Abstract
Objective
To study the antioxidant efficacy of Commiphora mukul (C. mukul) gum resin ethanolic extract in streptozotocin (STZ) induced diabetic rats.
Methods
The male Wistar albino rats were randomly divided into four groups of eight animals each: Control group (C), CM-treated control group (C+CMEE), Diabetic control group (D), CM- treated diabetic group (D+CMEE). Diabetes was induced by intraperitoneal injection of STZ (55 mg/kg/ bwt). After being confirmed the diabetic rats were treated with C. mukul gum resin ethanolic extract (CMEE) for 60 days. The biochemical estimations like antioxidant, oxidative stress marker enzymes and hepatic marker enzymes of tissues were performed.
Results
The diabetic rats showed increased level of enzymatic activities aspartate aminotransaminase (AST), alanine aminotransaminase (ALT) in liver and kidney and oxidative markers like lipid peroxidation (LPO) and protein oxidation (PO) in pancreas and heart. Antioxidant enzyme activities were significantly decreased in the pancreas and heart compared to control group. Administration of CMEE (200 mg/kg bw) to diabetic rats for 60 days significantly reversed the above parameters towards normalcy.
Conclusions
In conclusion, our data indicate the preventive role of C. mukul against STZ-induced diabetic oxidative stress; hence this plant could be used as an adjuvant therapy for the prevention and/or management of diabetes and aggravated antioxidant status.
Keywords: Commiphora mukul, Antioxidants, Lipid peroxidation, Streptozotocin
1. Introduction
Diabetes mellitus, a life threatening as well as life style modifying metabolic disorder, is manifested mainly by hyperglycemia, which is due to defect in insulin secretion, function and or both. Hyperglycemia leads to several acute and long-term complications if it persists for longer time. Diabetes mellitus exhibits wide geographic variation in its incidence and prevalence. According to the World Health Organization, approximately 171 million people worldwide are affected by diabetes mellitus. It has been assumed that 57.2 million Indian will be affected by diabetes mellitus by the year 2025, mainly due to a vast growth in population[1]. It is well documented that chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction and eventually the failure of organs especially the eyes, kidneys, nerves, heart and blood vessels[2].
Streptozotocin (STZ) induces diabetes mellitus by destroying pancreatic β-cells, possibly through generating excess reactive oxygen species (ROS). STZ generated lipid peroxidation and DNA breaks in pancreatic islets cells have been demonstrated[3]. Exaggerated production of these reactive species in diabetes can lead to very serious problems including cardiovascular disease, liver and kidneys failure, blindness, and nerve injure[4]. Thus antioxidant therapy is one of the strategies for diabetes treatment. Many herbal extracts or derivatives with high antioxidant activity are useful for treatment of diabetes and other metabolic syndrome[5]. Over production of ROS and insufficient antioxidant defense mechanism were well documented in diabetic patients as well as in experimental diabetes mellitus[6],[7].
Several plant extracts are known to have antidiabetic properties and a large number of compounds from plant extracts have been reported to have beneficial effects for treatment of diabetes mellitus[8]. Diabetes can be managed by diet, exercise, and chemotherapy. However, the pharmacological drugs are either too expensive or have undesirable side effects or contraindications[9]. Throughout the world, many traditional plant treatments for diabetes exist, and therein lies a hidden wealth of potentially useful natural products for the control of diabetes[10]. Natural plant drugs are frequently considered to be less toxic and freer from side effects than synthetic ones[11].
Commiphora mukul (C. mukul) (synonym Commiphora whighitti) (family: Burseraceae) commonly called as gum guggulu is highly valued in Ayurveda an Indian system of medicine practiced in India, Bangladesh and Pakistan. The gum resin extract of C. mukul tree has been used in Ayurvedic medicine for more than 2 000 years to treat a variety of ailments like obesity, lipid disorders, rheumatoid arthritis, diabetes[12], bone fracture, cardiovascular disorder disease[13]. Traditional (India) uses of C. mukul are for its anti-inflammatory, antispasmodic, carminative, emmenagogue, hypoglycemic, alternative, antiseptic, and astringent, a thyroid stimulant, anthelminitic and antihyperlipidemia properties. It is an important herb used in the treatment of several degenerative disorders in modern medicine too and established as a hypolipidemic drug[14]. Ayurvedic medicines containing gum guggul often contain guggul in their names, such as Shunthi-guggul, and Yogaraja guggul. All the formations used for traditional Ayurvedic treatments for obesity contained gum guggul among its herbal ingredients. Guggulipid, an ethyl acetate extract of the resin of plant C. mukul is an established hypolilpidemic agent. It is used extensively for the treatment of different ailments including dysmenorrhea, endometritis, hypercholesteremia, hypertension, bronchitis, inflammation, arthritis, cancer and cardiovascular disorders[15].
