Abstract
Objective
To evaluate the effects of Mirazid® and myrrh volatile oil on adult Fasciola gigantica (F. gigantica ) under laboratory conditions.
Methods
The effects of oleoresin extract of myrrh (Mirazid®) and myrrh volatile oil on the surface morphology of adult F. gigantica following treatment in vitro had been determined by scanning electron microscopy. The results were compared with those observed in the fluke tegument following incubation in triclabendazole sulphoxide (TCBZ-SO), active form, (Fasinex®, Ciba-Geigy).
Results
Observations of the efficacy of Mirazid® oleoresin extract and myrrh volatile oil indicated that both products showed dose-dependent anthelmintic efficacy. The anterior half of the fluke was consistently more severely affected than the posterior half. The surface changes induced by Mirazid® oleoresin extract were less severe than those observed after exposure to either myrrh volatile oil or TCBZ-SO. Flukes showed swelling after these treatments, but its level and blebbing were much greater with myrrh volatile oil; in which patches of tegumental sloughing were observed in the apical cone and the posterior mid-body region of flukes. This was not observed after treatment with Mirazid® oleoresin extract.
Conclusions
The comparatively more disruption, observed in myrrh volatile oil exposed specimens, compared to that exposed to Mirazid® oleoresin extract might suggest that the anthelmintic activity of Mirazid® oleo resin extract was attributed to its content of volatile oil. So, increasing the concentration of myrrh volatile oil in Mirazid® might possibly help to developing its anthelmintic activity.
Keywords: Fasciola gigantic, Mirazid®, Myrrh volatile oil, In vitro effect
1. Introduction
Fasciolosis is an economically important disease of ruminants world-wide, causing significant animal welfare problems and may threaten the production of food in many regions of the world[1]. It is now also recognized as a human disease of large public health importance[2]. In recent years, the number of humans infected by Fasciola spp. has increased significantly, and several geographical areas are now considered endemic for the disease in humans[3]. In Egypt, high infection levels had been described in livestock[4]. Such infection rates induced important economic problems. The annual loss in milk and meat due to fasciolosis was being estimated to be 30%[5]. Also, the number of human cases had dramatically increased since 1980, especially in the Nile Delta region, which was meso- to hyperendemic[6]. Both Fasciola gigantica (F. gigantica) and Fasciola hepatica coexisted but the former; with a tropical and subtropical distribution, was considered the endogenous fasciolid species in Egypt[3].
At present, effective and commercial vaccines are not yet available[7], therefore anthelmintic drugs are the main method employed for controlling the fluke infection. Triclabendazole (TCBZ) is the drug of choice and the most effective flukicide against both juvenile and adult flukes. It has been used to control fasciolosis since 1983, as a veterinary drug (Fasinex®)[8]. As an almost inevitable consequence of its success, problems with resistance against TCBZ have emerged. This may pose a serious problem as no other effective drug is available[9]. The emergence of resistance has prompted the search for new compounds or for better use of existing drugs. Alternative drugs tested include nitazoxanide, artemisinin derivatives, the TCBZ derivative, compound alpha and Mirazid®[10].
Mirazid®is a myrrh derived drug used for treatment of schistosomiasis and fasciolosis[11]. Myrrh is an aromatic oleo gum resin obtained from the stem of Commiphora molmol (family: Burseraceae), a tree that grows in the northeast Africa and the Arabian Peninsula. It contains 7%-17% volatile oil, 25%-40% resin, 57%-61% gum, and 3%-4% impurities[12]. The gum contains polysaccharides and proteins, while the volatile oil is composed of steroids, sterols and terpenes. The characteristic odor of myrrh is derived from furanosesquiterpenes[13]. One of the advantages of myrrh is that, like many herbal and botanical products, it is safe to humans and animals. This natural flavoring substance has been approved by the U.S. Food and Drug Administration[14]. The well-organized scientific studies on myrrh as an antiparasitic agent are a recent event. It is in Egypt where the first published studies have documented that this traditionally used natural resin might have trematodicidal properties. Myrrh proved to be an effective fasciolicidal drug. This could be evidenced from the very promising results obtained with myrrh in experimental animals[15], clinical trials[16] as well as in field studies[17]. A pharmaceutical company, in Egypt, now produces a special myrrh preparation and markets it as gelatin capsules (Mirazid®) containing 300 mg of purified oleoresin alcoholic extract of myrrh[11].
The mode of action of myrrh as an antiparasitic agent is still unclear. On the other hand, the fluke's tegument is the interface between parasite and host, and it is the principal route by which drug metabolites enter the fluke[10]. It also plays a number of important roles in fluke biology, including protection against immune attack and anthelmintic action[18]. Thus, examination of tegumental changes induced by drug is important to shed light on the mechanism by which the drug may exert its fasciolicidal property. In the present study, the effects of Mirazid® and myrrh volatile oil on the surface morphology of adult F. gigantica following treatment in vitro had been determined by scanning electron microscopy. The results were compared with those observed in the fluke tegument following incubation in triclabendazole sulphoxide (TCBZ-SO), active form, (Fasinex®, Ciba-Geigy).
2. Materials and methods
2.1. Parasites
Adult worms of F. gigantica were collected from the bile ducts and gall bladder of buffalo slaughtered in a local abattoir in Cairo province, Egypt. They were washed several times with warm (37 °C) sterile complete RPMI 1640 culture medium. Only intact and actively mobile worms were used in this study.
2.2. Drugs
Oleoresin extracts in Mirazid® capsules were obtained from Pharco Pharmaceuticals Company, Alexandria, Egypt. TCBZ “Fasinex® 10%” was a previously known drug for treatment of fasciolosis. It was purchased from Ciba-Geigy Company.
2.3. Myrrh volatile oil
The method of extracting volatile oil from myrrh included the steps of grinding up the myrrh, covering the ground up plant with a layer of water to create a mixture of plant and water, passing steam through the mixture, condensing volatile oil from the steam in a condensing chamber, and separating the layer of oil from the aqueous layer[13].
