Abstract
Objective
To characterize, identify and investigate the anticancer properties of two new soil fungal isolates, Emericella nidulans and Fusarium solani isolated from Wady El-Natron in Egypt against colon cancer Caco-2 (ATCC) cell line.
Methods
Soil sample was cultured and two strains were chosen for morphological and phenotypical characterization. Partial sequences of the 18s rRNA gene and the internal transcribed spacer region ITS of the two isolates were amplified by PCR. Phylogenetic tree construction and analysis of the resulted multiple sequences from the two fugal isolates were also carried out. In vitro anticancer activity of the two strains was done against colon Caco-2 cancer cell line. Reverse transcription – PCR was carried out to detect level of expression of p53 in Caco-2 cell line.
Results
HF.1 displayed morphological and genotypic characteristics most similar to that of Fusarium solani while HF.2 was most similar to Emericella nidulans with high similarity of 99% and 97% respectively. The multiple sequence alignment of the two fungal isolates showed that, the maximum identical conserved domains in the 18s rRNA genes were identified with the nucleotide regions of 51st to 399th base pairs, 88th to 525th base pairs respectively. While those in the ITS genes were identified with the nucleotide regions of 88th to 463rd and 51st to 274th. The two isolates showed IC50 value with (6.24±5.21) and (9.84±0.36) µg/mL) concentrations respectively at 28h. Reverse transcription – PCR indicated that these cells showed high level of expression for p53 mRNA.
Conclusions
The morphology and molecular analysis identified HF.1 and HF.2 to be Fusarium solani and Emericella nidulans; new isolates of anticancer producing fungi from Wady El-Natroon city in Egypt. Treatment with the two isolates caused P53 expression in Caco-2 cell line. These two isolates can be used as an anticancer agents.
Keywords: Fungi, Colon cancer, Caco-2, Phylogenetic tree, Anticancer property, Multiple sequence analysis, Anticancer agent, Cell line
1. Introduction
Cancer still remains one of the most serious human health problems and colon cancer is the fourth most universal cause of cancer deaths[1]. Therapeutic methods for cancer treatment are surgery, radiotherapy, immunotherapy and chemotherapy[2] and these techniques are individually useful in particular situations and when combined, they offer a more efficient treatment for tumor. Many pharmaceutical agents have been discovered by screening natural products from plants, animals, marine organisms and microorganisms. In fact, more than 60% of approved drugs are derived from natural compounds[3]. Many of the developed antitumor drugs are derived from algal metabolites[4] and these metabolites play an important role in identification of new pharmaceutical compounds[5],[6]. Also actinomycetes are one of the most important sources for new bioactive compounds such as antibiotics and enzymes[7],[8]. The use of fungi for the production of commercial products is ancient, but it has increased rapidly over the last 50 years. They are known to produce a wide biodiversity of secondary metabolites such as volatile metabolites, mycotoxins, nonribosomal peptides, polyketides, terpenes, diketopiperazines and alkaloids[9], some of which could be involved in their pathogenicity; among which many have been reported to have an anticancerous activity[10]. Soil has the largest population of microbes of any habitat. Cultured soil microbes have been an incredibly productive source of drugs, for example the cancer chemotherapeutics doxorubicin hydrochloride, bleomycin, daunorubicin and mitomycin. Aspergilli represent a group of filamentous fungi that plays a key role in industrial biotechnology. One of the Emericella species is Emericella nidulans which serves as a working horse in industrial production of enzymes and chemicals. But, studies related with the biopotential activities of anticancer metabolites from soil fungi based drug discovery are too limited. In this connection, the present study was made an attempt to find out the anticancer property from some soil fungi. A soil sample taken from highly exposed locality to pollution in Egypt was used as the source of the microbial isolates to be experimented for anticancerous activities. Among the wide range of fungal pattern isolated, two common fungal species were chosen and purified. The purified two species were identified using molecular techniques, 18s ribosomal RNA gene and internal transcribed spacer (ITS) region gene sequencing analysis. Molecular characterization and sequence analysis for the isolated genes revealed that the two isolates are identical to Fusarium solani and Emericella nidulans (a sexual form, or teleomorph of Aspergillus nidulans). The two fungal isolates were tested against Caco-2(ATCC) cell line.
The Caco-2 cell line is a continuous line of heterogeneous human epithelial colorectal adenocarcinoma cells, developed by the Sloan-Kettering Institute for Cancer Research through research conducted by Dr. Jorgen Fogh[11]. Although derived from a colon (large intestine) carcinoma, when cultured under specific conditions the cells become differentiated and polarized such that their phenotype, morphologically and functionally, resembles the enterocytes lining the small intestine[12],[13]. Caco-2 cells express tight junctions, microvilli, and a number of enzymes and transporters that are characteristic of such enterocytes: peptidases, esterases, P-glycoprotein, uptake transporters for amino acids, bile acids carboxylic acids, etc. The level of expression of mRNA after treatment with the cell free crude extract from the two isolates was then analyzed by using RT-PCR for the P53 gene.
