Abstract
Objective
To study morpho-anatomical characters and physicochemical analysis of Fumaria indica (F. indica) (Hausskn.) Pugsley, (Fumariaceae), an important medicinal plant used extensively for treating a variety of ailments in various system of indigenous medicine.
Methods
Evaluation of the different parts of the plant was carried out to determine the morpho-anatomical, physicochemical, phytochemical and HPTLC fingerprinting profile of F. indica and other WHO recommended methods were performed for standardization.
Results
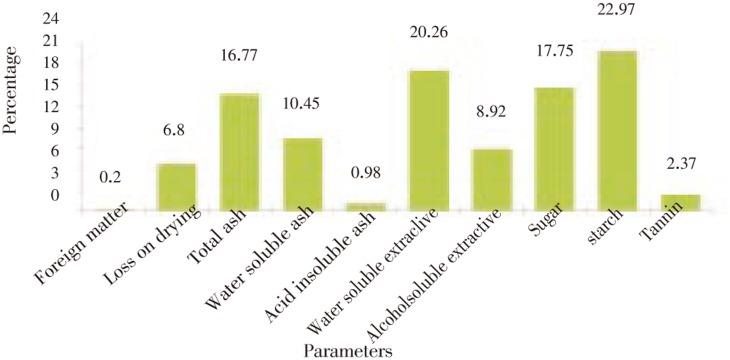
Morpho-anatomical studies showed compound and pinnatifid leaf, 4 to 6 cm in length, linear and oblong in shape and anomocytic arrangement of stomata, thin walled parenchymatous cells, scattered, sclerenchymatous, capped vascular bundles and radiating medullary rays. Physicochemical studies showed foreign matter 0.2%, loss on drying 6.8%, total ash 16.77%, alcohol and water soluble extractives 8.92% and 20.26%, respectively, sugar 17.75%, starch 22.97% and tannins 2.37%. Phytochemical evaluation revealed the presence of carbohydrate, alkaloids, flavonoids, saponins, tannins and sterol. Thin layer chromatography was carried out with different solvents and the best solvent system was chloroform and methanol in 80:20 ratio and revealed 12 spots with different Rf value under UV light 366λ.
Conclusions
The results of the study can serve as a valuable source of information and provide suitable standards for identification of this plant material for future investigations and applications.
Keywords: Morpho-anatomy, Transverse sections, HPTLC, Stomata, Xylem, Physicochemical
1. Introduction
Fumaria indica (Hausskn.) Pugsley, (Fumariaceae) (F. indica)commonly known as Pitpapra, is an annual herb, sub-erect or diffuse, probably scarcely scandent and distributed over the greater parts of India, Baluchistan, Afghanistan, and Persia. The plant is considered to be diuretic, diaphoretic, anthelmintic, laxative and is used to purify blood and in liver obstruction in ethnopharmacology[1],[2]. Pharmacological studies reported that F. indica possesses antidiarrhoeal[3], anti-inflammatory and anti-nocciceptive[4], hepatoprotective[5], anxiolytic[6], central nervous system depressant[7] and chemopreventive effect[8]. Antifungal activity of fuyuziphine isolated from F. indica have been reported[9]. Recently, it have been reported that F. indica is safe and devoid of toxic manifestations during various toxicity studies[10],[11]. Fumaria species contain some kind of fatty acids with antioxidant effects. A part of these lipids are phospholipids[12],[13]. Isoquinoline alkaloids isolated from Fumaria species showed significant antifungal and antiviral activity[14]. Phytochemical investigation revealed the presence of alkaloids, viz. protopine, parfumine, cryptopine, copticine, fumariline, fumaramine, fumaritine, paprafumicin, paprarine, papracinine, papraline, fumarophycine, narlumicine, narceimine, narlumidine, fuyuziphine; steroids, viz. b-sitosterol, stigmasterol, campesterol; organic acids viz. caffeic acid and fumaric acid[4],[15]. The genus Fumaria consist of 46 species in the world and Fumaria species look similar in appearance and hard to differentiate by local people[16]. The identification of Fumaria species is difficult due to the variability present in their vegetative and reproductive features, possibly due to the occurrence of inter-specific hybridisation[17]. Microscopy is an important tool for authentification of crude drugs and study of powdered drugs[18]. It is important to interpret morphological and anatomical descriptions of crude drugs as well as characteristic features of drugs and adulterants of commercial significance[19]. Establishment of the morpho-anatomical and physicochemical parameters of the plant will assist in standardization, which can guarantee quality, purity and proper identification of plant.