2.4. In vitro assays
Under sterile conditions in a laminar flow cabinet, flukes were transferred to fresh culture medium containing antibiotics (penicillin, 50 IU/mL; streptomycin, 50 mg/mL), 50% (v/v) heat denatured rabbit serum and 2% (v/v) rabbit red blood cells; as recommended by Hegazi et al[19]. Stock solutions of Mirazid® oleoresin and myrrh volatile oil at 10 mg/mL were prepared in Cremiphore EL 10%. The flukes were incubated with 10, 30 and 60 µg/mL of Mirazid® oleoresin and myrrh volatile oil for 24 h at 37 °C in an atmosphere of 5% CO2. A positive control group was prepared by incubating whole flukes for 24 h in RPMI 1640 culture medium containing 15 µg/mL TCBZ-SO. This level corresponded to maximum blood levels in vivo[20]. The TCBZ was initially prepared as a stock solution in dimethylsulfoxide (DMSO) and added to the culture medium to give a solvent concentration of 0.1% (v/v), based on the report by Shalaby et al[9], which demonstrated that no significant differences were observed between fresh fluke and control fluke incubated for 24 h in solvent; 0.1% (v/v) DMSO. Solvent control flukes were incubated for 24 h in RPMI 1640 culture medium containing 0.06% (v/v) Cremiphore EL. Normal control flukes were fixed immediately following the initial washing. Six flukes were examined for each concentration.
2.5. Scanning electron microscopy
Following incubation, the adult flukes were rinsed with 0.9% NaCl (w/v) and fixed with 2.5% (v/v) glutaraldehyde in a PBS buffer (pH 7.4) for 24 h at room temperature. After rinsing with PBS buffer, the specimens were washed with distilled water, dehydrated in ascending ethanol concentrations and critically point dried in carbon dioxide. Then, they were fixed to aluminum stubs and coated with gold-palladium. The specimens were viewed in a Jeol scanning electron microscope (Jeol Corp., Mitaka, Japan) operated at 20 kV.
3. Results
3.1. Scanning electron microscopy (SEM) of normal fresh and control flukes
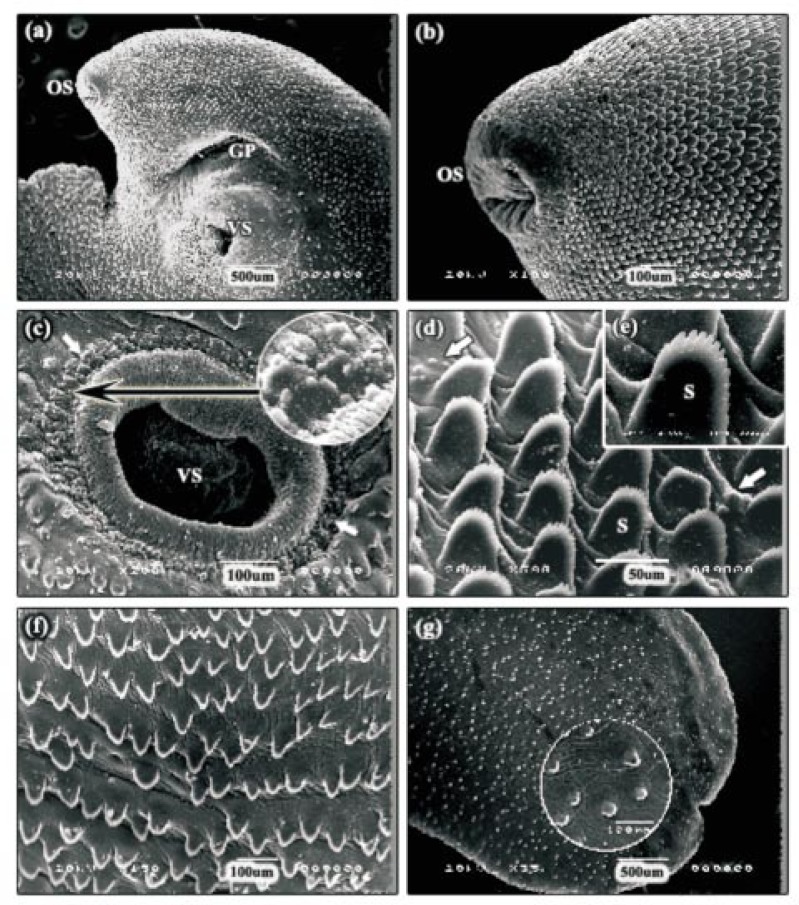
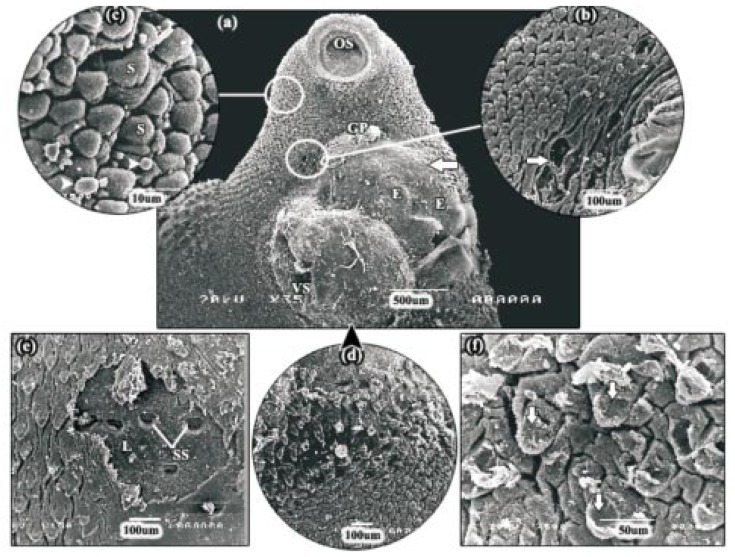
Adult F. gigantica was leaf-like in shape with tapered anterior and posterior ends. At the anterior end of the body was the apical cone, with the oral sucker at its tip. Visible on the ventral surface was the ventral sucker, at the apical cone-main body junction, and the gonopore, which lied just anterior to the ventral sucker (Figure 1a). The typical intact tegument surface exhibited microridges and grooves with numerous serrated spines, which were deficient at the rims of both suckers (Figure 1b and 1c). Sensory papillae surrounding the oral and ventral suckers were present in clusters, fungiform in shape, and solitaries. Also these papillae were scattered throughout the body surface between spines (Figure 1b, 1c and 1d). Each papilla appeared as a small dome with a smooth top and highly pitted base. Spines were most numerous on the anterior part especially around the oral and ventral suckers and reduced in number on posterior part of the body. The size and shape of these spines were dependent on the area of the fluke on which they were present. The spines on the apical cone were broad, tightly-packed and posteriorly-directed, with a number of finger-like protrusions at their tips (Figure 1d and 1e). The spines on the mid-body were not closely-arranged as those on the apical cone and the protrusions along their tips were not well-defined (Figure 1f). The spines on the tail surface were smaller than the spines covering the rest of the fluke's surface (Figure 1g and inset). No significant differences were observed between fresh fluke and control fluke incubated for 24 h in solvent; 0.06% (v/v) Cremiphore EL.
