Abstract
Objective
To conduct a systemic evaluation of the medicinal value of seeds which include macroscopic and microscopic characterization, physiochemical evaluation, preliminary phytochemical screening and experimental antipyretic activity.
Methods
Saraca asoca seed was studied for pharmacognostical, phytochemical and other recommended methods for standardizations. Also, the acetone extract of the seeds was evaluated for acute toxicity study and antipyretic activity using Brewer's yeast induced pyrexia in Wistar rats at oral doses of 300 mg/kg and 500 mg/kg.
Results
After phytochemical screening, the acetone extract showed the presence of saponin, tannins and flavonoids which inhibit pyrexia. The therapeutic efficacy achieved at both the dose levels of the research drug and standard drug aspirin (100 mg/kg) showed significant (P<0.01) antipyretic activity when compared to the control group. The highly significant antipyretic effect exhibited at the dose of 500 mg/kg was also found to be sustainable in nature.
Conclusions
The antipyretic effect of the acetone extract showed significant results in rats at the dose of 500 mg/kg after following the standard pharmacognostical and phytochemical methods.
Keywords: Antipyretic, Saraca asoca, Seed, Pharmacognosy, Acetone extract
1. Introduction
Numerous medicinal therapies treat their patients with herbal medicines for its extraordinary influence, though relatively little knowledge about their mode of action is available. In the Ayurvedic system of medicine, herbal extracts instead of purified compounds have been used since centuries because many constituents with more than one mechanism of action are considered essential for the required holistic therapeutic action. Ashoka is one of the most legendary and sacred trees of India which has been utilized from ancient times till date[1]-[3]. Ashoka tree, universally known by its binomial Latin name Saraca asoca (Roxb.) or Saraca indica belonging to the Caesalpiniaceae family[4], is found throughout India, especially in Kerala, West Bengal, regions of southern India and in the Himalayas up to an altitude of 750 m. It is a small, spreading evergreen tree of 7-10 m height whose bark is dark brown or almost grey with a warty surface. Its leaves are parpinnate, 15-20 cm long and the leaflets are 6-12 cm, oblong and rigidly subcoriaceous[5] while the flowers are fragrant and polygamous apetalous, yellowish orange turning to scarlet[6]. Stem bark of S. asoca is reported to contain glycosides, flavonoids, tannins and saponins[4],[7]. It is used as a spasmogenic, oxytocic, uterotonic, antibacterial and antidysentric agent[5],[8]. It has also been reported to possess antiprogestational and antioestrogenic activity against menorrhagia[4]. An extensive search of the literature reveals no proper studies on the pharmacological activity of the seeds of this plant although a large number of its seeds are readily found scattered near the trees without being put to any special use. Thus, the present investigation aims towards the pharmacognostical evaluation, determination of physiochemical parameters, preliminary phytochemical screening and assessment of the antipyretic efficacy of acetone extract of S. asoca seeds.
2. Materials and methods
2.1. Collection and identification of plant material
The seeds of S. asoca were collected from the medicinal plant garden of Narendrapur Ramakrishna Mission, Kolkata and the State Government Herbal Garden at Kalyani, West Bengal, India. The identification of the seeds was done at the Botanical Survey of India, Howrah, India vide Ref. No. BSI/CNH/AD/Tech./2010 and Sample Reg. No (AS-01). An authentic herbarium specimen was deposited in the herbarium museum of Department of Dravyaguna, IPGAE&R, Kolkata, India for future reference.
2.2. Chemicals
Aspirin was purchased from NICE Chem. Pvt. Ltd., Cochin, India. Gallic acid, ferric chloride, sodium hydroxide and all other chemicals used in different studies were of analytical grade.