2. Materials and method
2.1. Plant material
F. indica plant was collected from the rural areas around Lucknow, India in the month of December. The plant was identified, authenticated taxonomically by taxonomist of National Botanical Research Institute (NBRI), Lucknow, India and a voucher specimen was preserved in herbarium (NBR-21) for future reference.
2.2. Morpho-anatomical evaluation
Fresh plant of F. indica (Figure 1) was taken for morphological and anatomical studies. For the anatomical studies, transverse sections of leaves, stem and root were prepared and stained as per standard and well established methods[20],[21]. The powder microscopy was performed according to the standard method[20].
Figure 1. F. indica.

2.3. Physicochemical and phytochemical analysis
Physicochemical values such as percentage of foreign matter, loss on drying, ash values, extractive values, sugar, starch and tannins were determined and calculated for the powdered plant material according to the well established official methods and recommended procedures[22]-[24]. Preliminary phytochemical screening of petroleum ether and methanolic extract of F. indica was carried out using the standard procedure[20].
2.4. HPTLC studies
For proper and meaningful utilization it is important to have quality standards of material and for this quality standardization, HPTLC finger print profile of hydro alcoholic extract and hexane, chloroform, acetone and methanol fraction of methanolic extract of F. indica (10 µL of 1 mg/mL) was developed. The HPTLC analysis was carried out on precoated Silica gel 60 F254 plate (Merck, India) with the help of Camag Linomat IV applicator. The plates were then eluted with different solvent system in a CAMAG twin trough chamber up to a distance of 9 cm. After development, all the plates were dried and densitometrically scanned on a TLC scanner III at 366 nm using Wincat software (CAMAG, Switzerland) and peak area was recorded.
3. Results
3.1. Macroscopic characteristics
Macroscopically, the fresh leaf of F. indica is green in color, compound, pinnatifid, 4 to 6 cm in length, linear or oblong, more or less glaucous. Racemes with 10 to 12 flowers about 5-6 mm long, penduncle 2 to 3 mm, pedicels 2 mm long; fruit about 2.4 mm long, slightly broader, subrotund, obovate, obtuse or subtruncate. Stem light green, smooth, hollow about 3-4 mm thick; root brown color, cylindrical, branched about 2-3 mm.
3.2. Microscopical characteristics
3.2.1. Leaf microscopy
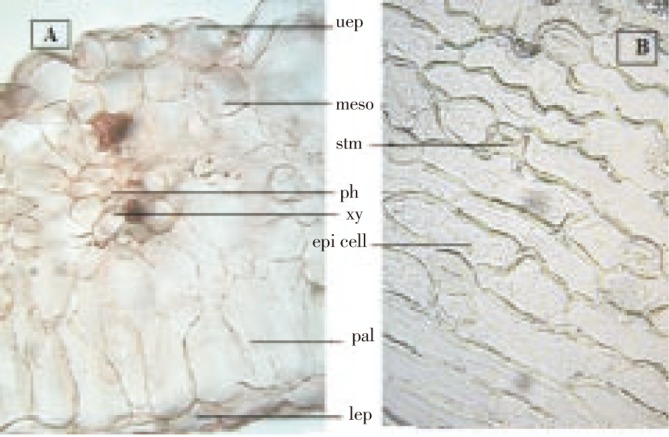
Transverse sections of leaf (Figure 2A) passing through lamina showed single layer epidermis on either side, consisting of thin walled, rectangular and oval shaped parenchymatous cells; mesophyll composed of oval to polygonal thin walled parenchymatous cells, filled with green pigment and not differentiated into palisade and spongy parenchyma; vascular bundle was scattered throughout the mesophyll; anomocytic stomata (Figure 2B) presented on both the surfaces.
Figure 2. Transverse sections of F. indica leaf.