The FXR antagonism by guggulsterone has been proposed as a mechanism for its hypolipidemic effect. Guggulsterones inhibited cholesterol synthesis in the liver via antagonism of the forsenoid X receptor and the bile acid receptor[16]. Various studies have been conducted to understand and illustrate the mechanism of action and potential of guggulsterone as a therapeutic agent using synthetic E and Z isomers[17]. A number of clinical trials have been conducted to evaluate the hypolipidemic effect of guggulipid. With proven hypolipidemic efficacy in rats, guggulsterone was used as a positive control to assess the hypolipidemic activity of other chemical compounds[18]. In more recent studies, the cardioprotective activity of guggulsterone was compared with that of a hypolipidemic drug, gemfibrozil. In a recent study, C. mukul and guggulsterone were effective antioxidants against LDL oxidation[19]. Hepatic microsomal lipid peroxidation was also significantly reduced by guggulipid. Very recently Ojha et al have reported the effect of C. mukul extract on cardiac dysfunction and ventricular function in isoprotereno induced myocardial infection[20]. This finding indicated that guggulsterone may be of therapeutic benefit in diseases associated with oxidative stress, such as myocardial ischemia and neurodegenerative diseases. The cardiovascular benefits of the therapy are derived from the multiple pharmacological activities associated with guggul or guggulsterone, notably its hypolipidemic, antioxidant, and anti-inflammatory activities. In addition, our earlier studies proved antihyperglycemic, hypolipidemic and antioxidant activities in fructose fed rats [21].
2. Materials and methods
2.1. Chemicals
Thiobarbituric acids, pyrogallol, STZ were obtained from the Sigma Chemical Co., St. Louis, MO, USA. All other chemicals and solvents were of analytical grade and procured from Sisco Research Laboratories (P) Ltd., Mumbai, India.
2.2. Plant material
Ethanolic extract of C. mukul gum resin (CMEE; brown, dry powder with Lot No. L5111031) was obtained from the manufacturers and exporters of herbal extracts, Ms Plantex Pvt. Ltd., Vijayawada, Andhra Pradesh, India. Procedure followed by the firm for the preparation of extract is as follows: the plant was identified by Dr. K Narasimha Reddy, Taxonomist, Lailaimpex R&D Center, Vijayawada. The collected plant sample (resin) was washed thoroughly with tap water, dried at room temperature away from sun light, cut into small pieces, and then powdered. Ethanolic extract was prepared by cold maceration of gum resin powder in ethanol for 7 days. The extract was filtered, concentrated under reduced pressure and finally dried in vacuum desiccators. Herb-to-product ratio was 8:1. A voucher specimen has been deposited in the Department of Biochemistry, Sri Krishnadevaraya University, Anantapur, under number DSK-CM-09. The extract was stored at (0-4 °C) and dissolved in water just before use.
2.3. Animals
Male Wistar rats weighing (160-190 g) were procured from Sri Venkateswara Enter prises (Bangalore, India), acclimatized for 7 days in our animal house (Regd. no. 470/01/a/ CPCSEA) before dietary manipulation. Animals were housed two per cage in an air-conditioned room (22±2 °C) with 12 h light/dark cycle and had free access to standard pellet diet and water. All the procedures were per formed in accordance with the Institutional Animal Ethics Committee.
2.4. Induction of diabetes
Diabetes was induced in overnight fasted adult Wistar albino rats weighing (160-190 g) by single intra peritoneal injection of 55 mg/kg of STZ dissolved in citrate buffer (pH 4.5). Hyperglycemia was confirmed by elevated glucose levels in plasma, determined at 72 h and then on day 7 after injection. The threshold value of fasting plasma glucose to diagnose diabetes was taken as >126 mg/dL. Only rats found with permanent noninsulin dependant diabetes mellitus (NIDDM) (except for control and control treatment) were selected for the antioxidant and liver marker enzymes study.
