Abstract
Objective
To investigate the antibacterial activity of marine actinobacteria against multidrug resistance Staphylococcus aureus (MDRSA).
Methods
Fifty one actinobacterial strains were isolated from salt pans soil, costal area in Kothapattanam, Ongole, Andhra Pradesh. Primary screening was done using cross-streak method against MDRSA. The bioactive compounds are extracted from efficient actinobacteria using solvent extraction. The antimicrobial activity of crude and solvent extracts was performed using Kirby-Bauer method. MIC for ethyl acetate extract was determined by modified agar well diffusion method. The potent actinobacteria are identified using Nonomura key, Shirling and Gottlieb 1966 with Bergey's manual of determinative bacteriology.
Results
Among the fifty one isolates screened for antibacterial activity, SRB25 were found efficient against MDRSA. The ethyl acetate extracts showed high inhibition against test organism. MIC test was performed with the ethyl acetate extract against MDRSA and found to be 1 000 µg/mL. The isolated actinobacteria are identified as Streptomyces sp with the help of Nonomura key.
Conclusions
The current investigation reveals that the marine actinobacteria from salt pan environment can be able to produce new drug molecules against drug resistant microorganisms.
Keywords: Marine actinobacteria, Salt pan, Multi drug resistance Staphylococcus aureus, Cross streak method, Kirby-Bauer method, MIC, Nonomura key
1. Introduction
Multi drug resistance in microorganism is an emerging serious problem in health care sector. The improper usage of antibiotics contributes a major role for drug resistance in pathogenic microbes. Microorganisms acquire resistance towards common antibiotics by altering their metabolism and genetic structure[1],[2]. There is an incessant need to find novel efficient drug molecules against multi drug resistant microbes.
Staphylococcus aureus (S. aureus) is a virulent pathogen that is responsible for a various infectious disease including sore throat, pneumonia, osteomyelitis, endocarditis, pimples and bacteremia. S. aureus has been reported resistance towards methicillin and vancomycin[3]. Methicillin resistance S. aureus (MRSA) was first reported in Britain, 1961 and was later found prevalent in hospitals. MRSA has been identified as an important nosocomial infection causing organism[4]. Due to the development of drug resistance in S. aureus, treatment of this bacterial infection has become a serious problem[5]. Recently, new antimicrobial agents like daptomycin, linezolid and streptogramin combination (quinupristin/dalfopristin) are useful to treat MRSA[6],[7]. However, due to certain defects and escalation of drug resistance it emphasizes the requirement of advance antimicrobial agents having potential activity towards Gram positive bacteria[8].
Natural products are boundless source for important novel compounds having antagonistic activity against pathogenic organisms. Marine environment covers almost 70% of the earth surface[9]. Organisms present in these environments are extremely rich sources of bioactive compounds[10],[11]. The ocean remains as an unexploited source for many drugs and pharmacologically active substances[12]. Actinobacteria are Gram positive, filamentous bacteria which are supreme secondary metabolite producers[13]. Among the members of actinomycetes genus, Streptomyces sp is a dynamic producer of functional, bio-effective metabolites with broad pharmaceutical range having antimicrobial, antihelminthic, antitumor and antiviral agents[14],[15].
Actinobacteria from terrestrial origin produce hundreds of antibiotics which are widely used at present. Some differences could be expected among organisms existing in marine and terrestrial environments due to variation in the physical, chemical and biological factors[16]. It is apparent that the marine environment is a potent source for finding new actinobacteria and new antibiotics or biologically active substances[17]. The present work was undertaken to isolate potent actinobacteria from salt pan soil sample to elucidate their antimicrobial activity against multi drug resistance S. aureus (MDRSA).
2. Materials and methods
2.1. Sample collection
Soil samples were collected from salt pans, costal area in Kothapattanam, Ongole, Andhra Pradesh (15°30′ 0″ N, 80°3′ 0″ E), India during December 2011. Soil samples were collected at the depth of 10-25 cm. Samples were collected in sterilized container and transferred to the laboratory and stored in refrigerator at 4°C until further processing.
