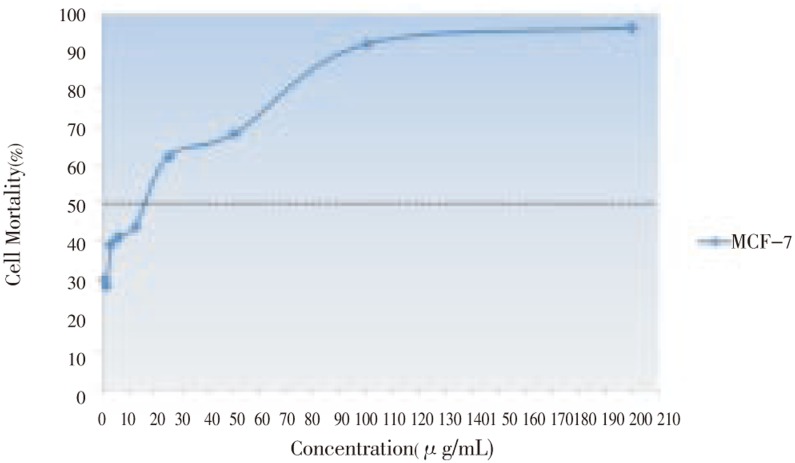
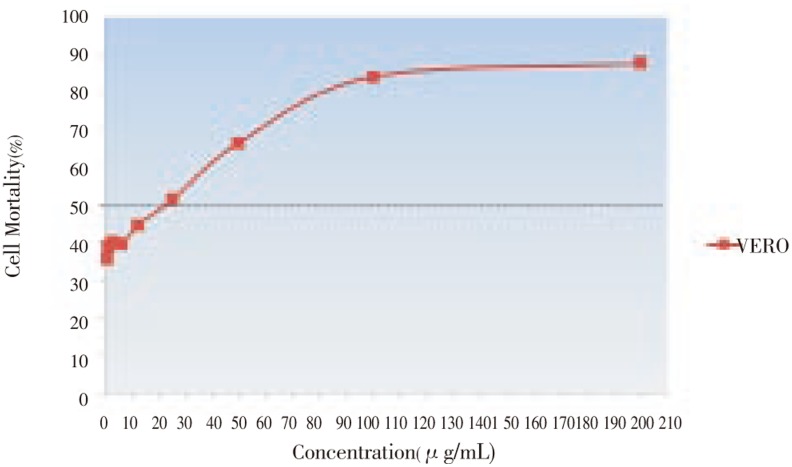
The effect of anticancer from E. guineensis on MCF-7 and Vero cell lines was evaluated thorugh micro-culture tetrazolium assay (MTT). The multiple concentrations of methanolic extract from E. guineensis were used and effective doses were calculated from dose-response curve. Results of the cytotoxicity evaluation against MCF-7 and Vero cell line of the E. guineensis extract are shown in Figure 1 and 2. The methanol extract of E. guineensis exhibited no significant activity against the Vero cell line achieving an IC50 value of 22.00 µg/mL. On the contrary, the methanol extract of E. guineensis exhibited significant activity against the MCF-7 cell line with an IC50 value of 15.00 µg/mL. The criteria of cytotoxicity for the crude extract, as established by the U.S. National Cancer Institute (NCI), is an IC50 < 20 µg/mL in the preliminary assay[4]. On treatment with E. guineensis extract, the MCF-7 cells showed an increased rate of cell death at a lower concentration of the extract when compared to that in the Vero cells (Figure 1 and 2).
Figure 1. Toxicity effects of the E. guineensis methanol extract against cancer cell line (MCF-7) after 24 hours of incubation.
Figure 2. Toxicity effects of the E. guineensis methanol extract against Vero cell line after 24 hours of incubation.
3.2. Evaluation on morphological changes upon treatment with extracts
Morphological alteration of MCF-7 and Vero cells lines upon exposure using E. guineensis extract was observed under phase contrast microscope. The cells indicated the most prominent effects after exposure to the E. guineensis extract. The microscopic observations revealed the E. guineensis extract to be having outstanding effect on treated MCF-7 cells compared to treated Vero cells and untreated cells (Figure 3). The number of dead cells increased correspondingly with concentration increment of the extract treatment in regard to observation. At high extract concentration, enlargement of the cells was conspicuosly observed. 40%-50% of the cells showed membrane blebbing (demonstrated with small protrusions of the membrane) and ballooning were apparent in the cells. The presence of apoptotic bodies could also be seen in the extract treated cells (Figure 3). Cells also showed extensive vacuolation in the cell cytoplasm, indicating autophagy like mechanism of cell death. Autophagosome like structures were clearly seen in the cells treated with E. guineensis extract (Figure 3A2 and 3B3). At highest concentration (100 µg/mL) the cells became rounder, shrunken and showed signs of detachment from the surface of the wells denoting cell death.