2.3. Processing and solvent extraction
Seeds were washed and cleaned thoroughly to remove any extraneous matter and dried under sun light for about twenty days. The sun-dried whole seeds were powdered with a grinding machine (Hammer mill) and passed through a #40 mesh sieve. Powdered material was stored properly in airtight containers for experimental purposes. The powder of research drug was subsequently extracted sequentially in petroleum ether (60-80 °C), chloroform, acetone, methanol and water in a Soxhlet's extractor and then filtered. The extract was concentrated under vacuum in a rotary evaporator to yield semi-solid mass. This was further dried under a vacuum oven drier to give solid residue and preserved in refrigerator below 10 °C for subsequent experiments.
2.4. Animals
Swiss albino mice of either sex, weighing about 20-30 g and albino (Wistar) rats of either sex, weighing about 120-150 g were used for in-vivo evaluation. All animals were procured from the Government of West Bengal approved breeder, M/s Ghosh Scientific, Kolkata and housed under standard environmental conditions with fixed 12 h light/dark cycles in animal house of IPGAE&R, registered by CPCSEA (Reg. No. 1180/ac/08/CPCSEA dated 27.03.08). The animals were kept in standard polypropylene cages and provided with food and water ad libitum. These animals were acclimatized for a period of 14 days prior to performing any experiments. All experimental protocols were approved by the Institutional Animal Ethical Committee.
2.5. Pharmacognostic study
Fresh seeds authenticated from the Botanical Survey of India were taken for morphological and histological studies. Coarse powder (#40 mesh) was used to find out different pharmacognostical (macroscopic and microscopic) characteristics in the department of Dravyaguna of IPGAE&R according to established procedures[9],[10].
2.6. Physiochemical parameters and preliminary phytochemical screening
Different physiochemical parameters (such as moisture content, ash values, extractive values, total phenolic content, saponification value, etc.) of the powdered seeds were estimated using standard methods[11]-[13]. The fluorescence analysis of the powdered material was done under visible and UV (254 and 365 nm) lights[14],[15]. The acetone extract of the seeds was then subjected to different qualitative tests to determine the presence of various phytoconstituents[16],[17].
2.7. Acute toxicity test
Acute toxicity study of acetone extract of the seeds of S. asoca was carried out on healthy Swiss albino mice following OECD guideline 423[18]. A single oral dose of the extract was administered orally at the level of 100 mg, 300 mg, 500 mg, 700 mg and 1 000 mg/kg body weight respectively to the 5 groups containing 6 mice each. These groups were observed for any signs of toxic symptoms, behavioral changes, locomotion, convulsions and mortality for 1, 2, 4, 8 and 24 h and further for a period of 14 days. During this period, their activity levels and behavior patterns were closely watched and meticulously noted[19].
2.8. Antipyretic activity
The assessment of antipyretic activity was carried out using Brewer's yeast induced pyrexia in Wistar rats by the method as described by Loux et al[20]. Rats were fasted overnight with water ad libitum before the experiment. The normal body temperature of each animal was measured by digital tele-thermometer (IMCORP, Ambala, India) and recorded. Pyrexia was induced by subcutaneously injecting 20% w/v Brewer's yeast (10 mL/kg), suspended in normal saline, into the animal's dorsum region. The peak pyrexia was observed to be at 18 h after yeast administration by conducting trial experiments. The animals that showed an increase in rectal temperature of at least 1 °C were used for the study. The drugs were administered orally at the time of peak pyrexia. The control group (group I) was administered 5% gum acacia, the standard group (group II) received aspirin (100 mg/kg) and the research groups (group III and IV) was given the research drug at doses of 300 mg/kg and 500 mg/kg respectively. The rectal temperature was recorded at a time interval of 1, 2, 3, 4 and 5 h after drug administration.
2.9. Statistical analysis
The data were statistically analyzed using one-way ANOVA followed by Dunnet's t test for individual comparison of the various groups with the control group[19],[21]. Results were expressed as Mean±SEM. P<0.01 was used to indicate statistical significance.