2.2. Isolation of actinobacteria
Isolation and enumeration of actinobacteria were performed on selective media such as actinomycetes isolation agar (AIA), Kuster's agars and Starch Caesin agar. The soil samples were serially diluted up to 10−7 and one milliliter of the serially diluted samples were inoculated into media. All these media are supplemented with cyclohexamide (100 µg/mL) to avoid fungal contamination. Inoculated plates were incubated at room temperature for 7 days[18].
2.3. Test organism
The clinical isolate of multi drug resistance S. aureus was collected from Narayani Hospital, Ariyur, Vellore District, Tamil Nadu, India. Test organism was maintained in glycerol stock and stored at -20 °C.
2.4. Antibiogram
The multi drug resistant S. aureus was screened for their sensitivity towards standard antibiotics including ampicillin (10 mcg/disc), methicillin (10 mcg/disc), vancomycin (30 mcg/disc), penicillin (10 U/disc), and chloramphenicol (30 mcg/disc). Drug sensitivity test was performed by disc diffusion method on Mueller Hinton agar plates. Bacterial test pathogen was inoculated into sterilized nutrient broth and incubated at 37 °C for 18-24 hours. Isolates were lawn cultured on Mueller Hinton agar plates using sterile cotton swabs. The standard antibiotics discs were placed on the agar surface using a sterile forceps. Plates were incubated at 37 °C for 24 hours and were observed for zone of inhibition[19].
2.5. Antimicrobial activity of actinobacterial isolates
2.5.1. Primary screening of actinobacteria for antibacterial activity against MDRSA by cross streak method
Primary screening of actinobacterial isolates were performed by cross streak method on modified nutrient agar plates (MNA)[20]. The actinobacterial isolates were inoculated in straight line on MNA plates and incubated for 7 days. MDRSA strain were cross streak on the same plate in perpendicular manner. The plates were incubated at 37°C for 24 hours. The plates were examined for the zone of inhibition of the MDRSA organisms.
2.5.2. Fermentation process
The potent actinobacterial isolate were inoculated into production broth (SS Media) containing soluble starch-25 g, glucose-10 g, yeast extract-2 g, CaCO3- 3 g, trace elements- 1 mL, distilled water-1 000 mL. Flasks were lodged on the flask shaker at a speed of 120 rpm at room temperature for 7 days. After fermentation, the medium was harvested and centrifuged to remove cell debris. Filtrate was collected and lyophilized stored at 4°C for further use[21].
2.5.3. Secondary screening (Agar well diffusion method)
Secondary antimicrobial screening of actinobacteria was detected by agar well diffusion method on Mueller Hinton agar[22]. Multi drug resistance S. aureus were inoculated in nutrient broth and incubated for 24 hours at 37°C. The turbidity of the broth was adjusted at 0.5 (optical density) using spectrophotometer. The bacterial cultures were inoculated on MHA plates using sterilized cotton swabs. In each of these plates, wells were cut out using a sterilized gel borer. The crude and solvent extracts were used against test pathogen, 100 µL of extracts were loaded into each well. Plates were incubated at 37°C for 24 hours. After incubation, all plates were examined for the presence of zone of inhibition around the wells[23].
2.5.4. Extraction of bioactive compounds
The bioactive metabolites were recovered from the harvested medium by solvent extraction method. The filtrate was mixed with ethyl acetate, chloroform, butanol (1:1 v/v) and shaken vigorously for 1 hour in a solvent extraction funnel. Solvent and filtrate mixture were stabilized for 24-48 hrs. After 48 hrs the solvent phase are separated from aqueous phase. The solvent extracts were concentrated and used for antibacterial activity[24],[25].
2.5.5. Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) for ethyl acetate extract was determined by modified agar well diffusion method[26]. The concentration of test cultures was adjusted to 0.5 McFarland standards by using a spectrophotometer. Test organism was lawn cultured on the Muller Hinton Agar plates. Agar surface was bored by using a sterilize cork borer of 7 mm diameter. The extract was dissolved into ethyl acetate to obtain a concentration range from 25 to 1 000 µg. A 100 µL of extracts in different concentration were loaded in to wells. All test plates were incubated at 37°C for 24 hours.
2.6. Thin layer chromatography
Silica gel plates, 10 cm × 20 cm, 1 mm thick, were prepared. They were activated at 150°C for half an hour. Ten micro liters of the ethyl acetate fractions were applied on the plate and the chromatogram was developed using chloroform: methanol (4:1) as solvent system. The spots in the chromatogram were visualized in the iodine vapour chamber and UV chamber[27].