(A) Transverse sections of F. indica leaf-lamina, (B) Anomocytic stomata. uep: Upper epidermis; lep: Lower epidermis; meso: Mesophyll; pal: Palisade cell; ph: Phloem; xy: Xylem; epi cell; Epidermal cell; stm: Stomata.
3.2.2. Stem microscopy
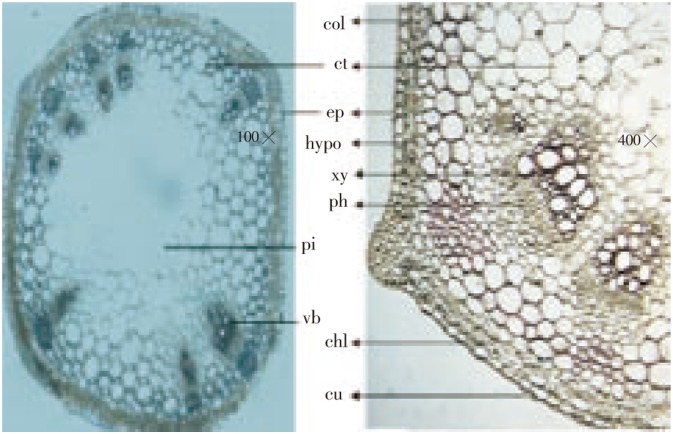
The stem (Figure 3) was quadrangular to pentagonal in shape. The outer most single layered epidermis was covered with cuticle; cortex was divided into two regions, outer 2-3 celled chlorenchymatous and inner 1-2 layered collenchymatous cells; endodermis was not distinct. Vascular bundle was collateral, either single or in a group of two, arranged at the ridges. Each vascular bundle was capped with sclerenchymatous cells. Phloem was well developed and made up of sieve tube, companion cells and phloem parenchyma. Xylem was also well developed and consisted of vessels, tracheids, fibers and xylem parenchyma. Major portion of the section was occupied by central collenchymatous pith. Pith cells were polygonal in shape with minor angular thickenings.
Figure 3. Transverse sections of F. indica stem.
cu: Cuticle; ep: Epidermis; hypo: Hypodermis; ct: Cortex; ph: Phloem; xy: xylem; col: Collenchyma; chl: Chlorenchyma; pi: Pith; vb: Vascular bundle.
3.2.3. Root microscopy
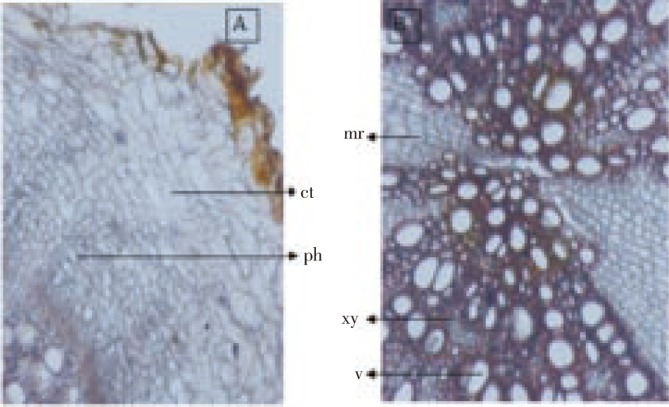
The root (Figure 4) was almost circular in outline. The epidermis was obliterated or crushed and cortex was consisted of thin walled, irregular shaped, parenchymatous cells, outer 1-2 layers crushed and brown in color (Figure 5A); endodermis was not distinct; secondary phloem was well developed and consisted of sieve tube, companion cells and phloem parenchyma; central core showed a wide zone of xylem and consisted of vessels, tracheids and fibers. Vessels were in radial rows having reticulate and spiral thickening, medullary ray broad and radiating (Figure 5B), fibers moderately long, thick walled, having narrow lumen and blunt tips.
Figure 4. Transverse sections of F. indica root.