Tumor protein P53 is a tumor suppressor protein that in humans is encoded by the TP53 gene[14]-[17]. p53 is crucial in multicellular organisms, where it regulates the cell cycle and, thus, functions as a tumor suppressor that is involved in preventing cancer. As such, p53 has been described as “the guardian of the genome” because of its role in conserving stability by preventing genome mutation[16]. p53 has many mechanisms of anticancer function, and plays a role in apoptosis, genomic stability, and inhibition of angiogenesis. In its anti-cancer role, p53 works through several mechanisms: 1) it can activate DNA repair proteins when DNA has sustained damage; 2) it can induce growth arrest by holding the cell cycle at the G1/S regulation point on DNA damage recognition (if it holds the cell here for long enough, the DNA repair proteins will have time to fix the damage and the cell will be allowed to continue the cell cycle); 3) it can initiate apoptosis, the programmed cell death, if DNA damage proves to be irreparable.
In a normal cell p53 is inactivated by its negative regulator, mdm2. Upon DNA damage or other stresses, various pathways will lead to the dissociation of the p53 and mdm2 complex. Once activated, p53 will induce a cell cycle arrest to allow either repair and survival of the cell or apoptosis to discard the damaged cell. How p53 makes this choice is currently unknown. Activated p53 binds DNA and activates expression of several genes including WAF1/CIP1 encoding for p21. p21 (WAF1) binds to the G1-S/CDK (CDK2) and S/CDK complexes (molecules important for the G1/S transition in the cell cycle) inhibiting their activity. When p21 (WAF1) is complexed with CDK2 the cell cannot continue to the next stage of cell division. A mutant p53 will no longer bind DNA in an effective way, and, as a consequence, the p21 protein will not be available to act as the “stop signal” for cell division. Thus, cells will divide uncontrollably, and form tumors[18]. Also mdm2 acts as ubiquitin ligase and covalently attaches ubiquitin to p53 and thus marks p53 for degradation by the proteasome. However, ubiquitylation of p53 is reversible. A ubiquitin specific protease, USP7 (or HAUSP), can cleave ubiquitin off p53, thereby protecting it from proteasome-dependent degradation. This is one means by which p53 is stabilized in response to oncogenic insults.
2. Materials and methods
2.1. Samples origin and isolation procedure
After removing the surface loose litter layer (approximately top 4 cm), the soil samples were taken from a depth of 5 to 10 cm of the superficial layers from Wady El-Natron City, El-Beheraa Governorate, Egypt. Soil samples were numbered and put in a sterilized paper sack, transferred immediately to the Microbiology Laboratory of the Faculty of Science, Al-Azhar University (Girls Branch). One gram of soil was aseptically transferred into 99 mL of presterilized water and kept for continuous shaking (100 rpm) for 1 h. About 100 µL of diluted sample was transferred to molten Czabec Dox agar medium consisting of 2 g/L sodium nitrate, 0.5 g/L potassium chloride, 0.5 g/L magnesium glycerophosphate, 0.01 g/L ferrous sulphate, 0.35 g/L potassium sulphate, 30.0 g/L sucrose, and 12.0 g/L agar (pH 6.8) and made up to 1 L with sterile water and incubated at 28 °C for 7 days.
2.2. Identification of fungal isolates
After incubation, colonies appeared were restreaked on the same agar medium and further subjected to identification by using conventional[19] and molecular[20] techniques.
2.3. Extraction of bioactive compounds
In order to detect any bioactive compounds that might be excreted extracellular or intracellular, the whole fungal broths were subjected to sonication, centrifugation to remove cell debris and the resulted supernatants were then lyophilized in order to concentrate the active components for later use.
2.4. Cytotoxicity assay
2.4.1. MTT method
The Caco-2 cell line was obtained through the American Type Culture Collection (ATCC; Manassas, VA, USA). The Caco-2 cells were seeded at 20 000 cells/well (80% cell confluence) in tissue culture plates and incubated at 37 °C for 28 h. Then the two isolates were added to the cells at serial concentrations of 20, 10, 5, 2.5 and 1.25 µg/mL, the control was also included (non treated cells). The final volume was adjusted to 100 µL/well. The plate was incubated overnight at 37 °C, 5% CO2. A volume of 30 µL of (1 mg/mL) MTT stain was added to each well and the plate was incubated at 37 °C for 4 h. And then 100 µL of dimethyl sulfoxide (DMSO) stop solution was added to each well. The plate was shaken at room temperature for 10 to 20 min. The plate was then read using ELIZA Micro plate reader at 570 nm. The percentage of viable cells was calculated from the formula: Survival fraction=OD of treatment cell/OD of control cells. Here, OD means optical density. The IC50 was calculated by fitting the survival curve using graphpad, Prizm software in corporate[21].