2.5. Experimental design
In the present experiment, a total of 32 rats (16 diabetic rats; 16 normal rats) were used. The rats were divided into four groups of 8 each: control (CON); control rats treated with C. mukul ethanolic extracts (CON+CMEE); diabetic (D); and diabetic rats treated with C. mukul ethanolic extracts (D+CMEE). Diabetic treated group and control treated group received an ethanol extract of the gum resin of C. mukul (2 ml of 5% Tween-80) by orogastric tube at a dose of 200 mg/kg bw for 60 days, whereas 2 mL of distilled water with 5% Tween-80 was administered to control and diabetic control rats. Based on preliminary experiment on dose-dependent antihyperglycemic effect of CMEE, a dose less than 200 mg/kg bw was not expected to be effective in rats[12]. At the end of experimental period of 60 days, animals were sacrificed by cervical decapitation. Pancreas, heart, liver and kidney were collected.
2.6. Oxidative stress markers and antioxidant enzymes
The concentration of lipid peroxidation intermediates, pancreas and heart tissues thiobarbituric acid reactive substances (TBARS) were measured following the method of Utley et al[22], using 10% pancreas and heart homogenate in 0.15 M KCl and expressed as nmol MDA formed/15 min/mg protein. The extent of protein oxidation Levine et al.[23] and reduced glutathione (GSH) levels in liver were determined[24]. Protein content in the liver homogenate was measured by the method of Lowry et al.[25].
Ten percent pancreas and heart homogenate was prepared in ice-cold 0.15 M KCl, centrifuged at 12 000 rpm for 45 min in Sigma Laboratory centrifuge 3K 18 models, rotor no. 12150. The clear supernatant thus obtained was used for the assay of superoxide dismutase (SOD; E.C.1.15.1.1); Soon and Tan[26], catalase (CAT; E.C.1.11.1.6); Beers and Sizer[27], glutathione peroxidase (GPX; E.C.1.15.1.9); Rotsruck et al[28], glutathione-S-transferase (GST; E.C.2.5.1.14); Habig et al.[29] and glutathione reductase (GR; E.C.1.8.1.7); Pinto and Bartley[30].
2.7. Evaluation of aspartate transaminase (AST) and alanine transaminase (ALT)
The frozen liver and kidney tissues were thawed, and 10% tissue homogenate was prepared in ice cold 0.1 M Tris-HCl buffer, pH 7.4 and centrifuged at 12 000 rpm for 45 min. Transaminases of cytosolic fraction were assayed.
Pyruvate gives a brown coloured compound with 2, 4-dinitrophenyl hydrazine (DNPH) which is measured colorimetrically at 520 nm[31].
To 1.0 mL of GOT buffered substrate (0.19 M of DL-aspartic acid, 0.02 M of α- ketoglutarate in 0.1 M disodium and monopotassium phosphate buffer, pH 7.4) or GPT buffered substrate (0.202 M of L-alanine and 0.02 M of α-ketoglutarate in 0.1 M disodium and monopotassium phosphate buffer, pH 7.4), 0.2 mL of enzyme source was added and incubated at 37 °C for 60 min. The reaction was arrested by the addition of 1.0 mL of 1 mM 2,4-DNPH in 1.1 N HCl. After 20 min, 10 mL of 0.4 N NaOH was added and left at room temperature for another 10 min. A series of pyruvate standards (10-50 µg) were also treated in a similar manner. The reddish brown colour developed was read at 520 nm against the reagent blank. The enzyme activities are expressed as µg of pyruvate liberated /min/mg protein.
2.8. Statistical analysis
The results were expressed as means±SEM. Data were analyzed for significant difference using Duncan's Multiple Range (DMR) test (P <0.05)[32].
3. Results
3.1. Pancreas and heart oxidative stress markers and antioxidants
Table 1, Figure 1, 2 and 3 summarizes the levels of MDA, GSH, protein carbonyl groups and activities of enzymatic antioxidants i.e. SOD, CAT, GST, GPx and GR in the pancreas and heart of control and experimental animals.