3. Results
3.1. Macroscopic characteristics
The legumes of S. asoca are 6-10 inches long containing 4-8 grey dicotyledonous seeds like a chest nut. The seeds are 3-5 cm long with average diameter of 8-9 cm, smooth surface, ellipsoid-oblong and compressed. The seed coating is brown or slightly black in color while sun-dried seeds are dark brown colored having a smooth surface with hard texture. Coarse powder of the seed is light brown in color with an aromatic odor and having a slightly sweet taste.
3.2. Microscopic characteristics
The seed shows a very thin membranous aril composed of several layers of parenchymatous and collenchymatous cells containing vessels, starch grains, prismatic crystals, etc. (Figure 1). The fine powder was mounted in glycerin as well as stained with different reagents. Observation under microscope (Dewinter, Italy) showed presence of cells containing tannins, stone cells, crystals, endospermic cells, starch grains, vessels, etc.
Figure 1. T.S. of S. asoca seed.
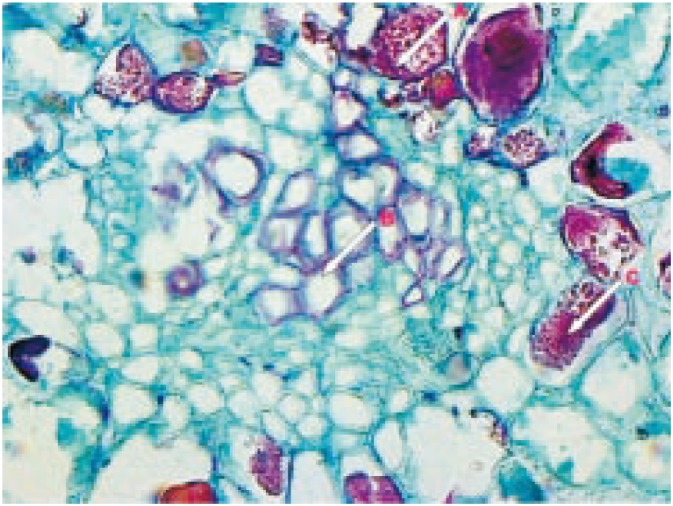
A: Starch grain; B: Vessel; C: Crystal
3.3. Physiochemical parameters
Different physiochemical parameters for the purpose of standardization such as total solids (93.5%), moisture content (6.5%), total ash (6.7%), water soluble ash (6.0%) and acid insoluble ash (0.7%) were determined. The saponification value of the seed oil was calculated as 145 mg KOH/g of oil. The extractive value of different extracts was also calculated. Petroleum ether soluble extractive was 0.18% w/w, chloroform soluble extractive was 0.05% w/w, acetone soluble extractive was 1.27% w/w, methanol soluble extractive was 3.22% w/w and water soluble extractive was 0.63% w/w. The powdered seeds were subjected to fluorescence analysis (Table 1) following standard procedure. The total phenolic content in 100 mg powder of the seeds of S. asoca was estimated as equivalent to 3.7 mg of gallic acid using the absorbance calibration curve generated with different concentrations of gallic acid.
Table 1. Fluorescence analysis of S. asoca seed powder.
| Reagent | Normal light | UV 254 nm | UV 365 nm |
| 1M Sodium hydroxide | Light brown | Dark brown | Violet |
| Acetic acid | Light brown | Light brown | Light brown |
| 1M Hydrochloric acid | Brown | Greenish brown | Dark greenish brown |
| dil. Nitric acid | Brown | Greenish brown | Dark green |
| 5% Iodine | Blackish brown | Greenish brown | Black |
| 5% Ferric chloride | Blackish brown | Black | Black |
| Methanol | Light brown | Light green | Olive green |
| 50% Nitric acid | Orange | Green | Dark green |
| 1M Sulphuric acid | Light yellow | Light green | Olive green |
| dil. Ammonia | Orange | Green | Dark green |
| Sodium hydroxide in MeOH | Brown | Green | Olive green |
3.4. Preliminary phytochemical screening
The extracts were subjected to preliminary phytochemical analysis using standard chemical methods which mainly revealed the presence of carbohydrates, flavonoids, polyphenols, tannins and saponins.