2.7. Taxonomic exploration
The efficacious actinobacteria were characterized by morphological and biochemical method and the results were compared with Nonomura key 1974, Shirling and Gottlieb 1966 and with Bergey's manual of Determinative Bacteriology[28]
2.7.1. Morphological characteristics
Actinobacteria isolate were inoculated in seven different international streptomyces project (ISP) mediums (ISP 1 to ISP 7) and incubated for 7 days at room temperature. The colonies were observed under a high power magnifying lens and colony morphology was noted with respect to aerial mycelium color, nature of colony and reverse side color.
2.7.2. Assimilation of carbon source
The ability of different actinobacteria species in utilizing various carbon sources is analyzed. viz., arabinose, xylose, inositol, mannitol, fructose, rhamnose, sucrose and raffinose as sources of energy were studied based on the method recommended by ISP. These carbon molecules were sterilized by ether sterilization[29].
2.7.3. Generic investigation
The genus of actinobacteria with good antagonistic activity against the multi drug resistant S. aureus was identified by using cell wall composition analysis (amino acids and whole cell sugars analysis)[30].
2.7.4. Statistical analysis
All tests were conducted in triplicate. Data are reported as means ± standard deviation (SD). Results were analyzed statically by using Microsoft Excel 2007 (Roselle, IL, USA).
3. Results
3.1. Antibiogram
S. aureus were screened for antibiogram by disc diffusion method on MH agar plates. The result exhibits that tested drugs did not showed any zone of inhibition against the S. aureus.
3.2. Isolation of actinobacteria
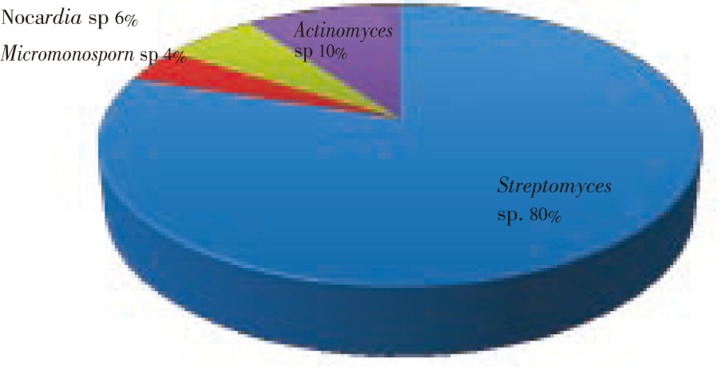
Soil samples were collected from salt pans near costal area in Kothapattanam, Ongole, Andhra Pradesh, India. The diversity of actinobacteria population was represented in Figure 1. A total of 51 actinobacteria colonies were isolated based on colony morphology and microscopic appearance (Table 1). The actinomycetes isolation agar enhanced more actinobacteria colonies, when compared to other media. The isolated strains were designated as SRB1-SRB51.
Figure 1. The diversity of actinobacteria population in marine salt pan soil.
Table 1. Isolation of actinomycetes using different media.