Table 1. Effect of CMEE on pancreas and heart oxidative stress markers and antioxidants (mean±SEM) (n=8).
| Parameter | Tissue | CON | CON+CMEE | D | D+CMEE |
| SOD (A) | Pancreas | 27.830±2.040a | 30.410±0.784a | 18.690±5.290c | 29.930±2.070a |
| Heart | 30.840±3.290a | 32.080±2.730a | 22.140±1.760b | 29.020±0.910a | |
| CAT (B) | Pancreas | 15.870±0.620a | 17.100±0.410b | 11.230±0.500c | 16.140±0.660a |
| Heart | 26.390±0.970a | 26.560±1.630a | 20.910±0.610c | 25.720±1.200a | |
| GST (C) | Pancreas | 0.042±0.001a | 0.046±0.001a | 0.038±0.002c | 0.042±0.001a |
| Heart | 0.050±0.003a | 0.051±0.002a | 0.040±0.002b | 0.050±0.002a | |
| GPX (D) | Pancreas | 4.980±0.290a | 5.010±0.100a | 4.020±0.150b | 5.040±0.150a |
| Heart | 6.020±0.250a | 6.130±0.190a | 5.100±0.110c | 6.130±0.230a | |
| GR (E) | Pancreas | 33.720±1.890a | 34.600±0.750a | 29.700±0.810b | 34.100±0.630a |
| Heart | 27.000±0.400a | 26.920±0.640a | 22.880±0.520c | 26.780±0.720a |
A: Units/mg protein; B: µmol of H2O2 consumed/min/mg protein; C: mmol of CDNB-GSH conjugate formed/min/mg protein; D: µg of GSH consumed/min mg protein; E: µmol of NADPH oxidized/min/mg protein. Values with different superscripts within the row are significantly different at P<0.05 (Duncan's multiple range test).
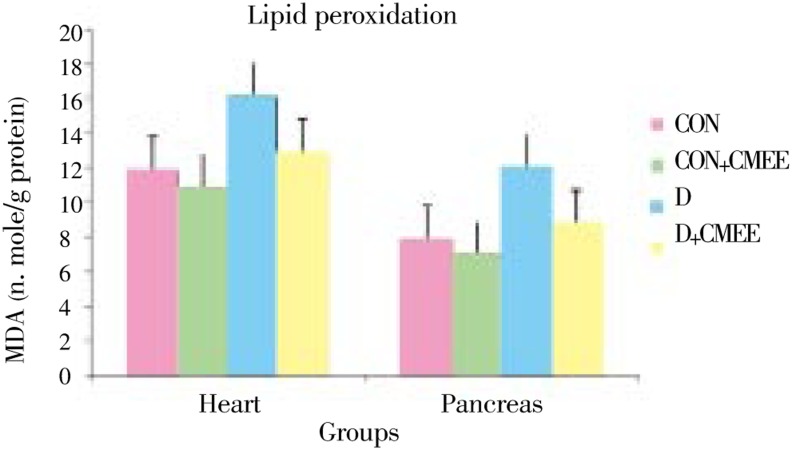
Figure 1. Change in Malondialdehyde (MDA) level in pancreas and heart tissues of diabetic rats after 60 days of treatment with C. mukul gum resin ethanolic extract. Data are the Mean (Value n=8) with ± SE Data are significant at P<0.05 compared to diabetic control.
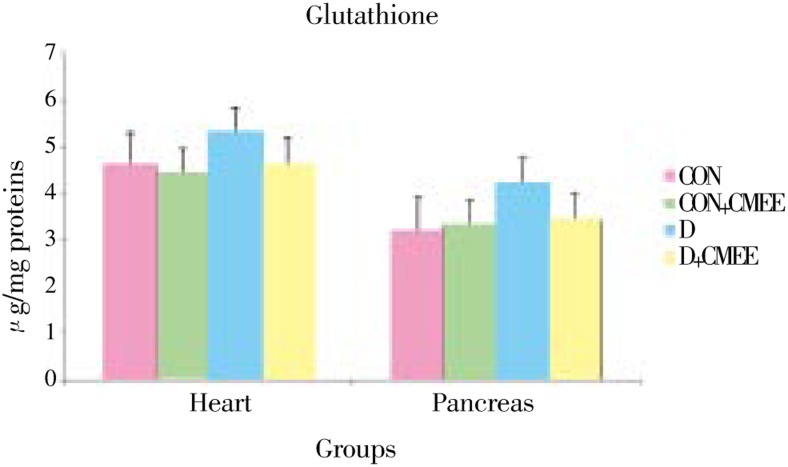
Figure 2. Change in reduced glutathione (GSH) level in pancreas and heart tissues of diabetic rats after 60 days of treatment with C. mukul gum resin ethanolic extract. Data are the Mean (Value n=8) with ± S.E. Data are significant at P<0.05 compared to diabetic control.