Figure 3. Morphological changes of the (A) MCF-7 and (B) Vero cells after E. guineensis methanol extract.
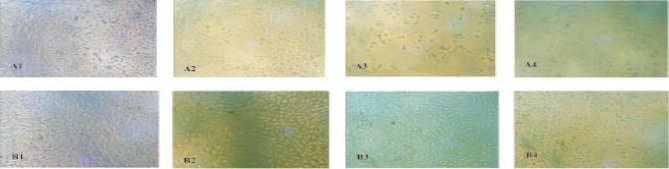
A1&B1: Control, A2&B2: 25 µg/mL, A3&B3: 50 µg/mL and A4&B4: 100 µg/mL treatment at various concentrations for 24 hours.
4. Discussion
The contribution of new and novel products from potential bioactive plants or their extracts for disease treatment and prevention is still vast, despite the overshadowing by recent synthetic chemistry as a method of drug discoveries and drug productions[5]. Moreover, plant derived drugs like vinblastine, vincristine, taxol, and camptothecin had lead to greatest extend within the vicinity of antitumor upon where, the drugs were reported to improvise the chemotherapy of some cancers[6]. Plants contain almost unlimited capacity to generate compounds that fascinates researchers in the quest for new and novel chemotherapeutics[7]. The persistency search for new anticancer compounds in plant medicines and traditional foods is a realistic and promising strategy for its prevention[8]. Numerous compounds found in plants with anticancer properties are such as alkaloids, phenylpropanoids, and terpenoids[9],[10].
Therefore, in this study E. guineensis leaf extract was evaluated as new anticancer agent by using MTT assays. Plants used in folk and traditional medicines have been accepted as leads for therapeutic drug development in modern medicine. E. guineensis was chosen for this study due to its use as a wound healing agent among the natives of Africans and as therapeutic agent in other parts of the world[11]. Hence this study the cytotoxicity was evaluated in vitro. Studies have observed the presence of a large number of bioactive compounds in the methanolic extracts of this plant including tannins, alkaloids, steroids, saponins, terpenoids, and flavonoids which exhibit various biological activities[11]-[17]. These compounds are present in a number of food items and hold great potential as drug candidates due to their safety, low toxicity and wide acceptance amongst the public.
In order to understand the characteristic of the cytotoxicity effect of Elaeis guineensis extract on cancer cells, two cells lines were selected to be investigated throughout this study; the cancerous MCF-7 cell lines and the control serving non- cancerous Vero cell lines. The present study also demonstrated the cytotoxicity indices as a measure of percentage cell mortality calculated by MTT assay in MCF-7 and Vero cells respectively, in a dose dependent manner at the end of 24 hours incubation with extract. Breast cancer cell line MCF-7 was used as the test system in this study which was prompted by the requirement of more effective treatment for the increasing incidence of breast cancers worldwide. The extract was able to inhibit the proliferation of the cancer cell at (15 µg/mL) and the normal Vero cells at (22 µg/mL). The American National Cancer Institute (NCI) guidelines set the limit of activity for crude extracts at 50% inhibition (IC50) of proliferation of less than 30 µg/mL after the exposure time of 72 hours[4]. However a crude extract with IC50 less than 20 µg/mL is considered highly cytotoxic[18]. The results of the present study showed potent cytotoxic effects on MCF-7 cells with E. guineensis extract. The IC50 value was found to be lower than that specified by NCI, USA for categorization of a pure compound as anticancer agent. The reduction in viable cell number was evident as 24 hours of treatment with both the extracts. The morphological effects were more prominent in the acetone extract treated cells showing extensive blebbing and vacuolation suggesting autophagic mechanism of cell death.
An IC50 value below this stringent value was noted for MCF-7 which falls within the NCI criteria thus to be considered as a promising anticancer potential. These data is also of interesting as it suggests that the extract is more toxic for cancer cells than on normal cells. The investigation provides evidence for cytotoxicity in MCF-7 which may be due to existing phytochemicals in the extract since E. guineensis as mention previously. The sensitivities of cancer cells to cell death by flavanoids[19] are accordance with this finding from previous reports in literature. In another study, the presence of alkaloids with flavonoids in Onobis hirta was reported expressing superior activity against cancer cells[20].