3.5. Pharmacological study
3.5.1. Acute toxicity study
No signs and toxic symptoms were observed during the acute toxicity study using the acetone extract after oral administration of dose up to 1 000 mg/kg.
3.5.2. Antipyretic activity
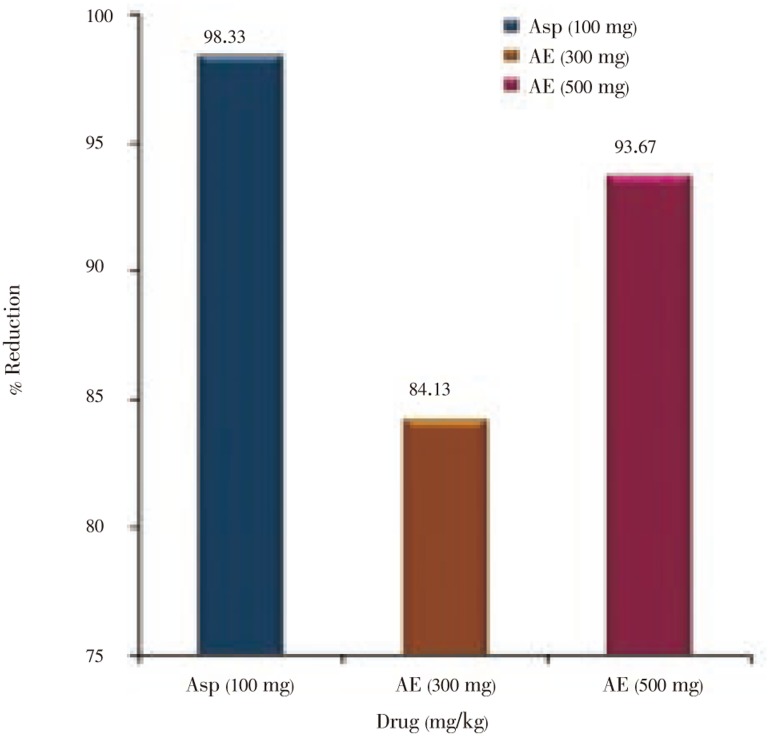
The acetone extract of the seeds of S. asoca showed significant antipyretic effect at both the dose levels (300 mg/kg and 500 mg/kg) when tested on yeast-induced pyrexia in rats (Table 2). The antipyretic activity of extract was found to be highly significant and dose dependent when compared with the control group. The antipyretic effect which persisted up to 5 h was observed at the dose of 500 mg/kg of the research drug as compared to the standard drug and the research drug at the dose of 300 mg/kg. Inhibition of pyrexia in terms of percentage reduction exhibited by the research drug at 500 mg/kg dose was significantly comparable to that in case of aspirin (Figure 2).
Table 2. Effect of acetone extract of the seeds of S. asoca on brewer's yeast induced pyrexia in rats (mean±SEM) (n=6).
| Group | Treatment | Dose (mg/kg) | Rectal temperature at different time intervals (°C) |
% Reduction | ||||||
| -18 ha | 0 hb | 1 h | 2 h | 3 h | 4 h | 5 h | ||||
| I | Control (vehicle) | - | 36.10±0.13 | 37.40±0.16 | 37.37±0.17 | 37.25±0.18 | 37.10±0.12 | 36.95±0.15 | 36.80±0.12 | - |
| II | Standard (aspirin) | 100 | 36.87±0.06 | 38.37±0.10 | 37.77±0.05** | 37.37±0.09*** | 37.05±0.06*** | 36.90±0.07*** | 36.95±0.06** | 98.33 |
| III | Acetone extract | 300 | 36.55±0.07 | 38.12±0.05 | 37.95±0.06 | 37.60±0.11* | 37.25±0.09*** | 36.80±0.07** | 37.00±0.09* | 84.13 |
| IV | Acetone extract | 500 | 36.47±0.21 | 38.05±0.17 | 37.80±0.11 | 37.27±0.09*** | 36.95±0.03* | 36.75±0.07* | 36.57±0.06** | 93.67 |
***: P<0.0001, **: P<0.001, *: P<0.01, when compared with control; a: temperature just before yeast injection; b: temperature just after drug administration.