| Medium | No. of plates inoculated | Total no. of actinomycetes isolated | No.of actinomycetes recovered |
| Actinomycetes isolation agar | 18 | 135 | 27 |
| Starch casein agar | 18 | 76 | 11 |
| Kusters agar | 18 | 92 | 13 |
3.3. Antibacterial activity of isolated actinobacteria
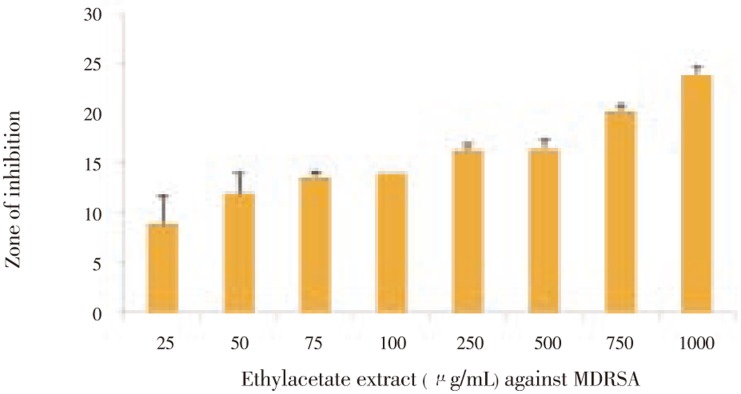
Actinobacteria isolates were screened for antimicrobial activity against MDRSA. Among 51 actinobacteria isolates only five isolates (SRB1, SRB20, SRB25, SRB32 and SRB38) showed activity towards test organism in cross streak method. The zone of inhibition was (6.40±0.25) mm, (5.10±0.40) mm, (18.16±0.35) mm, (4.70±0.40) mm and (6.20±0.30) mm, respectively.Out of 5 strains, SRB25 showed good antibacterial activity in agar well diffusion method. The potential strain SRB25 were inoculated into production medium (SS media), the bioactive compound was extracted in different polarity solvents and the extracts were screened for antimicrobial activity against multi drug resistant S. aureus. The crude extract [(23.33±1.15) mm]and ethyl acetate extract [(24.66±0.57) mm] exhibited potent activity against test organism, other solvent extracts did not showed any activity against the bacteria. MIC test was performed with the extract against MDRSA and found to be 1 000 µg/mL (Figure 2).
Figure 2. Minimum inhibitory concentration of SRB25 against MDRSA.
3.4. Thin layer chromatography
The ethyl acetate extraction of SRB25 strain was carried out by thin layer chromatography using solvent system composed of chloroform: methanol (4:1). Total three different circular spots were obtained from the extract with a Rf values of 0.81, 0.54, 0.37. The fluorescents compound was observed under UV light. This compound was partially identified as actinomycin-D (Rf = 0.38).
3.5. Identification of efficient actinobacteria
The strain SRB25 possessed LL-DAP and containd glycine in its cell wall. Presence of LL-DAP along with glycine indicated the cell wall chemotype I. The cell wall analysis of the strain SRB25 revealed that the strain belonged to the genus Streptomyces. The physiological characteristic of the strain SRB25 was compared with those of the Streptomyces species given in the key of Nonomura. The strain SRB25 showed more similarity when compared to the reference strain, Streptomyces parvulus (S. parvulus) (Table 2).
Table 2. Comparison of morphological characteristics of strain SRB25 and S. parvulus.
| Characteristics | Strain SRB25 | S. parvulus | |
| Colour of aerial mycelium | Grey | Grey | |
| Melanoid pigment | - | - | |
| Reverse side pigment | - | - | |
| Soluble pigment | - | - | |
| Sporechain morphology | Spiral | Spiral | |
| Utilization of sole carbon sources | Arabinose | + | + |
| Xylose | + | + | |
| Inositol | + | + | |
| Mannitol | + | + | |
| Fructose | + | + | |
| Rhamnose | + | + | |
| Sucrose | + | + | |
| Raffinose | + | + | |
4. Discussion
Natural compounds obtain from marine source plays important key to discover various new drug molecules[31],[32]. Actinobacteria are the most potent industrially important microorganism which are capable for the synthesis of bioactive compounds like enzymes, hormones, vitamins and other secondary metabolites. These bioactive compounds are highly difficult to synthesize artificially. Hence, these microbial compounds are most prominent sources for discover and production for new drugs[33]-[35]. Marine microorganism are entirely varies from terrestrial microbes. The marine actinobacteria producing bioactive compounds are varying from terrestrial actinobacteria. Most of the 70% commercial antibiotics are obtained from soil actinobacteria[36].
Multi drug resistant S. aureus serves as a hospital borne pathogen and plays a dominant role in many clinical problems globally[37]. In Indian hospitals, MRSA is one of the common causes of hospital acquired infections and different hospitals reported about 30% to 80% methicillin resistance. The organism has the ability to cause several outbreaks in hospitals. The choice of drugs against MRSA is very less due to their genetic alteration, enzyme variation and permeability changes. Due to harmful effects, there is a need to find out new drug molecules against MDRSA[38].
In 2011, Karthik et al reported marine sediments were good sources for isolation of actinobacteria and M2 media good for isolation of marine actinobacteria. Supporting to that our study also revealed that marine salt pan soil harbored more actinobacteria[39]. Baskaran et al reports that starch casein agar, Kuster's agar was found to be good for isolation of marine actinobacteria population. However, in the present study the maximum number of colonies was isolated on AIA followed by kuster's agar[40]. Hence, the soil physicochemical properties may play a major role in the selection of isolation media.