Figure 1. Scanning electron micrographs (SEMs) of normal fresh fluke.
a: SEM of the apical cone surface showing smooth oral sucker at its tip, the ventral sucker and the gonopore. OS: oral sucker, VS: ventral sucker, GP: gonopore. b: SEM of the apical cone tegument showing ridged tegument with numerous serrated spines and clusters, fungiform in shape, of sensory papillae surrounding the oral sucker. OS: oral sucker. c: SEM of the ventral sucker showing clusters of sensory papillae (arrows). VS: ventral sucker. d, e: High power SEMs of the spines on the apical cone revealing tightly-packed, posteriorly-directed and broad spines, with a number of finger like protrusions at their tips. There are clusters of sensory papillae scattering throughout the body surface between spines (arrows). S: spine. f: SEM of the mid-body region. Note the spines are not closely-arranged as these on the apical cone. g: SEM of the tail region showing smaller spines (inset) than that covering the rest of the fluke's surface.
3.2. Effects of oleoresin extract of myrrh (Mirazid®)
After 24 h incubation with 10 µg/mL Mirazid® oleoresin, the apical cone region appeared to be slightly more swollen than normal. The swelling was more prominent on the ventral surface; around the oral sucker. In the ventral sucker, some blebs were present. The swollen tegument along the apical cone region was accompanied with clusters of normal sensory papillae. The most severe disruption occurred along its lateral margins and took the form of tegumental swelling, blebbing and distortion of the spines. The swelling of the tegument between the spines decreased posteriorly. In the majority of the specimens, the mid-body region showed little disruption. In the tail region, very little, if any, disruption was seen.
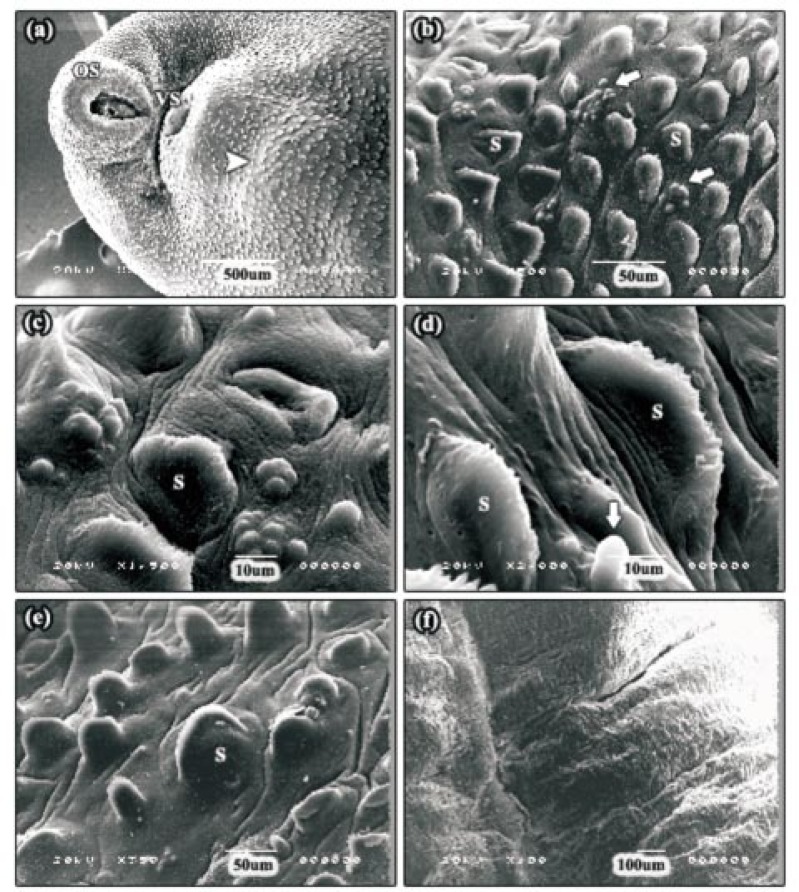
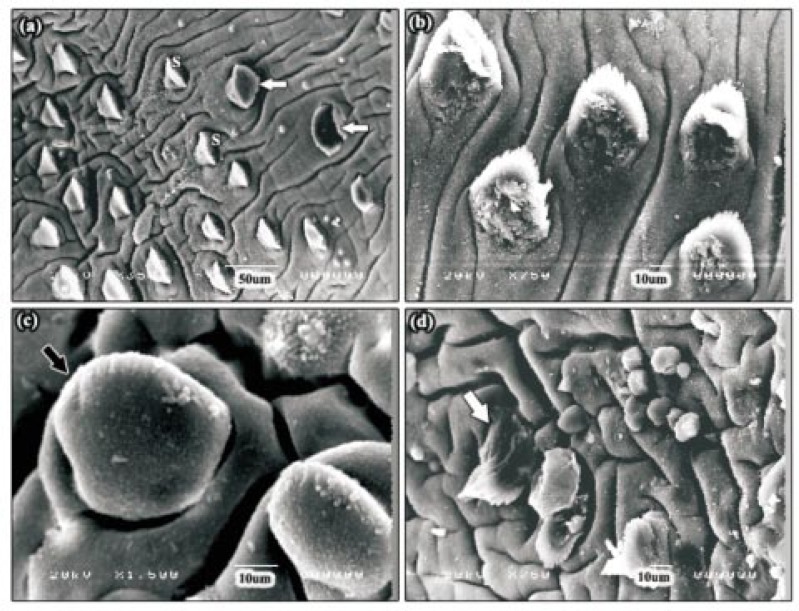
After 24 h incubation with 30 µg/mL Mirazid® Oleo resin, moderate swelling of the tegumental surface covering and between the spines was seen in the apical cone region, in a large number of the specimens examined (Figure 2a). This disruption was most often concentrated around the ventral sucker. At the meantime, numerous patches of normal sensory papillae were confined to the tegument of this area between the spines (Figure 2b and 2c). In some specimens, the severity of tegumental swelling was greater and the spines and some sensory papillae appeared to be swollen (Figure 2d). In the majority of the specimens examined, an often large area of tegumental swelling was observed just posterior to the ventral sucker in the anterior mid-body (Figure 2a). In these areas, the spines appeared sunken due to the swelling of the tegument around them. In the posterior mid-body region, the tegument was moderately swollen and spines could be distinguished, although they were partially submerged (Figure 2e). In the tail region, fissuring of the tegument was present. In some of the specimens, the tegument was severely swollen to the extent that the spines were not visible (Figure 2f).