2.4.2. RT-PCR analysis
These assays were performed as described by Olano et al[8]. Total RNA was isolated from frozen cells using a Trizol reagent as recommended by the manufacturer (Life Technologies). cDNA was amplified from total RNA (1µgne-Step RT-PCR System with Platinum Taq (Life Technologies). PCR was conducted for 30-40 cycles in a thermal controller (Programmable Thermal Controller; M) Research Inc., Watertown, MA). The primers used for amplification of p53 gene were shown in Table 1. Each amplification cycle consisted of 0.5 min at 94 °C for denaturation, 0.5 min at 55 °C for primer annealing, and 1 min at 72 °C for extension. After PCR amplification, the fragments were analyzed by agarose gel electrophoresis and stained with ethidium bromide.
Table 1. Oligonucleotide primers used for PCR amplification of 18s rRNA, ITS and p53 gene from Fusarium solani and Emericella nidulans.
| Name of the gene | Primer sequence | Product size | Reference |
| 18s rRNA | Forward HM1 (5′-TGCCAGCMGCCGCGGTA-3′) | 538 bp | [23] |
| Reverse HM2 (5′-GACGGGCGGTGTGTRCAA-3′) | 411 bp | ITS | |
| Forward | HM3 (5′-TCCGTAGGTGAACCTGCGG-3′) | 463 bp | [24] |
| Reverse HM4 (5′-CCTCCGCTTATTGATATGC-3′) | 311 bp | ||
| P53 | Forward P1 (5′-TCAGATCCTAGCGTCGAGCCC-3′) | 438 bp | [25] |
| Reverse P2 (5′-GGGTGTGGAATCAACCCACAG-3′) |
2.5. 18s rRNA and ITS gene amplification and sequencing
Genomic DNA was isolated by using standard method[22] and amplified by PCR with forward and reverse primers HM1 and HM2 as shown in Table 1. The reaction mixture contained 25 to 50 ng of DNA, ExTaq PCR buffer, 1.5 mM MgCl2, 10 mM deoxynucleoside triphosphate mixture, 50 pmol of each primer, and 0.5 U of ExTaq polymerase. PCR conditions consisted of an initial denaturation at 95 °C for 5 min; 30 cycles at 94 °C for 30 sec, annealing at 55 °C for 30 sec and 72 °C for 1 min; and final 7 min extension at 72 °C and 4 °C pause. The amplification products were examined by using QIA quick PCR clean up kit with the protocol suggested by Qiagen Inc. The complete 18s rRNA gene was sequenced by using the PCR products directly as sequencing template with the primers shown in Table 1. All sequencing reactions were carried out with ABI 377 automated DNA sequencer.
All the same was done for amplification of the ITS gene using primers HM3 and HM4 shown in Table 1. Tthe PCR conditions used were as follows: 95 °C for 5 min; 32 cycles: 94 °C for 30 sec, annealing at 56 °C for 30 sec and 72 °C for 1 min; and final 72 °C for 7 min extension and 4 °C pause.
2.6. Construction of phylogenetic tree
The retrieved gene sequences were compared with other fungal sequences by using NCBI BLAST search for their pair wise identities. Multiple sequence alignment and the phylogenetic tree were constructed with MEGA 4.0 software (http://www.megasoftware.net) by using the neighborjoining (NJ) method with 100 replicates as bootstrap value and NJ belongs to the distance-matrix method[22].
3. Results
Two different soil fungi were isolated from Wady El-Natron City, El-Beheraa Governorate. The microscopic and macroscopic characteristics of the two fungi were determined. The mycelial characteristics of the isolated fungi were similar to Fusarium solani and Emericella nidulans.
Fusarium isolate HF.1 colonies grew rapidly, on Czabec Dox agar at 26 °C giving 4.5 cm in 4 days, aerial mycelium white to cream, darken with maturity to purple-brown when sporodochia had formed. Macroconidia were formed after 4-7 days from short multi-branched conidiophores which may form sporodochia. They are 3-septate, fusiform, cylindrical, often moderately curved, with an indistinctly pedicellate foot cell and a short blunt apical cell, (28-42 µm × 4-6 µm). Microconidia are usually abundant, cylindrical to oval, one- to two-celled and formed from long lateral phialides, (8-16 µm × 2-4.5 µm). Chlamydospores are hyaline, globose, smooth to rough-walled, borne singly on short lateral hyphal branches.