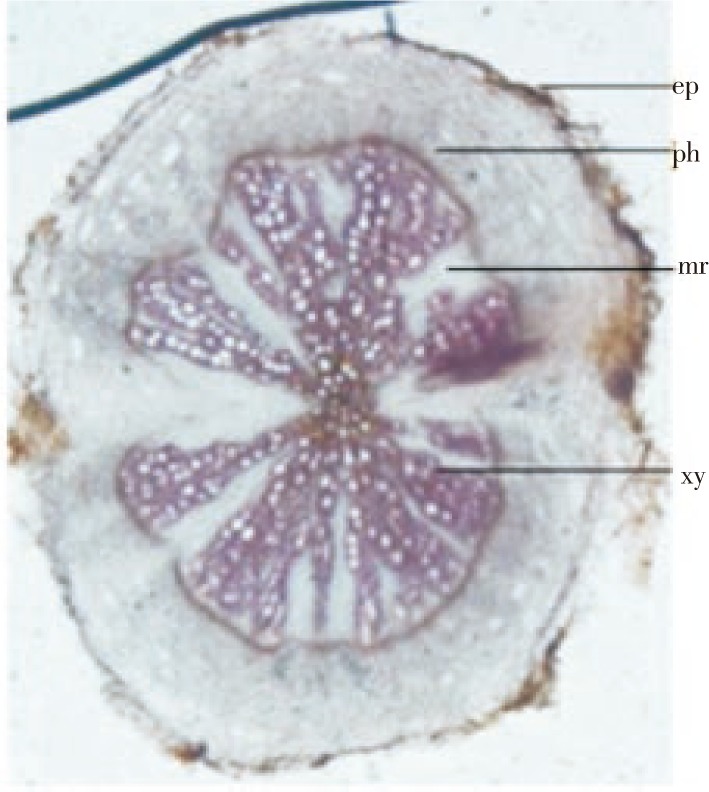
ep: Epidermis; ph: Phloem; xy: Xylem; mr: Medullary ray.
Figure 5. Transverse sections of root.
(A)cortex region; (B) xylem and radiating medullary ray. ct: Cortex;; ph: Phloem; xy: Xylem; mr: Medullary ray; v- Vessel.
3.3. Powder microscopic characteristics
The powder plant material was green in color, showing fragments of fibers, tracheids, spiral reticulate and pitted vessels and epidermal cells with stomata in surface view.
3.4. Physicochemical parameter
Physicochemical values such as percentage of foreign matter, loss on drying, ash values and extractive values, sugar, starch and tannins were determined and results are shown in Figure 6.
Figure 6. Physicochemical parameters of F. indica.
3.5. Preliminary phytochemical screening
Petroleum ether and methanolic extract of F. indica were qualitatively analyzed for the major chemical groups (carbohydrate, alkaloid, protein, sterol, flavanoid, saponin, tannin, gum and resin) and results are shown in Table 1.
Table 1. Phytochemical analysis of F. indica.
| Phytochemical group | Petroleum ether | Methanol |
| Alkaloid | - | + |
| Carbohydrate | - | + |
| Flavonoid | - | + |
| Protein and amino acid | - | + |
| Saponin | - | + |
| Sterol | + | + |
| Gum and resin | - | - |
+ Present, - Absent.
3.6. HPTLC studies
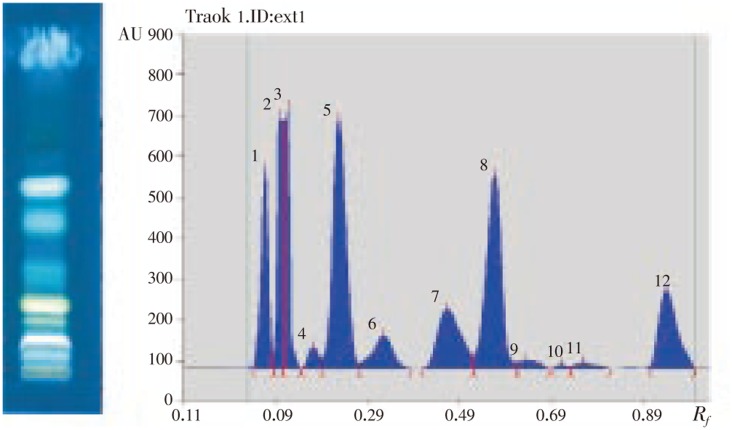
A densitometric HPTLC analysis was performed for the development of characteristic finger print profile which may be used as marker for quality evaluation and standardization of the drug. We have previously reported the concentration of caffeic acid (396 µg/g extract) present in 50% ethanolic extract of F. indica through quantitative analysis by HPTLC study[4]. The preliminary HPTLC studies revealed that the solvent system chloroform: methanol (80:20) was ideal for the hydro alcoholic extract and gave well resolved peaks of crude extract of F. indica. The band in the sample were obtained at Rf 0.04, 0.08, 0.10, 0.15, 0.19, 0.27, 0.40, 0.52, 0.61, 0.69, 0.73 and 0.90 which can be used as identifying marker (Figure 7), while haxene, chloroform, acetone and methonalic fraction gave well resolved peak in chloroform: methanol (90:10) (Figure 8).