Figure 2. SEMs of adult F. gigantica following 24 h incubation in 30 µg/mL Mirazid®.
a: SEM of the apical cone region showing moderate swelling of the tegumental surface. In the majority of the specimens examined, an often large area of tegumental swelling was observed just posterior to the ventral sucker in the anterior mid-body (head arrow). OS: oral sucker, VS: ventral sucker. b, c: SEMs of the swollen tegument along the apical cone region revealing clusters of normal sensory papillae (arrows). S: spine. d: High power SEM of the tegumental surface, in some specimens, showing severe tegumental swelling. The spines and some sensory papillae (arrow) appeared to be swollen. S: spine. e: SEM of the posterior mid-body region showing moderately swollen tegument and the spines were partially submerged. S: spine. f: SEM of the tail region. In this specimen, the tegument was severely swollen to the extent that the spines were not visible.
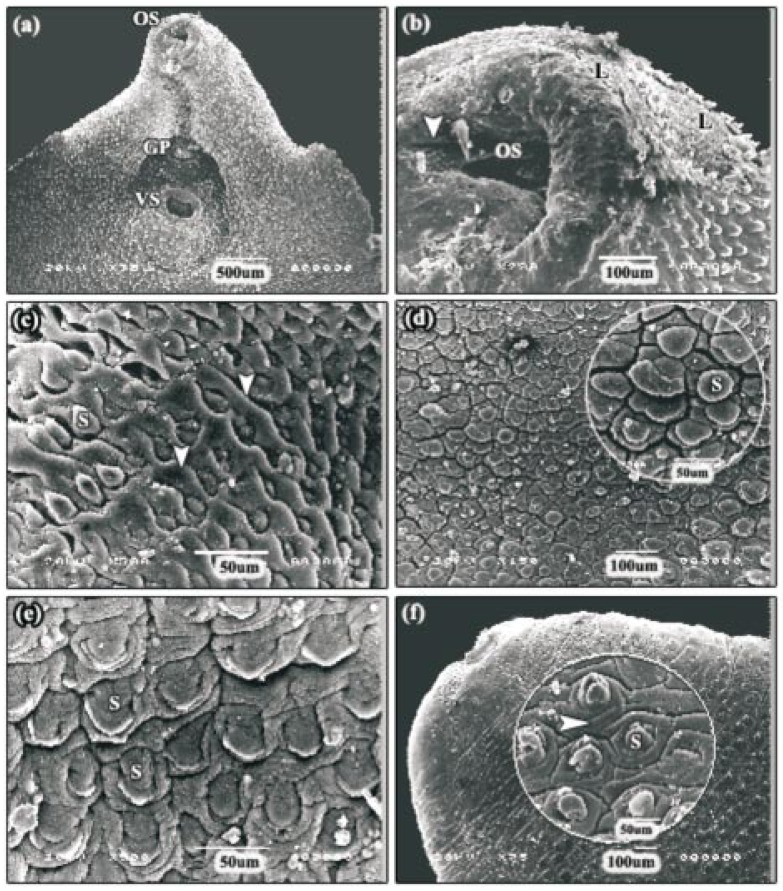
After 24 h incubation with 60 µg/mL Mirazid® oleoresin, disruption of the tegument observed in the apical cone region was considerably more severe than that observed with the 30 µg/mL Mirazid® oleoresin. The majority of the specimens examined had severe swelling on the surface of the apical cone (Figure 3a). The swelling was most evident towards the lateral margins and around the oral sucker; which accompanied with some blebs (Figure 3b). No tegumental sloughing had occurred in any area except that around the oral sucker. In this region, and in patches, neither spines nor sensory papillae remained and the entire tegument had been sloughed away to expose the basal lamina (Figure 3b). In areas in which the tegument remained intact, disruption generally took the form of swelling of the tegument between the spines. Where this disruption was sever, the tegument covering the spines was also swollen ,and these spines often appeared sunken due to the swollen tegument around them (Figure 3c). Along the mid-line of the anterior mid-body region, small patches of severe swelling and blebbing were seen (Figure 3d). In this region, the spines appeared sunken as the tegument had swollen to engulf them, and the tegument covering the spines also appeared very swollen, giving the spines a bulbous appearance. Less severe disruption was evident in the posterior mid-body and lateral margins of the flukes examined, and took the form of swelling of the tegument between the spines; accompanied with some blebs (Figure 3e). The tail region was the least severely affected region, where mild furrowing of the tegumental syncytium was the only change observed. Some of the specimens, however, did show distortion of the spines (Figure 3f). Overall, the anterior region of the fluke was more disrupted than the posterior region as well as no noticeable difference in the severity or distribution of the disruption was observed between the dorsal and ventral surfaces.