Figure 2. Percentage reduction of rectal temperature of aspirin and acetone extracts of the seeds of Saraca asoca.
Asp: Aspirin; AE: Acetone extract.
4. Discussion
For the purposes of quality control, assessment of purity and identification of any sample, standardization is very much essential. In the present research, pharmacognostic study, physiochemical analysis, toxicity assessment and evaluation of antipyretic activity on rats of the seeds of S. asoca were carried out. Pharmacognostical studies and determination of different physiochemical parameters are very much essential for the standardization of drug and establishing its pharmacological efficacy. Hence, these studies help in identification and authentication of the plant material[22]-[27]. The acetone extract of the seed was also evaluated for its antipyretic activity on the basis of phytoconstituents present in it.
Fever may occur as a result of infection or one of the sequels of tissue damage, inflammation, graft rejection, or other diseases. It is the body's natural function to create an environment where infectious agents or damaged tissues cannot survive. Yeast induced fever is called pathogenic fever. Its etiology includes production of prostaglandins, which set the thermoregulatory center at a higher temperature. Most of the antipyretic drugs like aspirin or paracetamol inhibit COX-2 expression to reduce the elevated body temperature by inhibiting PgE2 biosynthesis[28]-[32].
Results of the present research work indicate no toxic effect up to the maximum dose of the drug at 1 000 mg/kg during acute toxicity study and also significant (P<0.01) antipyretic effect of acetone extract of the seeds of S. asoca found especially at the dose of 500 mg/kg in yeast-provoked elevation of body temperature when compared with standard drug aspirin at 100 mg/kg. The administration of the research drug at 500 mg/kg resulted in its inhibitory action on pyrexia up to 5 h while aspirin (100 mg/kg) and research drug at 300 mg/kg exhibited their impact up to 4 h. Similarly, the reduction in temperature at dose level of 500 mg/kg (92.30%) was significantly comparable with the effect in case of standard drug aspirin (98.01%). So, inhibition of prostaglandin biosynthesis by our research drug on yeast induced pyrexia in rats may be the possible mechanism of antipyretic action as that of aspirin.
The antipyretic effect of different plant species could be attributed to the presence of flavonoids since flavonoids normally exhibit antipyretic, analgesic and anti-inflammatory properties[33]-[36]. The acetone extract of the seeds of S. asoca contains different phytoconstituents including high amounts of flavonoids and tannins. So, these bio active constituents may be responsible for the antipyretic effect exhibited by the seed extract. This research drug may be used as a safe, economic, non-toxic and potent antipyretic herbal drug. It is well known that there are several mediators or multi-processes underlining the pathogenesis of fever and inhibition of any of these mediators may bring about antipyretic effect. Therefore, further studies regarding other important pharmacological activities as well as isolation and characterization of the active principle constituents responsible for the observed antipyretic activity need to be undertaken.
Acknowledgments
This research work was carried out at the Department of Dravyaguna (Medicinal pharmacology), Institute of Post Graduate Ayurvedic Education & Research, Kolkata, India using funds from the University Grants Commission, New Delhi, India ( Grant sanctioned vide no- F. No- 37-496/2009(SR) dated- 16 . 02.2010)
Footnotes
Foundation Project: Supported by University Grants Commission, New Delhi [Grant sanctioned vide no-F. No. 37-496/2009 (SR)].