The 51 isolates which are isolated from marine salt pan soil were subjected to primary screening, among them only five strains showed anti bacterial activity. As the strain SRB25 showed maximum inhibition zone, it was selected for secondary screening. Pickup et al reported the 134 actinobacteria isolates are subjected to antimicrobial activity, 51 isolates showed good activity in primary screening but failed to manifest activity in secondary screening[41]. The Streptomyces sp isolated from saline farmlands possess both antibacterial and antifungal activity[42]-[48]. The Streptomyces sp PM-32 isolated from offshore sediments collected at the Bay of Bengal coast has shown antimicrobial activity against a group of bacterial and fungal pathogens[49].
The bioactive compounds are extracted from natural sources through several techniques. Solvent extraction is usually employed for the extraction of secondary metabolites from the culture filtrates. Different polarities of organic solvents have been utilized for the extraction of bioactive compounds from actinobacteria[50]. The extracts from ethyl acetate showed maximum antimicrobial activity against bacteria and fungi, other solvents extracts showed moderate activity against test organism[51]. Same trend was observed in this study and it is also found that ethyl acetate solvent was most appropriate for compound extraction. The ethyl acetate extract of SRB25 showed potent activity against MDRSA. The MIC of extrat from SRB25 was 1 000 µg/mL. According to thin-layer chromatography separation, the three fluorescence spots were detected under UV radiation. The ethyl acetate extract yielded components showed Rf value 0.37 which are similar to Rf value of actinomycin-D[52]. Actinomycin-D acts as potent antineoplastic drug molecules produced by S. parvulus, and is used to inhibit cell proliferation in tumor cells[53]. This molecule may be responsible for inhibition of growth of MDRSA. Based on the chemotaxonomy, the genera belonging to the cellwall type-I are Streptomyces, Streptoverticillium, Chainia, Actinopycnidium, Actinosporangium, Elyptrosporangium, Microellobosporia, Sporichthya and Intrasporangium[54]. The morphological observations and cell wall analysis of SRB25 reveals that these isolate belong to the genus Streptomyces. The sugar utilization test and other characters are exactly similar to those of S. parvulus. Hence, the strain SRB25 has been identified as S. parvulus. The antibiotic producing Streptomyces which are isolated from mangrove environment are identified with the help of Nonomura key (1974) and those species described in the Bergey's Manual of Determinative Bacteriology[55]. Based on the importance of marine actinobacteria, current studies focused on bioactive compounds from marine actinobacteria of unexplored salt pan environment. Actinobacteria were isolated from salt pan soil samples which show high tremendous activity against drug resistant strain. The identification and production of new drug molecules from marine actinobacteria are necessary to counteract drug resistance in microbial population. Further investigations are needed in order to determine the active metabolites.
Acknowledgments
Authors wish to thank management of VIT University, Vellore, Tamil Nadu, India, for providing financial support for the completion of this work.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci. 2008;33:593–603. doi: 10.1007/s12038-008-0077-9. [DOI] [PubMed] [Google Scholar]
- 2.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6:751–763. doi: 10.1586/14787210.6.5.751. [DOI] [PubMed] [Google Scholar]