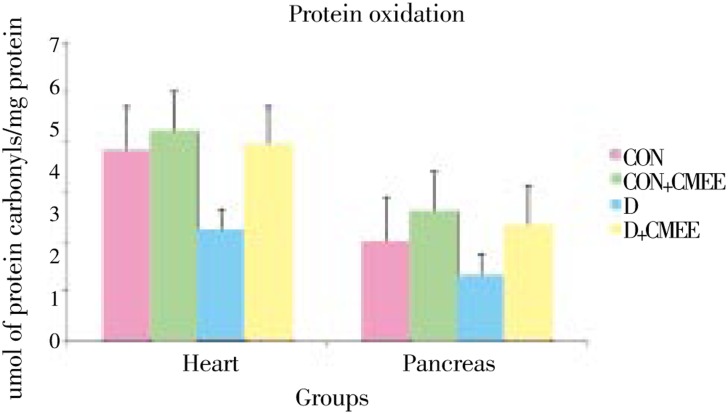
Figure 3. Change in protein oxidation (PO) level in pancreas and heart tissues of diabetic rats after 60 days of treatment with C. mukul gum resin ethanolic extract. Data are the Mean (Value n=8) with ±SE Data are significant at P<0.05 compared to diabetic control.
Group-D showed significantly higher levels of TBARS (52.5, 35.5%) and protein carbonyl groups (28.1, 15.2%) as compared to group-CON rats. Group-D+CMEE showed significantly lower TBARS (26.8, 20.0%) and protein carbonyl groups (17.0, 13.2%) when compared with group-D. Group-D showed depleted pancreas and heart GSH levels (53.8% and 42.1%) as compared to control, but treatment with CMEE limited the depletion to only 76.9% and 77.2%. Group-CON+CMEE showed 33.0% and 10.5% increase in GSH levels when compared to group-CON rats.
The activities of pancreas and heart enzymatic antioxidants SOD, CAT, GST, GPx and GR were significantly lower (47.8%, 38.6%, 21.4%, 26.5%, 21.9% and 33.4%, 32.6%, 26.5%, 25.0%, 27.4%, respectively) in group-D rats than in group-CON rats. In group-D+CMEE, the activities were significantly higher (80.6%, 60.8%, 30.3%, 33.3%, 23.0% and 39.5%, 30.5%, 30.5%, 31.1%, 47.4%, respectively) as compared to group-D.
3.2. Biochemical assay of AST, ALT activities from hepatic and renal tissues
STZ induced diabetes resulted in an elevation of AST and ALT activities in liver and kidney at significant level when compared to control. After the treatment of C. mukul gum resin ethanolic extract, both parameters in liver as well as in kidney were in the levels of control group although the ethanolic extract was able to protect these parameters partially (Table 2).
Table 2. Effect of C. mukul treatment on tissue transaminases in STZ induced diabetic rats (mean±SEM) (n=8).
| Parameters | Tissue | CON | CON+CMEE | D | D+CMEE |
| ALT(A) | Liver | 1.330±0.098a | 1.300±0.025a | 2.050±0.062b | 1.470±0.035a |
| Kidney | 0.240±0.002a | 0.230±0.002a | 0.420±0.060b | 0.250±0.004a | |
| AST(B) | Liver | 0.830±0.016a | 0.810±0.010a | 1.310±0.016b | 0.830±0.033a |
| Kidney | 0.450±0.012a | 0.440±0.010a | 0.760±0.014b | 0.430±0.018a |
A: µg of pyruvate formed/min/mg protein; B: µg of pyruvate formed/min/mg protein. Values with different superscripts within the row are significantly different at P<0.05 (Duncan's multiple range test).
4. Discussion
In the present study, among the tissues in which oxidative stress studies were conducted pancreas and heart tissues have shown more prominent oxidative stress under insulin deficient conditions, respectively. The oxidative stress can be marked by LPO, PO, GSH, SOD, GST, GR, GPx and CAT levels. Oxidative stress results in increased LPO which plays an important role in several pathologies like atherosclerosis, diabetes, wound healing, liver disorder, inflammation, etc[33].