Emericella isolate HF.2, is one of many species of filamentous fungi in the phylum Ascomycota. Growth rate was rapid on complete Czabec Dox agar media at 27 °C giving wooly texture colonies. Surface colony color is smoky grey - purple with suede - like surface consisting of a dense felt of conidiophores and the reverse was yellow. A microscopic view of Emericella nidulans showed typical short columnar strigmata with primary and secondary smooth walled conidiophores, more or less browned, commonly less than 200 µ long and terminating in dome like vesicles; small echinulate conidia (3-4 µ) in diameter; brittle perithica with walls of one cell thickness; quickly repining ascospores, purple-red in color.
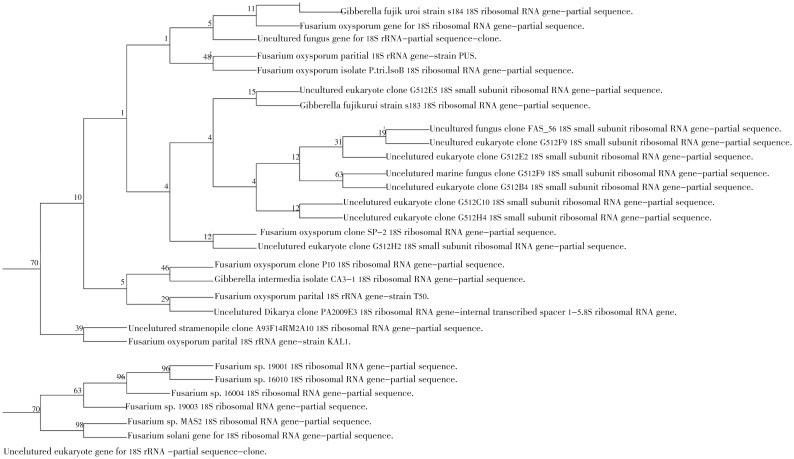
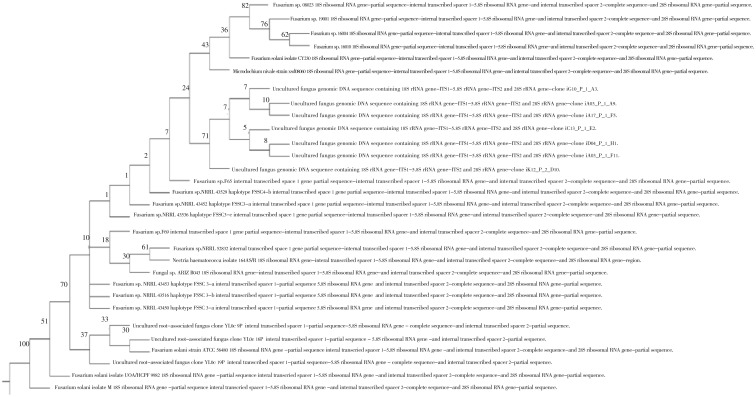
The 18s rRNA and ITS gene sequence analysis of Fusarium isolate showed 538 and 463 nucleotide base pairs, respectively. The NCBI BLAST analysis showed maximum identity of (99% and 94%, respectively) with Fusarium solani, while the 18s rRNA and ITS gene sequence analysis of the Emericella isolate showed 411 and 311 nucleotide base pairs, respectively. The NCBI BLAST analysis showed maximum identity of (97% and 92%, respectively) with Emericella nidulans species (Table 2). The phylogenetic analysis of the isolated fungi was categorized on the basis of evolutionary distance with NJ method (Figures 1-4). Moreover, the multiple sequence alignment was carried out with available sequences from NCBI data bank (first ten hits in BLAST results) for the two isolates. The results for Fusarium and Emericella isolates showed that, the maximum identical conserved regions in the 18srRNA genes were identified with the nucleotide regions of 51st to 399th base pairs, 88th to 525th base pairs respectively. While those in the ITS genes were identified with the nucleotide regions of 88th to 463rd and 51st to 274th base pairs respectively without the gap alignments (data not shown).
Table 2. NCBI alignment result showing maximum identity of the amplified 18s rRNA and ITS sequences of the two fungal isolates with the shown strains.
| NCBI accession number | Strain gene | Query coverage | Max ident |
| AB473810.1 | Fusarium solani ITS | 97% | 94% |
| HM214445.1 | Fusarium solani 18s rRNA | 98% | 99% |
| AF138289.1 | Emericella nidulans ITS | 80% | 92% |
| JQ082904.1 | Emericella nidulans 18s rRNA | 89% | 97% |
Figure 1. Neibour joining phylogenetic tree analysis based on 18s rRNA sequence data of HF.1.