Figure 3. SEMs of adult F. gigantica following 24 h incubation in 60 µg/mL Mirazid®.
a: SEM of the apical cone region showing severe swelling of the tegumental surface. OS: oral sucker, VS: ventral sucker, GP: gonopore. b: SEM of the swollen tegument around the oral sucker showing some blebs (head arrow) and tegumental sloughing (L). OS: oral sucker. c: SEM of the area around the oral sucker in which the tegument remained intact. In this specimen, the spines often appeared sunken due to the swollen tegument around them (head arrows). S: spine. d: SEM of the anterior mid-body region showing small patches of severe swelling and blebbing. In this region, the tegument covering the spines also appeared very swollen, giving the spines a bulbous appearance (inset). S: spine. e: SEM of the posterior mid-body region showing less severe disruption. S: spine. f: SEM of the tail region showing mild furrowing of the tegumental syncytium (head arrow). S: spine.
3.3. Effects of myrrh volatile oil
After 24 h incubation with 10 µg/mL myrrh volatile oil, in the apical cone region of almost all the specimens examined, only very mild disruption was observed. The disruption most commonly took the form of swelling of the tegument between the spines. However, swelling was most often concentrated just posterior to the ventral sucker in the anterior mid-body region. In the posterior mid-body region, furrows had formed in the tegumental surface and, in these furrowed areas; the tegument was usually swollen between the spines. No obvious disruption was seen in the tail region, apart from tegumental swelling as that was evident in the posterior mid-body region.
After 24 h incubation with 30 µg/mL myrrh volatile oil, the disruption in the apical cone region in a large number of the specimens examined was extremely severe. The oral and ventral suckers showed broken tegument with neither spines nor sensory papillae could be observed (Figure 4a). Small areas of tegumental sloughing did occur around both oral and ventral suckers. Blebbing of tegumental surface was also present in all of the specimens. The severity of the blebbing varied from a carpet of blebs on the oral sucker (Figure 4b and 4c) to isolated patches of small blebs, between the spines, covering most of the apical cone region. Sever tegumental damage was apparent around the ventral sucker. At higher magnification, networks of fine fibrous or thread-like structures were present (Figure 4d). The apical tegumental membrane between the spines and also that covering the spines was severely swollen and a large number of spines were carpeted in blebs (Figure 4e and 4f). This was especially severe towards the lateral margins, where furrowing and sloughing small areas of the tegument had occurred (Figure 4g). In mid-body region, swelling of the tegument was apparent in the interspinal region and also swelling of the tegument on the backs of the spines (Figure 5a). This disruption was more severe in the posterior than the anterior mid-body region and some of the spines were split and took the form of opened jaws (Figure 5a). In the tail region, swelling and furrowing of the tegument was observed in several specimens. Another prominent feature of this treatment was the disruption to the upper surfaces of the spines, and took the form of a build up of ‘rubble’ (Figure 5b). However, some areas showed either severely swollen (Figure 5c) or completely damaged spines (Figure 5d).
Figure 4. SEMs of the anterior part of adult F. gigantica following 24 h incubation in 30 µg/mL myrrh volatile oil.
a: SEM of the apical cone region showing broken tegument of oral and ventral suckers with neither spines nor sensory papillae could be observed. OS: oral sucker, VS: ventral sucker, GP: gonopore. b, c: SEMs of the oral sucker showing small areas of erosion (E) and blebbing (head arrows) that varied from a carpet of blebs on the oral sucker to isolated patches of small blebs between the spines. OS: oral sucker. d: SEM of the ventral sucker showing severe tegumental damage (white arrow) and networks of fine fibrous or thread-like structures (black arrow). VS: ventral sucker. e: SEM of the apical tegument membrane showing severely swollen tegument, furrows (arrow) and blebs between the spines (head arrows). S: spine. f: High power SEM of the spine showing a carpet of blebs. g: High power SEM of the lateral margin showing furrows, small areas of tegumental sloughing (arrows) and severely swollen tegument that covering the spines and carpeting in blebs (inset). S: spine.
Figure 5. SEMs of the posterior part of adult F. gigantica following 24 h incubation in 30 µg/mL myrrh volatile oil.
a: SEM of the mid-body region showing tegumental swelling in the interspinal region. In this specimen, some of the spines were split and took the form of opened jaws (arrows). b, c, d: SEMs of the tail region revealing swelling and furrowing of the tegument, and disruption to the upper surfaces of the spines. Some areas showed either severely swollen (black arrow) or completely damaged (white arrow) spines.