Figure 7. HPTLC profile and densitometric scanning of 50% ethanolic extract of F. indica.
Solvent system: Chloroform: Methanol (80: 20), Detection: Under UV light λ366 nm.
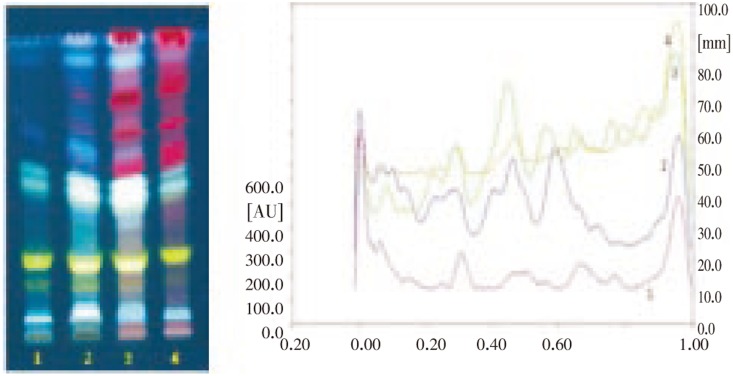
Figure 8. HPTLC finger print profile of different fractions of F. indica in solvent system Chloroform: Methanol (90:10), Detection: Under UV light λ366 nm.
Track 1: Methanol fraction, Track 2: Acetone fraction, Track 3: Chloroform fraction, Track 4: Hexane fraction.
4. Discussion
According to wealth of India, Indian plant bearing the name Pitpapra (F. indica) has been wrongly referred to by many authors as Fumaria officinalis Linn. or Fumaria paviflora lamm., which are common fumitory of Europe and not found in India[25]. The best condition to identify a Fumaria species is to study fresh material, as many changes occur in the herbarium specimens during drying, and significant changes in flower colour occur after drying[26]. Microscopic method is one of the simplest and cheapest methods to start with for establishing the correct identity of the source materials[27]-[31]. The results of these investigations could, therefore, serve as a basis for proper identification, collection and investigation of the plant. Microscopy, physicochemical and HPTLC studies are the parameters that are unique to the plant and are required in its standardization. The presence of anomocytic stomata, scattered vascular bundle in leaf, sclerenchymatous capped vascular bundle in stem and broad, radiating medullary rays of root are some of the diagnostic features noted from anatomical study of the plant. Ash values and extractive values can be used as reliable aid for detecting adulteration. These studies help in identification of the plant materials[32]. Total ash (16.77%) and acid insoluble ash (0.975%) are considered to be an important and useful parameter for detecting the presence of inorganic substances. Similarly alcohol (8.92%) and water soluble extractives (20.26%) are indicators of the total solvent soluble component. Ash values of drug also give an idea of earthy matter and other impurities present along with drug. Extractive values are primarily useful for the determination of exhausted and adulterated drugs. Extractive values are also useful to evaluate the chemical constituents present in the crude drug and help in estimation of specific constituents soluble in particular solvents[33],[34]. Likewise sugar (17.75%), starch (22.97%) and tannins (2.37%) which are a biochemical parameters, will be helpful for standardizing the drug for its various pharmacological potentials and to check the adulteration in natural valuable drug. In last two decades HPTLC method has employed as an important tool for the qualitative and quantitative phytochemical analysis of herbal drugs and formulations[35]. HPTLC fingerprint profile along with their Rf values were recorded, which would serve as a reference standard for the scientist engaged in research on the medicinal properties of plant.