Both enzymatic and non-enzymatic antioxidant defense systems were impaired in STZ induced diabetic rats[34]. Lowered activities of enzymatic antioxidants and reduced levels of non-enzymatic antioxidants observed in plasma and liver of STZ induced diabetic rats suggest that these antioxidants are exhausted to combat the deleterious effects of increased oxidative stress. Oral administration of CMEE to diabetic rats significantly improved the antioxidant defense mechanism, which suggests its role in the protection of vital tissues from oxidative damage during diabetic condition.
Profound studies have shown enhanced oxidative stress in both experimental and human diabetes mellitus[35],[36]. Increased levels of liver TBARS has been documented well in diabetic rats[12]. Our results corroborate these observations. Increased pancreas and heart TBARS noticed in diabetic rats could be due to over production and diffusion of lipid peroxidation by products from damaged pancreatic and liver tissues with subsequent leakage into plasma. Oral administrations of CMEE brought back the levels of pancreas and heart TBARS to normal range in diabetic rats, which indicates its free radical scavenging property. The free radical scavenging property of CMEE indicates that C. mukul gum resin ethanol extract may contain potent antioxidant principles.
Recent studies have clearly demonstrated the importance of medicinal plants in the treatment of experimental diabetes, where oxidative stress induced apoptosis of β-cells[37]. Oral administration of CMEE showed antioxidant effects against STZ-induced diabetes in rats. The extract significantly lowered the levels of hepatic enzymes and TBARS and significantly increased the levels of GSH, SOD and CAT[38],[39].
The activities of GPx, GST and GR were observed to decrease significantly in diabetic rats. Glutathione peroxidase, an enzyme with selenium, and GST catalyse the reduction of hydrogen peroxide and hydroperoxides to non-toxic products. The depletion in the activity of these enzymes may result in deleterious oxidative changes due to the accumulation of toxic products. In this context, other workers also reported a decrease in the activities of these antioxidant enzymes (SOD, CAT, GPx, GR and GST) in the liver of diabetic rats[12]. As the alterations produced in the antioxidant activities indicate the involvement of deleterious oxidative changes, increased activities of the components of this defense system would therefore be important in protection against radical damage. Administration of CMEE increased the activities of GPx, GR and GST in the pancreas and heart of diabetic rats.
C. mukul supplementation prevented the depletion in tissue GR activity in D + CMEE-group by maintaining the normal levels of this enzyme in these animals. Enhanced GR activity in D + CMEE group when compared to D-group, respectively reveals the protective effect of C. mukul against oxidative damage by keeping normal GSH levels in tissues in STZ diabetic conditions which is further reflected by enhanced activities of GPx and GST in C. mukul treated insulin deficient animal models.
The liver is regarded as the central metabolic organ in the body, with an important role in glucose and lipid homeostasis[40]. AST and ALT are considered as liver toxicity markers[41]. The increase in the activities of liver and kidney AST and ALT indicated that diabetes may induce hepatic dysfunction. Therefore, the increment of the activities of AST, ALT, in liver and kidney may be mainly due to infective utilization of glucose in insulin deficient state which lead to enhanced breakdown of protein thereby enhancing amino acid catabolism and thus providing substrates for gluconeogenesis. In addition, enhanced non-enzymatic glycation of protein under hyperglycemic conditions may decrease the half life of proteins, thus contributing to the enhanced protein degradation[42]. The potentially toxic nitrogen of amino acids is eliminated via transamination, deamination and urea formation; the carbon skeletons are generally conserved as carbohydrate, via gluconeogenesis or as fatty acid via fatty acid synthesis pathways. Treatment of the diabetic rats with ethanolic extract caused reduction in the activity of these enzymes in liver and kidney compared to the diabetic untreated group and consequently alleviated liver damage caused by STZ-induced diabetes.
There is an improvement noticed in the levels of AST and ALT are as a consequence of improvement in the carbohydrate, fat and protein metabolism due to the therapy of ethanolic extract of C. mukulgum resin. The restoration of AST and ALT to their normal levels may be due to the presence of flavonoids in the ethanolic C. mukul extract, which are reported to be hepatoprotective agents[43].
Higher ALT concentrations were cross-sectionally associated with obesity and whole-body and hepatic insulin resistance and prospectively associated with a decline in hepatic insulin sensitivity and the development of type-2 diabetes[44]. According to Barbora et al.[44], high ALT is a marker of risk for type-2 diabetes and suggests a potential role of the liver in the pathogenesis of type-2 diabetes. Earlier literature[45] is in accordance with our observations of enhanced transaminases activity under insulin deficient condition (STZ diabetic condition).