- 3.Loomba PS, Taneja J, Mishra B. Methicillin and vancomycin resistant S. aureus in hospitalized patients. J Glob Infect Dis. 2010;2:275–283. doi: 10.4103/0974-777X.68535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enright MC. The evolution of a resistant pathogen-the case of MRSA. Curr Opinion Pharmacol. 2003;5:474–479. doi: 10.1016/s1471-4892(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Ray P, Das A, Sharma M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol. 2009;58:1067–1073. doi: 10.1099/jmm.0.009720-0. [DOI] [PubMed] [Google Scholar]
- 6.Jevitt LA, Smith AJ, Williams PP, Raney PM, McGowan JE, Tenover FC. In vitro activities of daptomycin, linezolid, and quinupristin-dalfopristin against a challenge panel of Staphylococci and Enterococci including vancomycin-intermediate Staphylococcus aureus and vancomycin- resistant Enterococcus faecium. Microbial Drug Resistance. 2003;9:389–393. doi: 10.1089/107662903322762833. [DOI] [PubMed] [Google Scholar]
- 7.Meka VG, Gold HS. Antimicrobial resistance to linezolid. Clin Infect Dis. 2009;39:1010–1015. doi: 10.1086/423841. [DOI] [PubMed] [Google Scholar]
- 8.Guskey MT, Tsuji BT. A comparative review of the lipoglycopeptides: oritavancin, dalbavancin and telavancin. Pharmacotherapy. 2010;30:80–94. doi: 10.1592/phco.30.1.80. [DOI] [PubMed] [Google Scholar]
- 9.Valli S, Suvathi Sugasini S, Aysha OS, Nirmala P, Vinoth KumarP, Reena A. Antimicrobial potential of Actinomycetes species isolated from marine environment. Asian Pac J Trop Biomed. 2012;2:469–473. doi: 10.1016/S2221-1691(12)60078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solanki R, Khanna M, Lal R. Bioactive compounds from marine actinomycetes. Indian J Microbiol. 2008;48:410–431. doi: 10.1007/s12088-008-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong K, Gao AH, Xie QY, Gao H, Zhuang L, Lin HP, et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar Drugs. 2009;7:24–44. doi: 10.3390/md7010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivasubramanian K, Ravichandran S, Vijayapriya M. Antagonistic activity of marine bacteria Pseudoalteromonas tunicata against microbial pathogens. Afr J Microbiol Res. 2011;5:562–567. [Google Scholar]
- 13.Olano C, Mendez C, Salas JA. Antitumor compounds from marine actinomycetes. Mar Drugs. 2009;7:210–248. doi: 10.3390/md7020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravi Kumar S, Inbaneson SJ, Uthiraselvam M, Priya SR, Ramu A, Banerjee MB. Diversity of endophytic actinomycetes from Karangkadu mangrove ecosystem and its antibacterial potential against bacterial pathogens. J Pharm Res. 2011;4:294–296. [Google Scholar]
- 15.Reddy NG, Ramakrishna DPN, RajaGopal SV. A morphological, physiological and biochemical studies of marine Streptomyces rochei (MTCC 10109) showing antagonistic activity against selective human pathogenic microorganisms. Asian J Biol Sci. 2011;4:1–14. [Google Scholar]
- 16.Saurav K, Kannabiran K. Diversity and optimization of process parameters for the growth of Streptomyces VITSVK9 spp. isolated from Bay of Bengal, India. J Nat Env Sci. 2010;1:56–65. [Google Scholar]
- 17.Gartner A, Ohlendorf B, Schulz D, Zinecker H, Wiese J, Imhoff JF. Levantilides A and B, 20-membered macrolides from a Micromonospora strain isolated from the mediterranean deep sea sediment. Mar Drugs. 2011;9:98–108. doi: 10.3390/md9010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deepika L, Kannabiran K. Biosurfactant and heavy metal resistance activity of Streptomyces spp. isolated from saltpan soil. Br J Pharm Toxicol. 2010;1:33–39. [Google Scholar]
- 19.Aravamuthan N, Kumar G, Karthik L, Bhaskara Rao KV. In vitro antagonistic activity of soil actinobacteria against multi drug resistant bacteria. Pharmacologyonline. 2010;2:507–516. [Google Scholar]
- 20.Santina M, Luigi M, Consolazione Matteo B, Vivia B, Renato F, Angelina LG. Antagonistic interaction between psychrotrophic cultivable bacteria isolated from Antartic sponges: A preliminary analysis. Res Microbiol. 2009;160:27–37. doi: 10.1016/j.resmic.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Umasankar ME, Kumar G, Karthik L, Bhaskara Rao KV. Exploration of antagonistic actinobacteria from Amirthi forest. Int J Curr Pharm Res. 2010;2:16–19. [Google Scholar]