Numbers above branches are bootstrap values. Scale bar represents genetic distance calculated by the Kimura 2-parameter model[23].
Figure 4. Neibour joining phylogenetic tree analysis based on ITS sequence data of HF.2.
Numbers above branches are bootstrap values. Scale bar represents genetic distance calculated by the Kimura 2-parameter model[23].
Figure 2. Neibour joining phylogenetic tree analysis based on ITS sequence data of HF.1.
Numbers above branches are bootstrap values. Scale bar represents genetic distance calculated by the Kimura 2-parameter model[23].
Figure 3. Neibour joining phylogenetic tree analysis based on 18s rRNA sequence data of HF.2.
Numbers above branches are bootstrap values. Scale bar represents genetic distance calculated by the Kimura 2-parameter model[23].
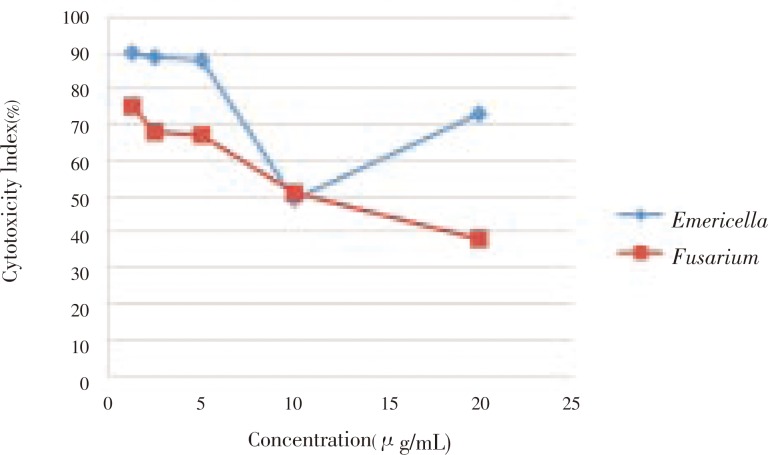
The anticancer property of the crude extracts against Caco-2 cell line revealed that the cytotoxicity index of the two isolates against the cell line showed IC50 value of (6.24±5.21 and 9.84±0.36 µg/mL) at 28 h, respectively (Figure 5).
Figure 5. Percentage of cytotoxic index of different concentrations of cell free crude extract from Emericella nidulans, Fusarium solani strains against Caco-2 colon cancer cell line.
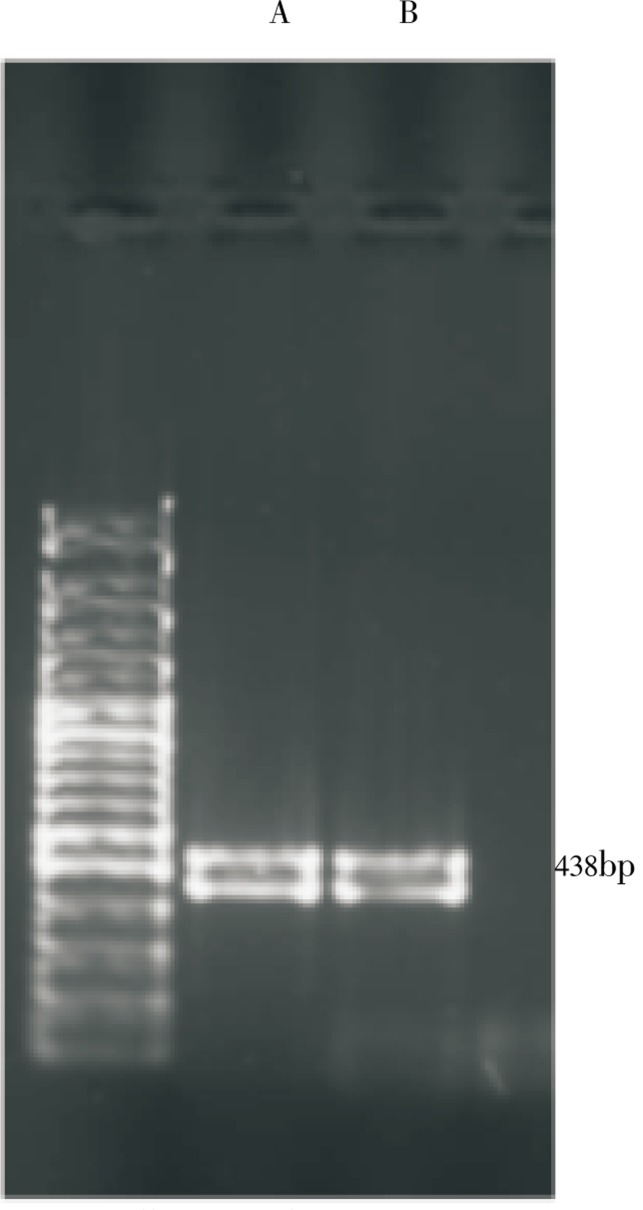
The cell free crude extract for both fungal isolates caused a rapid increase in the level of expression of P53 mRNA in Caco-2 (ATCC) cell line. In the present study, it was found that the free cell crude extract for both fungal isolates inhibit the growth of Caco-2 cell line with an IC50 value of (6.24±5.21 and 9.84±0.36 µg/mL) concentration, respectively. The RNA were then isolated at 3 h after the addition of the extract and examined by RT-PCR in order to detect and assay the level of expression of P53 gene (Figure 6). An intense band was obtained at 438 base pair in both cases of the two isolates in contrast with the control lane with no treatment which showed no bands.