In conclusion, these parameters which are being reported for the first time, could be useful in setting some diagnostic indices for the identification and preparation of a monograph of F. indica plant.
Acknowledgments
Authors thanks Dr. Sayyada Khatoon, Scientist E1, NBRI, for her kind help in identifying the microscopical features.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Kirtikar KR, Basu BD. In: Indian medicinal plants. Basu LM, editor. Allahabad: 1985. p. 138. [Google Scholar]
- 2.Chopra RN, Nayar SL, Chopra SN. Glossary of Indian medicinal plants. New Delhi: National Institute of Science Communication and Information Resources (CSIR); 2002. p. 122. [Google Scholar]
- 3.Gilani AH, Bashir S, Janbaz KH, Khan A. Pharmacological basis for the use of Fumaria indica in constipation and diarrhea. J Ethnopharmacol. 2005;96(3):585–589. doi: 10.1016/j.jep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Rao ChV, Verma AR, Gupta P, Vijaykumar M. Anti-inflammatory and anti-nociceptive activities of Fumaria indica whole plant extract in experimental animals. Acta Pharm. 2007;57:491–498. doi: 10.2478/v10007-007-0039-z. [DOI] [PubMed] [Google Scholar]
- 5.Rathi A, Srivastava AK, Shirwaikar A, Singh AK, Mehrotra S. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts fractions and an isolated alkaloid protopine. Phytomedicine. 2008;15:470–477. doi: 10.1016/j.phymed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Singh GK, Kumar V. Anxiolytic activity of standardized extract of Fumaria indica. Ann Neurosci. 2009;16:81. [Google Scholar]
- 7.Singh GK, Kumar V. Neuropharmacological screening and lack of antidepressant activity of standardised extract of Fumaria indica: l study. E J Pharmacol Ther. 2010;3:19–28. [Google Scholar]
- 8.Rao ChV, Kumar MV, Hussain T, Siddiqui HH, Fareed S, Sweety K. Evaluation of chemopreventive effect of Fumaria indica against N-nitrosodiethylamine and CCl4-induced hepatocellular carcinoma in Wistar rats. Asian Pac J Trop Biomed. 2011 doi: 10.1016/S1995-7645(12)60128-X. In press. [DOI] [PubMed] [Google Scholar]
- 9.Pandey MB, Singh AK, Singh AK, Singh UP. Inhibitive effect of fuyuziphine isolated from plant (Pittapapra) (Fumaria indica) on spore germination of some fungi. Mycobiology. 2007;35(3):157–158. doi: 10.4489/MYCO.2007.35.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh GK, Kumar V. Acute and sub-chronic toxicity study of standardized extract of Fumaria indica in rodents. J Ethnopharmacol. 2011;134:992–995. doi: 10.1016/j.jep.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 11.Singh GK, Chauhan SK, Rai G, Kumar V. Fumaria indica is safe during chronic toxicity and cytotoxicity: A preclinical study. J Pharmacol Pharmacother. 2011;2:191–192. doi: 10.4103/0976-500X.83287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazal H, Ahamad N, Khan MA. Physicochemical, phytochemical evaluation and DPPH-scavenging antioxidant potential in medicinal plants used for herbal formulation in Pakistan. Pak J Bot. 2011;43:63–67. [Google Scholar]
- 13.Habibi Tirtash F, Keshavarzi M, Fazeli F. Antioxidant components of Fumaria species. World Acad Sci, Engin & Technol. 2011;74:238–241. [Google Scholar]
- 14.Orhan I, Ozcelik B, Karaoglu T, Sener B. Antiviral and antimicrobial profiles of selected isoquinoline alkaloids from Fumaria and Corydalis species. Zeitschrift fur Naturforsch. 2007;62:19–26. doi: 10.1515/znc-2007-1-204. [DOI] [PubMed] [Google Scholar]