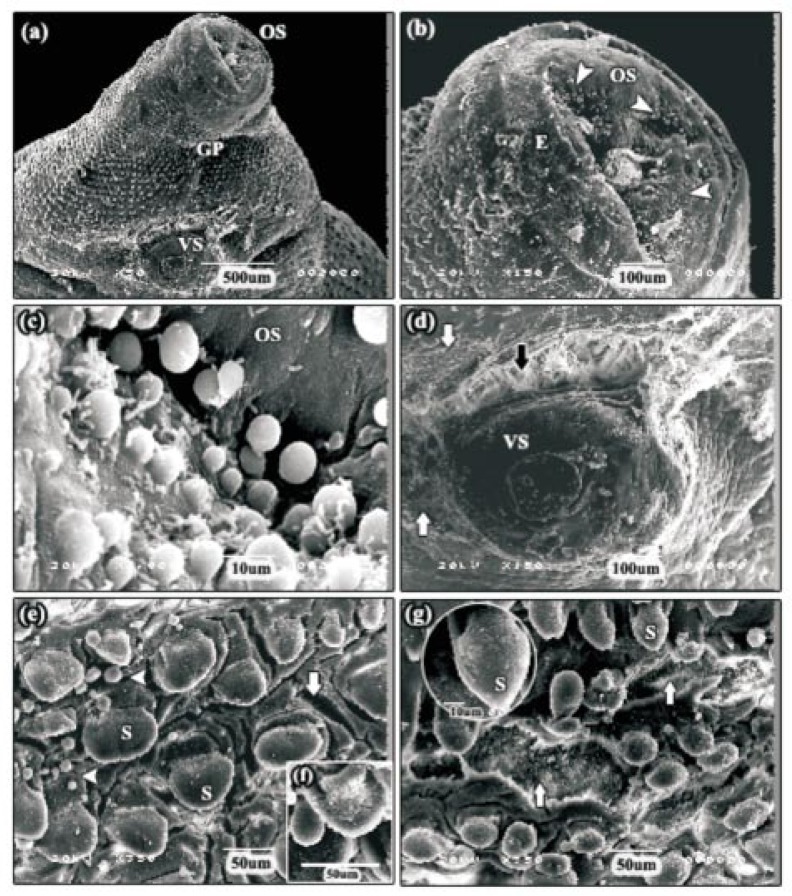
After 24 h incubation with 60 µg/mL myrrh volatile oil, tegumental disruption in the apical cone region became more pronounced and the ventral surface was more severely affected than the dorsal surface (Figure 6a). Tegumental disruption, in the form of a huge swelling was only evident on the ventral surface of the apical cone, between the gonopore and the ventral sucker (Figure 6a). In this region, patches of tegument had been completely removed, exposing the basal lamina. Immediately, beside the gonopore, small holes had begun to form which penetrated the basal lamina in areas where tegument had been removed (Figure 6b). Tegumental disruption was also seen in the lateral margins of the apical cone, where the tegument between and covering the spines was severely swollen and large areas were fissured and accompanied by blebbing (Figure 6c). These changes were most evident directly behind the ventral sucker in the anterior mid-body region of the fluke and the swelling had become so severe that the spines were barely visible (Figure 6d). Patches of tegumental sloughing were observed in the posterior mid-body region, revealing the underlying basal lamina and empty spine sockets (Figure 6e). In most of the specimens, mild furrowing of the tegumental syncytium was evident in the tail region. However, in some of the specimens, the tegument covering the spines was seen to be partially sloughed off (Figure 6f)
Figure 6. SEMs of adult F. gigantica following 24 h incubation in 60 µg/mL myrrh volatile oil.
a: SEM of the apical cone region showing more pronounced tegumental disruption, in the form of huge swelling (arrow) accompanied with erosions (E), on the ventral surface between the gonopore and the ventral sucker. OS: oral sucker, VS: ventral sucker, GP: gonopore. b: SEM of the tegument close to the gonopore showing small holes (arrow) which penetrated the basal lamina in areas where tegument had been removed. c: SEM of the lateral margin of the apical cone. In this specimen, the tegument was severely swollen and large areas were fissured and accompanied by blebbing (head arrows). S: spine. d: SEM of the anterior mid-body region, directly behind the ventral sucker. in this area, the tegument swelling had become so severe that the spines were barely visible. e: SEM of the posterior mid-body region showing patches of tegumental sloughing (L) and empty spine sockets (SS). f: SEM of the tail region showing mild furrowing of the tegumental syncytium. The tegument covering the spines was seen to be partially sloughed off (arrows).
3.4. Effects of TCBZ-SO (reference drug)
After 24 h incubation with 15 µg/mL TCBZ-SO, tegument disruption; in the apical cone region of almost all the specimens examined, was pronounced and the ventral surface was more severely affected than the dorsal one, as described previously by Shalaby et al[9]. Briefly, due to the swelling of the tegument around them, the spines did not protrude as clearly from the surface. Tegumental disruption, in the form of sloughing of the tegument and complete spine loss, was restricted on the apical cone around the ventral sucker. Disruption of the spines, in the form of distortion of the tips, was observed in some areas at lateral margins of the apical cone. Immediately behind the ventral sucker, in the anterior mid-body region, sever tegumental swelling was evident. In most of the specimens examined, the disruption continued down the centre of the body, where it still appeared to be severely swollen accompanied with spine loss and blebbing at the lateral margins of the flukes.
4. Discussion
This study is the first to demonstrate the comparative effects of Mirazid® oleoresin, myrrh volatile oil and the reference drug (TCBZ-SO) on the adult F. gigantica. Observations of the efficacy of Mirazid® oleoresin and myrrh volatile oil indicated that both products showed dose-dependent anthelmintic efficacy with the anterior half of the fluke consistently more severely affected than the posterior half. Scanning electron microscopy could be used to determine the target of the tested products, as morphological changes in tegumental surface could be observed. These changes consisted of swelling, blebbing, which was later disrupted, leading to erosion and desquamation of the tegument, resulting in the lesion, and finally the exposure and disruption of basal lamina and the dislodging of spines. Swelling and blebbing were characteristic of a stress response by the fluke; they had been seen in the present study with TCBZ-SO and in previous studies with other drugs (clorsulon, albendazole, closantel, nitroxynil, compound alpha, and artemether)[9],[10],[21]-[24]. Also, they were similar to changes on the surface of, a closely related trematode, Schistosoma mansoni treated with Mirazid® oleoresin[25].