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Pradhan P, Joseph L, Gupta V, Chulet R, Arya H, Verma R, et al. Saraca asoca (Ashoka): A Review. J Chem Pharm Res. 2009;1(1):62–71. [Google Scholar]
- 2.Rathee P, Rathee S, Rathee D, Rathee D. Quantitative estimation of (+)-Catechin in stem bark of Saraca asoka Linn using HPTLC. Der Pharma Chemica. 2010;2(1):306–314. [Google Scholar]
- 3.Gupta M, Shaw BP, Mukherjee A. A new glycosidic flavonoids from jwarhar mahakashay (antipyretic) Ayurvedic preparation. International Journal of Ayurveda Research. 2010;1(2):106–111. doi: 10.4103/0974-7788.64401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma PC, Yelne MB, Dennis TJ. New Delhi: Central Council for Research in Ayurveda and Siddha, Department of ISM&H, Ministry of Health and Family Welfare (Govt. of India); 2005. Database on medicinal Plants used in Ayurveda; pp. 3, 76–87. [Google Scholar]
- 5.Chaterjee A, Pakrashi SC. New Delhi: National Institute of Science Communication and Information Resources, CSIR; 2006. The treatise on Indian medicinal plants; pp. 46–47. [Google Scholar]
- 6.Ali M. New Delhi: CBS Publishers & Distributors; 2008. Text Book of Pharmacognosy; pp. 668–669. [Google Scholar]
- 7.Vol. 2. New Delhi: Indian Council Medical Research; 2005. Quality standards of Indian Medicinal Plants; pp. 201–208. [Google Scholar]
- 8.Satyavati GV, Prasad DN, Sen SP, Das PK. Oxytotic activity of a pure phenolic glycoside (P2) from Saraca indica Linn. (Ashoka) Indian J Med Res. 1970;58:660–663. [PubMed] [Google Scholar]
- 9.Kokate CK. 4th ed. New Delhi: Vallabh Prakashan; 2005. Practical Pharmacognosy; pp. 107–111. [Google Scholar]
- 10.Bhatia D, Gupta MK, Gupta A, Singh M, Kaithwas G. Pharmacognostical studies on seeds of Centratherum anthelminticum Kuntze. Natural Product Radiance. 2008;7(4):326–329. [Google Scholar]
- 11.Ministry of Health and Family Welfare . 1st ed. 1. Part-1. New Delhi: Government of India, Ministry of H and FW, Department of Indian System of Medicine & Homoeopathy; 2001. Ayurvedic Pharmacopoeia of India; p. 14, 143. [Google Scholar]
- 12.Siddiqua A, Premakumari KB, Sultana R, Vithya, Savitha Antioxidant activity and estimation of total phenolic content of Muntingia calabura by colorimetry. International Journal of ChemTech Research. 2010;2(1):205–208. [Google Scholar]
- 13.Kyari MZ. Extraction and characterization of seed oils. Int. Agrophysics. 2008;22:139–142. [Google Scholar]
- 14.Madhav NVS, Upadhyaya K, Bisht A. Phytochemical screening and standardization of polyherbal formulation for dyslipidemia. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3:235–238. [Google Scholar]
- 15.Shukla SH, Mistry HA, Patel VG, Jogi BV. Pharmacognostical, preliminary phytochemical Studies and analgesic activity of Amomum subulatum Roxb. Pharma Science Monitor. 2010;1(1):90–102. [Google Scholar]
- 16.Evans WC. 16th ed. London: Saunders Elsevier; 2009. Trease and Evans Pharmacognosy. [Google Scholar]
- 17.Harborne JB. 2nd ed. London: Chapmann and Hall; 1984. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis; p. 192. [Google Scholar]
- 18.OECD Acute Oral Toxicity - Acute Toxic Class Method. Paris: OECD Guideline 423 for Testing of Chemicals. 2001. pp. 1–14.