- 15.Pandey MB, Singh AK, Singh JP, Singh VP, Pandey VB. Fuyuziphine, a new alkaloid from Fumaria indica. Nat Prod Res. 2008;22(6):533–536. doi: 10.1080/14786410701592596. [DOI] [PubMed] [Google Scholar]
- 16.Orhan I, Sener B, Musharraf SG. Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exp Toxicol Pathol. 2010 doi: 10.1016/j.etp.2010.08.007. In press. [DOI] [PubMed] [Google Scholar]
- 17.Murphy JR. Fumitories of Britain and Ireland. London: Botanical Society of British Isles Press; 2009. pp. 1–12. [Google Scholar]
- 18.Rangari VD. Pharmacognosy and phytochemistry. 1st ed. New Delhi: Career Publications; 2006. pp. 103–115. [Google Scholar]
- 19.Evans WC. Trease and evans pharmacognosy. 16th ed. Edingburgh: Saunders Elsevier; 2009. pp. 541–570. [Google Scholar]
- 20.Khandelwal KR. Practical pharmacognosy. 19th ed. Pune, India: Nirali publication; 2008. pp. 7–29. [Google Scholar]
- 21.Kokate CK. Practical pharmacognosy. 4th ed. New Delhi: Vallabh Prakashan; 2010. pp. 17–26. [Google Scholar]
- 22.Peach K, Tracy MV. Modern methods of plant analysis. Vol. III and IV. Springer: Heidelberg; 1955. pp. 258–261. [Google Scholar]
- 23.Official methods of analysis. USA: Association of official chemists; 1984. pp. 55–56. [Google Scholar]
- 24.Government of India . The Ayurvedic pharmacopoeia of India. 1st ed. New Delhi: Ministry of Health and Family Welfare, Department of Indian System of Medicines and Homeopathy; 2009. pp. 242–244. [Google Scholar]
- 25.Anonymous . The wealth of India: Raw materials. New Delhi: Publication and Information Directorate, Council of Scientific and Industrial Research; 1956. p. 68. [Google Scholar]
- 26.Ebrahimzadeh Araii F, Keshavarzi M, Sheidaii M, Ghadam P. Fruit and seed morphology of the Fumaria L. species (Papaveraceae) of Iran. Turk J Bot. 2011;35:167–173. [Google Scholar]
- 27.Singh S, Machawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacognosy Phytother. 2010;2(5):71–75. [Google Scholar]
- 28.Mansour A, Enayat K, Neda MS, Behzad A. Antibacterial effect and physicochemical properties of essential oil of Zataria multiflora Boiss. Asian Pac J Trop Med. 2010;3(6):439–442. [Google Scholar]
- 29.Refaat AT, Shahat AA, Ehsan NA, Yassin N, Faiza Phytochemical and biological activities of Crataegus sinaica growing in Egypt. Asian Pac J Trop Med. 2010;3(4):257–261. [Google Scholar]
- 30.Obianime AW, Uche FI. The phytoconstituents and the comparative effects of aqueous extract of Irvingia gabonensis seeds and proviron on the biochemical parameters of male guinea pigs. Asian Pac J Trop Med. 2010;3(2):101–104. [Google Scholar]
- 31.Jadeja RN, Thounaojam MC, Singh TB, Devkar RV. Traditional uses, phytochemistry and pharmacology of Clerodendron glandulosum Coleb - a review. Asian Pac J Trop Med. 2012;5(1):1–6. doi: 10.1016/S1995-7645(11)60236-8. [DOI] [PubMed] [Google Scholar]
- 32.Nayak BS, Patel KN. Pharmacognostic studies of the Jatropha curcas leaves. Int J Pharm Tech Res. 2010;2(1):140–143. [Google Scholar]
- 33.Thomas S, Patil DA, Patil AG, Chandra N. Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L. fruit. J Herb Toxicol. 2008;2(2):51–54. [Google Scholar]
- 34.Kumar S, Kumar V, Prakash OM. Pharmacognostic study and anti-inflammatory activity of Callistemon lanceolatus leaf. Asian Pac J Trop Biomed. 2011;1(3):177–181. doi: 10.1016/S2221-1691(11)60022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sajeeth CI, Mann PK, Manavalan R, Jolly CI. Quantitative estimation of gallic acid, rutin and quercetin in certain herbal plants by HPTLC method. Der Chimica Sinica. 2010;1:80–85. [Google Scholar]