- 22.Kumar G, Karthik L, Bhaskara Rao KV. In vitro anti-candida activity of Calotropis gigantea against clinical isolates of candida. J Pharm Res. 2010;3:539–542. [Google Scholar]
- 23.Kumar S, Kannabiran K. Antifungal activity of Streptomyces VITSVK5 sp. against drug resistant Aspergillus clinical isolates from pulmonary tuberculosis patients. Journal de Mycologie Medicale. 2010;20:101–107. [Google Scholar]
- 24.Atta HM, Ahmad MS. Antimycin-A antibiotic biosynthesis produced by Streptomyces Sp. AZ-AR-262: taxonomy, fermentation, purification and biological activities. Aust J Basic Appl Sci. 2009;3:126–135. [Google Scholar]
- 25.Manivasagan P, Gnanam S, Sivakumar K, Thangaradjou T, Vijayalakshmi S, Balasubramanian T. Antimicrobial and cytotoxic activities of an actinobacteria (Streptomyces Sp. PM-32) isolated from offshore sediments of the Bay of Bengal in Tamilnadu. Advan Biol Res. 2009;3:231–236. [Google Scholar]
- 26.Okunji CO, Okeke CN, Gugnani HC, Iwu MM. An antifungal saponin from fruit pulp of Dracaena manni. Int J Crude Drug Res. 1990;28:193–199. [Google Scholar]
- 27.Usha R, Ananthaselvi P, Venil CK, Palaniswamy M. Antimicrobial and antiangiogenesis activity of Streptomyces parvulus KUAP106 from mangrove soil. European J Biol Sci. 2010;2:77–83. [Google Scholar]
- 28.Cross T. Bergey's manual of systematic bacteriol. Baltimore: Williams&Wilkins Company; 1989. Growth and examination of actinomycetes some guidelines; pp. 2340–2343. [Google Scholar]
- 29.Shirling EB, Gottlieb D. Methods for characterization of Streptomyces sp. Int J Syst Bacteriol. 1996;16:313–340. [Google Scholar]
- 30.Karthik L, Kumar G, Bhaskara Rao KV. Mutational effects on the protease producing marine actinomycetes isolated from Scylla serrata. Pharmacologyonline. 2010;1:221–227. [Google Scholar]
- 31.Zhang LH, Zhang W, Jin Y, Jin M, Yu X. A comparative study on the phylogenetic diversity of culturable actinobacteria isolated from five marine sponge species. Antonie Van Leeuwenhoek. 2008;93:241–248. doi: 10.1007/s10482-007-9196-9. [DOI] [PubMed] [Google Scholar]
- 32.Demain AL, Sanchez S. Microbial drug discovery: 80 Years of progress. J Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Li ZY, Miao XL, Zhang FL. The screening of antimicrobial bacteria with diverse novel nonribosomal peptide synthetase (NRPS) genes from South China sea sponges. Mar Biotechnol. 2009;11:346–355. doi: 10.1007/s10126-008-9148-z. [DOI] [PubMed] [Google Scholar]
- 34.Yuan L, Zhang Y, Yu L, Sun C, Wei Y, Li H, et al. Actinopolymorpha Cephalotaxi sp. nov., a novel actinomycete isolated from rhizosphere soil of the plant Cephalotaxus fortune. Int J Syst Evol Microbiol. 2010;60:51–54. doi: 10.1099/ijs.0.011197-0. [DOI] [PubMed] [Google Scholar]
- 35.Imhoff JF, Labes A, Wiese J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol Adv. 2011;29:468–482. doi: 10.1016/j.biotechadv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Williams PG. Panning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotechnol. 2008;27:45–52. doi: 10.1016/j.tibtech.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Sharma D, Kaur T, Chadha BS, Manhas RK. Antimicrobial activity of actinomycetes against multidrug resistant Staphylococcus aureus, E. coli and various other pathogens. Trop J Pharm Res. 2011;10:801–803. [Google Scholar]
- 38.Parasa LS, Tumati SR, Kumar CA, Chicurupati SP, Rao GS. In vitro antimicrobial activity of cashew (Anacardium occidentale, L.) nuts shell liquid against methicillin resistant Staphylococcus aureus (MRSA) clinical isolates. Int J Pharm Pharm Sci. 2011;3:436–440. [Google Scholar]