The surface changes induced by Mirazid® oleoresin were less severe than those observed after exposure to either myrrh volatile oil or TCBZ-SO. Flukes showed swelling after these treatments, but its level and blebbing were much greater with myrrh volatile oil; in which patches of tegumental sloughing were observed in the apical cone and the posterior mid-body region of flukes. This was not observed after treatment with Mirazid® oleoresin; where no tegumental sloughing had occurred in any area of the flukes except that around the oral sucker. At the meantime, swelling of the tegument between and covering the spines was the major change seen, over the rest of the body surface.
According to the PDR for Herbal Medicines, 2nd Edition, some of the chief components in myrrh were sesquiterpenes. Sesquiterpenes were a large family of C15-isoprenoid molecules found in plants, microbes, and some marine organisms. Isoprenoids also called terpenoids, were unsaturated hydrocarbons found in essential oils and oleoresins of plants. When distilled from plants, these bitter constituents stimulated the glands and the liver, and had antibacterial, antifungal, anti-allergen, antispasmodic, and anti-inflammatory properties. The Department of Food Science at Rutgers University, New Brunswick, New Jersey, found that the gum exudates of Commiphora myrrha contained six sesquiterpenes including two new furanosesquiterpenes. One of the latter exhibited cytotoxic activity. Myrrh oil had one of the highest levels of sesquiterpenes[13]. In vitro physicochemical assays characterized these components as antioxidants[26]. They passed through the cell wall and cytoplasmic membrane, disrupted the structure of their different layers of polysaccharides, fatty acids and phospholipids and permeabilized them. Cytotoxicity appeared to include such membrane damage. This raised the obvious question how such antioxidants could be cytotoxic. They could provoke depolarization of the mitochondrial membranes by decreasing the membrane potential, affecting ionic Ca++ cycling[26] and other ionic channels and reducing the pH gradient, affecting the proton pump and the ATP pool. They changed the fluidity of membranes, which became abnormally permeable resulting in leakage of radicals, cytochrome C, calcium ions and proteins, as in the case of oxidative stress and bioenergetic failure. Permeabilization of outer and inner mitochondrial membranes led to cell death by apoptosis and necrosis[27],[28].
On the other hand, TCBZ-SO inhibited the movement of tegumental secretory bodies from the cell body to the apical surface of the tegument[32]. This might lead to the progressively severe damage visible externally, culminating in the loss of the tegument after 24 h treatment in vitro[29]. Inhibition of proteolytic enzyme secretion in F. hepatica by triclabendazole had also been attributed to disruption of microtubule-based secretory processes. Such changes were typical of microtubule inhibition and were similar to those induced by microtubule inhibitors, particularly tubulozole-C[30]-[32].
Most research effort into the effects of plants on parasite infections had been undertaken using aqueous or alcoholic extractions, yet purified plant essential oils could also be efficacious in treating or preventing parasitic diseases. Until now, because of their mode of action affecting several targets at the same time, generally, no particular resistance or adaptation to essential oils has been described. In this study, the comparatively more disruption, observed in myrrh volatile oil exposed specimens, compared to that exposed to Mirazid® oleoresin extract might suggest that the anthelmintic activity of Mirazid® oleoresin extract was attributed to its content of volatile oil. So, increasing the concentration of myrrh volatile oil in Mirazid® might possibly help to developing its anthelmintic activity.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.McKinstry B, Halferty L, Brennan GP, Fairweather I. Morphological response of triclabendazole-susceptible and triclabendazole-resistant isolates of Fasciola hepatica to treatment in vitro with nitroxynil (Trodax) Parasitol Res. 2009;104:645–655. doi: 10.1007/s00436-008-1241-8. [DOI] [PubMed] [Google Scholar]
- 2.El-Shazly AM, El-Beshbishi SN, Azab MS, El-Malky M, Abdeltawab AH, Morsy AT. Past and present situation of human fascioliasis in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2009;39:247–262. [PubMed] [Google Scholar]
- 3.Mas-Coma S, Valero MA, Bargues MD. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009;69:141–146. doi: 10.1016/S0065-308X(09)69002-3. [DOI] [PubMed] [Google Scholar]
- 4.Degheidy NS, Shalaby HA. Scanning electron microscopic observations of adult Fasciola gigantica after immunization with glutathione S-transferase in goats. Res J Parasitol. 2010;5:79–89. [Google Scholar]
- 5.Hussein AA, Khalifa RMA. Fascioliasis prevalences among animals and human in Upper Egypt. J King Saud Univ. 2010;22:15–19. [Google Scholar]
- 6.Esteban JG, Gonzalez C, Curtale F, Munoz-Antoli C, Valero MA, Bargues MD, et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am J Trop Med Hyg. 2003;69:429–437. [PubMed] [Google Scholar]