This finding suggests that the reduction observed in the viable cells following treatment with E. guineensis extract is due to cell death. In conclusion, the present observations provide preliminary data exposing E. guineensis extract to have potent cytotoxic activity against MCF-7 cells. This calls for further studies on the active components for proper assessment of their chemotherapeutic properties as well as their possible development as promising anticancer drugs.
Acknowledgments
This work was partly supported by USM Incentive Grant (Grant Number: 2009/167) from Universiti Sains Malaysia. S. Vijayarathna is supported by the Graduate Assistant Scheme from Institute for Postgraduate Studies of Universiti Sains Malaysia.
Footnotes
Foundation project: This work was partly supported by USM Incentive Grant (Grant Number: 2009/167) from Universiti Sains Malaysia.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Gueritte F, Fahy J. The vinca alkaloids. In: Cragg GM, Kingston DGI, Newman D, editors. Anticancer agents from natural products. Boca Raton: Taylor and Francis; 2005. pp. 123–136. [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:1022–1037. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Hameed ES, Salih A, Bazaid SA, Shohayeb MM, El-Sayed MM, El-Wakil EA. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. Eur J Med Plants. 2012;2:93–112. [Google Scholar]
- 5.Kviecinski MR, Felipe KB, Schoenfelder T, de Lemos Wiese LP, Rossi MH, Gonçalez E, et al. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol. 2008;117:69–75. doi: 10.1016/j.jep.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Yousefzadi M, Sharifi M, Behmanesh M, Moyano E, Bonfill M, Cusido RM, et al. Podophyllotoxin: Current approaches to its biotechnological production and future challenges. Eng Life Sci. 2010;10:281–292. [Google Scholar]
- 7.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–441. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 8.Yan-Wei H, Chun-Yu L, Chong-Min D, Wen-Qian W, Zhen-Lun G. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J Ethnopharmacol. 2009;123:293–301. doi: 10.1016/j.jep.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Kintzios E. Terrestrial plant-derived anticancer agents and plant species used in anticancer research. Crit Rev Plant Sci. 2006;25:79–113. [Google Scholar]
- 10.Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase 3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008;15:147–151. doi: 10.1016/j.phymed.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 11.Sasidharan S, Nilawatyi R, Xavier R, Yoga Latha L, Amala R. Wound healing potential of Elaeis guineensis Jacq leaves in an infected albino rat model. Molecules. 2010;15:3186–3199. doi: 10.3390/molecules15053186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulecha V, Sivakuma T. Anticancer activity of Tephrosia purpurea and Ficus religiosa using MCF 7 cell lines. Asian Pac J Trop Med. 2011;4(7):526–529. doi: 10.1016/S1995-7645(11)60139-9. [DOI] [PubMed] [Google Scholar]
- 13.Kumar RS, Rajkapoor B, Perumal P. In vitro and in vivo anticancer activity of Indigofera cassioides Rottl. Ex. DC. Asian Pac J Trop Med. 2011;4(5):379–385. doi: 10.1016/S1995-7645(11)60108-9. [DOI] [PubMed] [Google Scholar]
- 14.Kumar D Jaya, Santhi R Jaya. Antioxidant and cytotoxic effects of hexane extract of Morinda pubescens leaves in human liver cancer cell line. Asian Pac J Trop Med. 2012;5(5):362–366. doi: 10.1016/S1995-7645(12)60060-1. [DOI] [PubMed] [Google Scholar]
- 15.Hussain T, Fareed S, Siddiqui HH, Vijaykumar M, Rao CV. Acute and subacute oral toxicity evaluation of Tephrosia purpurea extract in rodents. Asian Pac J Trop Dis. 2012;2(2):129–132. [Google Scholar]
- 16.Kumbhare MR, Guleha V, Sivakumar T. Estimation of total phenolic content, cytotoxicity and in-vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac J Trop Dis. 2012;2(2):144–150. [Google Scholar]
- 17.Chairman K, Ranjit Singh AJA, Alagumuthu G. Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac J Trop Dis. 2012;2(2):234–238. [Google Scholar]
- 18.Mahavorasirikul W, Viyanant V, Chaijaroenkul W, Itharat A, Na-Bangchang K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern Med. 2010;10:55. doi: 10.1186/1472-6882-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116:164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talib WH, Mahasneh AM. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm. 2011;78:33–45. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]