Figure 6. Effect of cell free crude extract of Fusarium and Emericella isolates on the expression of P53 gene in Caco-2 cell line.
A: RT-PCR analysis of P53 mRNA from Fusarium solani; B: RT-PCR analysis of P53 mRNA from Emericella nidulans. RNA samples were analyzed by RT-PCR using the pair of primers. RNA was prepared from Caco-2 cells treated with DMSO (control) for 1 h.
4. Discussion
Cancer is considered to be the public health problem in developed and developing countries, among which colon cancer is the fourth most commonly diagnosed cancer in the world. Despite the intense efforts to develop treatments, effective agents are still not available. In this regard, natural product extracts continue to be the most promising source of new drugs for cancer. As fungi are known for being one of the most productive groups for useful natural products, the present study was made an attempt to find out the anticancer compounds from two isolated soil fungi. In the present study, Fusarium solani (HF.1) and Emericella nidulans (HF.2) showed IC50 value of (6.24±5.21 and 9.84±0.36 µg/mL) concentration, respectively in Caco-2 cell line. Suffness and Pezzuto reported that, the IC50 values less than 30 µg/mL in cancer cell lines can be considered as promising for anticancer drug development[26]. The anticancer activity of cell free crude extracts from the two fungal isolates may be due to the production of active secondary metabolites such as fumonisins compounds known to be produced by Fusarium solani[27] and polyketides, nonribosomal peptides, terpenes, indole alkaloids, and quinins known to be produced by Emericella nidulans[28].
Alkaloids are one of the major physiologically active nitrogenous compounds derived from many biogenetic precursors. Alkaloids are microtubule interfering agents which can bind with beta tublin, thus preventing the cell from making the mitotic spindle fibers necessary to move the chromosome around as the cell divides, inhibiting topoisomerase I[29], mitochondrial damage and inducing the release of cytochrome C and apoptosis inducing factor[1]. Moreover, quinine derivatives viz., driamycin, daunorubicin, mitomycin C, streptonigrin and lapachol, can interfere the DNA and RNA replication and mitochondrial oxidative pathways or the formation of super oxide, peroxide and hydroxide radicals as toxic products in the cell line. Several compounds of anthroquinone families showed antitumor activities[30]-[32]. The cytotoxic effect of the two fungal isolates seems to be the induction of apoptosis, partial cellular differentiation, and degradation of fusion transcripts, antiproliferation and inhibition of angiogenesis[1].
Molecular characterization and Identification of the two fungal isolates were done through uses of 18s ribosomal RNA and ITS RNA in phylogeny. The small subunit (SSU) 18s rRNA gene is one of the most frequently used genes in phylogenetic studies and an important marker for random target PCR in environmental biodiversity screening[33]. In general, rRNA gene sequences are easy to access due to highly conserved flanking regions allowing for the use of universal primers. Their repetitive arrangement within the genome provides excessive amounts of template DNA for PCR, even in smallest organisms. The 18s gene is part of the ribosomal functional core and is exposed to similar selective forces in all living beings. ITS refers to a piece of non-functional RNA situated between structural ribosomal RNAs (rRNA) on a common precursor transcript. Genes encoding ribosomal RNA and spacers occur in tandem repeats that are thousands of copies long. The ITS region is now perhaps the most widely sequenced DNA region in fungi[34]. It has typically been most useful for molecular systematics at the species level, and even within species (e.g., to identify geographic races). Because of its higher degree of variation than other genic regions of rDNA (for small- and large-subunit rRNA), ITS has proven especially useful for elucidating relationships among congeneric species and closely related genera in clinically important yeast species[35].