- 39.Karthik L, Kumar G, Bhaskara Rao KV. Diversity of marine actinomycetes from Nicobar marine sediments and its antifungal activity. Int J Pharm Pharm Sci. 2010;1:199–203. [Google Scholar]
- 40.Baskaran R, Vijayakumar R, Mohan PM. Enrichment method for the isolation of bioactive actinomycetes from mangrove sediments of Andaman Islands. India. Mal J Microbiol. 2011;7:26–32. [Google Scholar]
- 41.Pickup KM, Nolan RD, Bushell ME. A method for increasing the success rate of duplicating antibiotic activity in agar and liquid cultures of Streptomyces isolates in new antibiotic screens. J Ferment Bioeng. 1993;76:89–93. [Google Scholar]
- 42.Imran S, Clarisse BFFY, Khaled AS, Shahida H, Hartmut L. Antifungal and antibacterial activities of indigenous Streptomyces isolates from saline farmlands: prescreening, ribotyping and metabolic diversity. World J Microbiol Biotechnol. 2009;25:601–610. [Google Scholar]
- 43.Huang FY, Meng QP, Tan GH, Huang YH, Wang H, Mei WL, et al. Isolation and identification of multidrug-resistant Staphylococcus haemolyticus from a laboratory-breeding mouse. Asian Pac J Trop Med. 2011;4(6):421–425. doi: 10.1016/S1995-7645(11)60118-1. [DOI] [PubMed] [Google Scholar]
- 44.Motamedi H, Mirzabeigi H, Shirali T. Determining of antibiotic resistance profile in Staphylococcus aureus isolates. Asian Pac J Trop Med. 2010;3(9):734–737. [Google Scholar]
- 45.Jarrar Naser, Abu-Hijleh Awni, Adwan Kamel. Antibacterial activity of Rosmarinus officinalis L. alone and in combination with cefuroxime against methicillin-resistant Staphylococcus aureus. Asian Pac J Trop Med. 2010;3(2):121–123. [Google Scholar]
- 46.Vijayakumar A, Duraipandiyan V, Jeyaraj B, Agastian P, Raj MK, Ignacimuthu S. Phytochemical analysis and in vitro antimicrobial activity of Illicium griffithii Hook. f. & Thoms extracts. Asian Pac J Trop Dis. 2012;2(3):190–199. [Google Scholar]
- 47.Jombo GTA, Emanghe UE, Amefule EN, Damen JG. Nosocomial and community acquired uropathogenic isolates of Proteus mirabilis and antimicrobial susceptibility profiles at a university hospital in Sub-Saharan Africa. Asian Pac J Trop Dis. 2012;2(1):7–11. [Google Scholar]
- 48.Moussa A, Noureddine D, Abdelmelek M, Saad A. Antibacterial activity of various honey types of Algeria against pathogenic gram-negative bacilli: Escherichia coli and Pseudomonas aeruginosa. Asian Pac J Trop Dis. 2012;2(3):211–214. [Google Scholar]
- 49.Vicente MF, Basilio A, Cabello A, Pela ez F. Microbial natural products as a source of antifungals. Clin Microbiol Infect. 2003;9:15–32. doi: 10.1046/j.1469-0691.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 50.Selvameenal L, Radakrishnan M, Balagurunathan R. Antibiotic pigment from desert soil actinomycetes; biological activity, purification and chemical screening. Indian J Pharma Sci. 2009;71:499–504. doi: 10.4103/0250-474X.58174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vijakumar R, Murugesan S, Panneerselvam A. Isolation, characterization and antimicrobial activity of actinobacteria from point calimere coastal region, east coast of India. Int Res J Pharam. 2010;1:358–365. [Google Scholar]
- 52.Sousa MFVQ, Lopes CE, Pereira N. Development of a bioprocess for the production of Actinomycin-D. Brazilian J Chemical Engin. 2002;19:277–285. [Google Scholar]
- 53.Ajijur Rahman MD, Islam MZ, Khondkar P, Islam AU. Characterization and antimicrobial activities of a polypeptide antibiotic isolated from a new strain of Streptomyces parvulus. Bangladesh Pharm J. 2010;13:14–17. [Google Scholar]
- 54.Lechevalier MP, Lechevalier H. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol. 1970;20:435–443. [Google Scholar]
- 55.Siva kumar K, Sahu MK, Kathiresan K. Isolation and characterization of Streptomycetes, producing antibiotic from a mangrove environment. Asian Jr Microbiol Biotech Env Sc. 2005;3:457–464. [Google Scholar]