- 7.Saowakon N, Tansatit T, Wanichanon C, Chanakul W, Reutrakul V, Sobhon P. Fasciola gigantica: Anthelmintic effect of the aqueous extract of Artocarpus lakoocha. Exp Parasitol. 2009;122:289–298. doi: 10.1016/j.exppara.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Keiser J, Morson G. Fasciola hepatica: tegumental alterations in adult flukes following in vitro and in vivo administration of artesunate and artemether. Exp Parasitol. 2008;118:228–237. doi: 10.1016/j.exppara.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Shalaby HA, El Namaky AH, Kamel ROA. In vitro effect of artemether and triclabendazole on adult Fasciola gigantica. Vet Parasitol. 2009;160:76–82. doi: 10.1016/j.vetpar.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 10.McConville M, Brennan GP, Flanagan A, Hanna REB, Edgar HWJ, Castillo R, et al. Surface changes in adult Fasciola hepatica following treatment in vivo with the experimental fasciolicide, compound alpha. Parasitol Res. 2009;105:757–767. doi: 10.1007/s00436-009-1453-6. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani RA, Loutfy N, Hassan A. Myrrh and trematodoses in Egypt: An overview of safety, efficacy and effectiveness profiles. Parasitol Int. 2009;58:210–214. doi: 10.1016/j.parint.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Massoud A, El Sisi S, Salama O, Massoud A. Preliminary study of therapeutic efficacy of a new fasciolicidal drug derived from Commiphora molmol (myrrh) Am J Trop Med Hyg. 2001;65:96–99. doi: 10.4269/ajtmh.2001.65.96. [DOI] [PubMed] [Google Scholar]
- 13.Hanuš LO, Řezanka TS, Dembitsky VM, Moussaieff A. Myrrh-Commiphora chemistry. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:3–27. doi: 10.5507/bp.2005.001. [DOI] [PubMed] [Google Scholar]
- 14.Ford RA, Api MA, Letizia CS. Monographs on fragrance raw materials. Food Chem Toxicol. 1992;30:1S–138S. [PubMed] [Google Scholar]
- 15.Mahmoud MS, Dobal SA, Soliman K. Immune response in Fasciola gigantica experimentally infected rabbits treated with either carnosine or Mirazid®. Res J Parasitol. 2008;3:40–49. [Google Scholar]
- 16.Soliman OE, El-Arman M, Abdul-Samie ER, El-Nemr HI, Massoud A. Evaluation of myrrh (Mirazid) therapy in fascioliasis and intestinal schistosomiasis in children: immunological and parasitological study. J Egypt Soc Parasitol. 2004;34:941–966. [PubMed] [Google Scholar]
- 17.Abo-Madyan AA, Morsy TA, Motawea SM, Morsy AT. Clinical trial of Mirazid in treatment of human fascioliasis, Ezbet El-Bakly (Tamyia Center) Al-Fayoum Governorate. J Egypt Soc Parasitol. 2004;34:807–818. [PubMed] [Google Scholar]
- 18.Soliman MI, Taha HA. Tegumental alterations of Fasciola gigantica due to in vitro treatment with Ro-354. Trop Biomed. 2011;28:283–292. [PubMed] [Google Scholar]
- 19.Hegazi AG, Abd el-Hady FK, Shalaby HA. An in-vitro effect of propolis on adult worms of Fasciola gigantica. Vet Parasitol. 2007;144:279–286. doi: 10.1016/j.vetpar.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Toner E, Mcconvery F, Brennan GP, Meaney M, Fairweather I. A scanning electron microscope study on the route of entry of triclabendazole into the liver fluke, Fasciola hepatica. Parasitology. 2009;136:523–535. doi: 10.1017/S0031182009005642. [DOI] [PubMed] [Google Scholar]
- 21.Meaney M, Fairweather I, Brennan GP, McDowell LSL, Forbes AB. Fasciola hepatica: effects of the fasciolicide clorsulon in vitro and in vivo on the tegumental surface, and a comparison of the effects on young- and old-mature flukes. Parasitol Res. 2003;91:238–250. doi: 10.1007/s00436-003-0863-0. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan JF, Fairweather I, Brennan GP, Trudgett A, Hoey EM. Fasciola hepatica: surface and internal tegumental changes induced by treatment in vitro with the sulphoxide metabolite of albendazole (“Valbazen”) Parasitology. 2003;126:141–153. doi: 10.1017/s0031182002002664. [DOI] [PubMed] [Google Scholar]
- 23.McKinstry B, Fairweather I, Brennan GP, Forbes AB. Fasciola hepatica: tegumental surface alterations following treatment in vivo and in vitro with nitroxynil (Trodax) Parasitol Res. 2003;91:251–263. doi: 10.1007/s00436-003-0930-6. [DOI] [PubMed] [Google Scholar]
- 24.McConville M, Brennan GP, Flanagan A, Edgar HWJ, Hanna REB, McCoy M, et al. An evaluation of the efficacy of compound alpha and triclabendazole against two isolates of Fasciola hepatica. Vet Parasitol. 2009;162:75–88. doi: 10.1016/j.vetpar.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Hassan M, El-Motaiem M, Afify H, Abaza B, El-Shafei M, Massoud AM. In vitro effect of Mirazid on Schistosoma mansoni worms. J Egypt Soc Parasitol. 2003;33:999–1008. [PubMed] [Google Scholar]
- 26.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils- A review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 27.Yoon HS, Moon SC, Kim ND, Park BS, Jeong MH, Yoo YH. Genistein induces apoptosis of RPE-J cells by opening mitochondrial PTP. Biochem Biophys Res Commun. 2000;276:151–156. doi: 10.1006/bbrc.2000.3445. [DOI] [PubMed] [Google Scholar]
- 28.Rana IS, Rana AS. Efficacy of essential oils of aromatic plants as larvicide for the management of filarial vector Culex quinquefasciatus Say (Diptera: Culicidae) with special reference to Foeniculum vulgare. Asian Pac J Trop Dis. 2012;2(3):184–189. [Google Scholar]
- 29.Ravikumar S, Inbaneson SJ, Suganthi P. In vitro antiplasmodial activity of ethanolic extracts of South Indian medicinal plants against Plasmodium falciparum. Asian Pac J Trop Dis. 2012;2(3):180–183. doi: 10.1016/S2221-1691(12)60057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijayakumar A, Duraipandiyan V, Jeyaraj B, Agastian P, Karunai Raj M, Ignacimuthu S. Phytochemical analysis and in vitro antimicrobial activity of Illicium griffithii Hook. f. & Thoms extracts. Asian Pac J Trop Dis. 2012;2(3):190–199. [Google Scholar]
- 31.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays. 2006;28:253–260. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 32.Halferty L, Brennan GP, Trudgett A, Hoey L, Fairweather I. Relative activity of triclabendazole metabolites against the liver fluke, Fasciola hepatica. Vet Parasitol. 2009;159:126–138. doi: 10.1016/j.vetpar.2008.10.007. [DOI] [PubMed] [Google Scholar]