The ITS region is now perhaps the most widely sequenced DNA region in fungi[36]. It has typically been most useful for molecular systematics at the species level, and even within species (e.g., to identify geographic races).
The construction of phylogenetic tree was used to find out the relationship between the production of secondary metabolites, nature of product and pathogenic identities. It can be concluded from the phylogenetic tree analysis that, the Emericella nidulans was proved to have good anticancer activity. Similarly, the other species Fusarium solani was also proved to have potential antibacterial properties from the source of soil samples. The ribosome is an important component for the protein synthesis and their structural RNA subunits are highly conserved and these conserved regions can be used as a molecular marker to identify the evolutionary relationships between organisms[36]. In the present study, multiple sequence alignment of the of the 18s rRNA genes for the isolated fungi showed to have the conserved regions between the nucleotide regions of 5st to 399th base pairs, 88th to 525th base pairs, respectively. While those in the ITS genes were identified with the nucleotide regions of 88th to 463rd and 5st to 274th base pairs, respectively. Hence, these regions can be used as a molecular marker for their identification from other fungal species. It is concluded from the present study that some soil fungi could be used for the development of anticancer drugs after completing the successive clinical trials.
Acknowledgments
The author is thankful to the Authority of Al-Azhar University, Cairo, Egypt, for providing required facilities.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.American Joint Committee on Cancer . Colon and rectum. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 143–164. [Google Scholar]
- 2.American Cancer Society . Atlanta, Ga: American Cancer Society; 2012. Cancer facts & figures. [Google Scholar]
- 3.Raja M, Ravikumar S, Gnanadesigan M, Vijayaumar V. In vitro antibacterial activity of diterpene and benzoxazole derivatives from Excoecaria agallocha L. Int J Biol Chem Sci. 2010;4(3):692–70. [Google Scholar]
- 4.Boopathy NS, Kathiresan K. Anticancer drugs from marine flora: an overview. J Oncol. 2010 doi: 10.1155/2010/214186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravikumar S, Suganthi P, Moses FM. Crude bioactive compounds of actinomycetes from Manakkudy mangrove sediment. J Pharm Res. 2011;4(3):877–879. [Google Scholar]
- 6.Garcia MA, Jamel EM, Ward MM, Center Y, Hao RL, Siegel RL, et al. Atlanta: The American Cancer Society; 2007. Global cancer facts and figures. [Google Scholar]
- 7.Ravikumar S, Fredimoses M, Gnanadesigan M. Anticancer property of sediment actinomycetes against MCF-7 and MDA-MB-231 cell lines. Asian Pac J Trop Biomed. 2012;1:92–96. doi: 10.1016/S2221-1691(11)60199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olano C, Lombó F, Méndez C, Salas JA. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab Eng. 2008;10(5):281–292. doi: 10.1016/j.ymben.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed H. University of Sheffield; 2005. Exploiting genomics to identify secondary metabolic pathways in Aspergillus fumigates (Ph. D thesis) [Google Scholar]
- 10.Huang YJ, Wang JF, Li GL, Zheng ZH, Su WJ. Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol Med Microbiol. 2006;31(2):85–167. doi: 10.1111/j.1574-695X.2001.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 11.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21(1):1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 12.Smetanová L, Ste˘tinová V, Svoboda Z, Kvetina J. Caco-2 cells, biopharmaceutics classification system (BCS) and biowaiver. Acta Medica (Hradec Kralove) 2011;4(1):3–8. [PubMed] [Google Scholar]
- 13.Leonard F, Collnot EM, Lehr CM. A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa In vitro. Mol Pharm. 2010;7(6):2103–2119. doi: 10.1021/mp1000795. [DOI] [PubMed] [Google Scholar]