- 19.Ghosh MN. 4th ed. Kolkata: Hilton & Company; 2008. Hand Book of Experimental Pharmacology; pp. 178–182. [Google Scholar]
- 20.Loux JJ, Depalma PD, Yankell SL. Antipyretic testing of aspirin in rats. Toxicol Appl Pharmacol. 1972;22(4):672–675. doi: 10.1016/0041-008x(72)90295-5. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni SK. 3rd ed. New Delhi: Vallabh Prakashan; 2006. Hand Book of Experimental Pharmacology; pp. 178–180. [Google Scholar]
- 22.Kumar S, Kumar V, Prakash OM. Pharmacognostic study and anti-inflammatory activity of Callistemon lanceolatus leaf. Asian Pac J Trop Biomed. 2011;1(3):177–181. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas S, Patil AG, Chandra N. Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L. Fruit. Journal of Herbal Medicine and Toxicology. 2008;2(2):51–54. [Google Scholar]
- 24.Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Kumar AA, Manoj B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac J Trop Med. 2011;3(9):702–706. [Google Scholar]
- 25.Rajasekaran A, Arivukkarasu R, Murugesh S. Evaluation of antipyretic activity of ethyl acetate extract of Adenema hyssopifolium G. Don in a rat model. Asian Pac J Trop Med. 2011;3(7):523–526. [Google Scholar]
- 26.Anbarasu K, Manisenthil KKT, Ramachandran S. Antipyretic, anti-inflammatory and analgesic properties of nilavembu kudineer choornam: a classical preparation used in the treatment of chikungunya fever. Asian Pac J Trop Med. 2012;4(10):819–823. doi: 10.1016/S1995-7645(11)60201-0. [DOI] [PubMed] [Google Scholar]
- 27.Akpan EJ, Okokon JE, Etuk IC. Antiplasmodial and antipyretic studies on root extracts of Anthocleista djalonensis against Plasmodium berghei. Asian Pac J Trop Dis. 2012;2(1):36–42. [Google Scholar]
- 28.Gobianand K, Vivekababdan P, Pradeep K, Mohan CVR, karthikeyan S. Anti-inflammatory and antipyretic activities of Indian medicinal plant Cassia fistula Linn. (Golden shower) in Wister albino rats. International Journal of Pharmacology. 2010;6(5):719–725. [Google Scholar]
- 29.Roberts LJ, Morrow JD. Analgesic, Antipyretic and Anti-inflammatory Agents and Drugs Employed in the Treatment of Gout. In: Goodman and Gilman, editor. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 687–732. [Google Scholar]
- 30.Chomchuen S, Singharachai C, Ruangrungsi N, Towiwat P. Antipyretic effect of the ethanolic extract of Ficus racemosa root in rats. J Health Res. 2010;24(1):23–28. [Google Scholar]
- 31.Sing N, Gupta AK, Juyal V, Chettri R. Study on antipyretic activity of extracts of Bergenia ligulata Wall. International Journal of Pharma and Bio Sciences. 2010;1(3):1–5. [Google Scholar]
- 32.Begum S, Saxena B, Goyal M, Ranjan R, Joshi VB, Rao CV, et al. Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamus niger and isolation of a new coumarinolignan. Fitoterapia. 2010;81:178–184. doi: 10.1016/j.fitote.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Nanda BK, Jena J, Rath B, Behera BR. Analgesic and Antipyretic activity of whole parts of Sphaeranthus indicus Linn. Journal of Chemical and Pharmaceutical Research. 2009;1(1):207–212. [Google Scholar]
- 34.Gupta M, Shaw BP, Mukherjee A. Studies on antipyretic-analgesic and ulcerogenic activity of polyherbal preparation in rats and mice. International Journal of Pharmacology. 2008;4(2):88–94. [Google Scholar]
- 35.Ahlawat S, Mishra PK, dalal K, Patra A. Antipyretic activity of roots of Argyreia speciosa (burm. f.) Bojer. International Journal of PharmTech Research. 2010;2(4):2165–2167. [Google Scholar]
- 36.Deepa PK, Usha PTA, Chandrasekharan Nair, Prassankumari KT. Antipyretic activity of seeds from red and white type of lotus (Nelumbo nucifera) in Albino rat. Veterinary World. 2009;2(6):213–214. [Google Scholar]