- 14.Alsner J, Jensen V, Kyndi M, Offersen BV, Vu P, Børresen-Dale AL, et al. A comparison between p53 accumulations determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol. 2008;47(4):600–607. doi: 10.1080/02841860802047411. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Ip MM, Podgorsak MB, Das GM. Disruption of estrogen receptor alpha-p53 interaction in breast tumors: a novel mechanism underlying the anti-tumor effect of radiation therapy. Breast Cancer Res Treat. 2009;115(1):43–50. doi: 10.1007/s10549-008-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varna M, Bousquet G, Plassa LF, Bertheau P, Janin A. TP53 status and response to treatment in breast cancers. J Biomed Biotechnol. 2011;2011:284584. doi: 10.1155/2011/284584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camerini A, Donati S, Viacava P, Siclari O, Puccetti C, Tartarelli G, et al. Evaluation of HER2 and p53 expression in predicting response to docetaxel-based first-line chemotherapy in advanced breast cancer. J Exp Clin Cancer Res. 2011;11:30–38. doi: 10.1186/1756-9966-30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Biotechnology Information The p53 tumor suppressor protein. Genes and disease. United States National Institutes of Health. [Online] Available from: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowSection&rid=gnd.section.107. [Accessed on 20 Mar, 2012]
- 19.Bergey DH, Holt JG. 9th ed. Baltimore: Lippincott Williams & Wilkins; 1994. Bergey's manual of determinative bacteriology. [Google Scholar]
- 20.Singh V, Praveen V, Khan F, Kant C, Tripathi M. Phylogenetics of an antibiotic producing Streptomyces strain isolated from soil. Bioinformation. 2009;4(2):53–58. doi: 10.6026/97320630004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 22.Joseph S, Shanmugapriya S, Gandhimathi R, Kiran SG, Ravji RT, Natarajaseenivasan K, et al. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MA D08. Appl Microbiol Biotechnol. 2009;83:435–445. doi: 10.1007/s00253-009-1878-y. [DOI] [PubMed] [Google Scholar]
- 23.Bundt M, Widmer F, Pesaro M, Zeyer J, Blaser P. Biological ‘hot spots’ in soils. Soil Biol Biochem. 2001;33(6):729–738. [Google Scholar]
- 24.Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing In vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- 25.Harris N, Brill E, Shohat O, Prokocimer M, Wolf D, Arai N, et al. Molecular basis for heterogeneity of the human p53 protein. Mol Cell Biol. 1986;6(12):4650–4656. doi: 10.1128/mcb.6.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suffness M, Pezzuto JM. Assay related to cancer drug discovery. In: Hostettman K, editor. Methods in plant biochemistry: assay for bioactivity. London: Academic Press; 2009. pp. 71–133. [Google Scholar]
- 27.Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, FitzPatrick ES. 2nd ed. Chichester, West Sussex, UK: John Wiley & Sons; 2011. Veterinary microbiology and microbial disease. [Google Scholar]
- 28.Brakhage AA, Schroeckh V. Fungal secondary metabolites - Strategies to activate silent gene clusters. Fungal Genet Biol. 2011;48(1):15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 29.García MT, Blázquez MA, Ferrándiz MJ, Sanz MJ, Silva-Martín N, Hermoso JA, et al. New alkaloid antibiotics that target the DNA topoisomerase I of Streptococcus pneumoniae. J Biol Chem. 2011;286:6402–6413. doi: 10.1074/jbc.M110.148148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anibou M, Chait A, Zyad A, Taourirt M, Ouhdouch Y, Benherref A. Actinomycetes from Moroccan habitats: isolation and screening for cytotoxic activities. World J Microbiol Biotechnol. 2008;24:2019–2025. [Google Scholar]
- 31.Gulecha V, Sivakuma T. Anticancer activity of Tephrosia purpurea and Ficus religiosa using MCF 7 cell lines. Asian Pac J Trop Med. 2011;4(7):526–529. doi: 10.1016/S1995-7645(11)60139-9. [DOI] [PubMed] [Google Scholar]
- 32.Kumar RS, Rajkapoor B, Perumal P. In vitro and in vivo anticancer activity of Indigofera cassioides Rottl. Ex. DC. Asian Pac J Trop Med. 2011;4(5):379–385. doi: 10.1016/S1995-7645(11)60108-9. [DOI] [PubMed] [Google Scholar]
- 33.Meyer A, Todt C, Mikkelsen NT, Lieb B. Fast evolving 18S rRNA sequences from Solenogastres (Mollusca) resist standard PCR amplification and give new insights into mollusk substitution rate heterogeneity. BMC Evol Biol. 2010;10:70. doi: 10.1186/1471-2148-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peay KG, Kennedy PG, Bruns TD. Fungal community ecology: a hybrid beast with a molecular master. BioScience. 2008;58:799–810. [Google Scholar]
- 35.Garner CD, Starr JK, McDonough PL, Altier C. Molecular identification of veterinary yeast isolates by use of sequence-based analysis of the D1/D2 region of the large ribosomal subunit. J Clin Microbiol. 2010;48(6):2140–2146. doi: 10.1128/JCM.02306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smit S, Widmann J, Knight R. Evolutionary rates vary among rRNA structural elements. Nucleic Acids Res. 2007;35(10):3339–3354. doi: 10.1093/nar/gkm101. [DOI] [PMC free article] [PubMed] [Google Scholar]