The plant extract has no metabolic toxicity induction which has been studied here by AST and ALT activities in liver and kidney as these are sensitive biosensors in this purpose[46]. So, on the basis of aforementioned results it may be concluded that ethanolic extract of C. mukul has a promising antidiabetic effect in correlation with antioxidant activity without any toxicity induction.
In conclusion, the present investigation shows that CMEE possesses an antioxidant activity that may contribute to its protective action on lipid peroxidation and to enhancing its effect on cellular antioxidant defense. This activity contributes to the protection against oxidative damage in streptozotocin induced diabetes.
Acknowledgments
The authors are very much thankful to Sri Venkateswara University for financial support (BC-408) and to the Department of Biochemistry, Sri Krishnadevaraya University, Professor K Lakshmi devi, Professor NC Varadacharyulu and Professor C Suresh Kumar faculty members for providing lab facilities.
Footnotes
Foundation Project: This work is financially supported by Sri Venkateswara University (Grant No. BC-408).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Singh RB, Singh NK, Vajpeyee S. Prevalence and prevention of hypertension, diabetes mellitus and coronary artery disease in India: a scientific statement of the Indian society of hypertension, international college of nutrition and international college of cardiology. In: Singh NK, editor. Current trends in hypertension, diabetes and coronary artery diseases. Varanasi: BHU Press; 2008. pp. 186–198. [Google Scholar]
- 2.Susheela T, Balaravi P, Theophilus J, Reddy TN, Reddy PUM. Evaluation of hypoglycemic and antidiabetic effect of Melia dubia CAV fruits in mice. Curr Sci. 2008;94:1191–1195. [Google Scholar]
- 3.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;5:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 4.Neyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 biabetes. Metabolism. 2011;60:1–23. doi: 10.1016/j.metabol.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samad A, Shams MS, Ullah Z, Wais M, Nazish I, Sultana Y, et al. Status of herbal medicines in the treatment of diabetes. Curr Diabetes Rev. 2009;5:102–111. doi: 10.2174/157339909788166837. [DOI] [PubMed] [Google Scholar]
- 6.Kedziora-Kornatowska K, Szewczyk-Golec K, Kozakiewicz M, Pawluk H, Czuczejko J, Kornatowski T, et al. Melatonin improves oxidative stress parameters measured in the blood of elderly type 2 diabetic patients. J Pineal Res. 2009;46:333–337. doi: 10.1111/j.1600-079X.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 7.Pavana P, Sethupathy S, Manoharan S. Antihyperglycemic and antilipidperoxidative effects of Tephrosia purpurea seed extract in streptozotocin induced diabetic rats. Indian J Clin Biochem. 2007;22:77–83. doi: 10.1007/BF02912886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramesh Babu K, Rajasekhar MD, Sameena Fatima SK, Kumar EGTV, Swapna S, Ramesh B, et al. Antihyperglycemic and antihyperlipidemic activities of methanol: water (4:1) fraction isolated from aqueous extract of Syzygium alternifolium seeds in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48:1078–1084. doi: 10.1016/j.fct.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Sunil C, Latha PG, Suja SR, Shine VJ, Shyamal S, Anuja GI, et al. Effect of ethanolic extract of Pisonia alba Span. leaves on blood glucose levels and histological changes in tissues of alloxaninduced diabetic rats. Int J Appl Res Nat Prod. 2009;2:4–11. [Google Scholar]
- 10.Maiti A, Dewanjee S, Jana G, Mandal SC. Hypoglycemic effect of Swietenia macrophylla seeds against type II diabetes. Int J Green Pharm. 2008;2:224–227. [Google Scholar]
- 11.Sunil C, Latha G, Mohanraj KP, Kalichelvan V, Agastian P. α -Glucosidase inhibitory and antidiabetic activities of ethanolic extract of Pisonia alba Span. leaves. Int J Integr Biol. 2009;6:41–45. [Google Scholar]
- 12.Bellamkonda R, Rasineni K, Singareddy SR, Kasetti RB, Pasurla R, Chippada AR, et al. Antihyperglycemic and antioxidant activities of alcoholic extract of Commiphora mukul gum resin in STZ induced diabetic rats. Pathophysiology. 2011;18:255–261. doi: 10.1016/j.pathophys.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Ojha S, Bhatia J, Arora S, Golechha M, Kumari S, Arya DS. Cardioprotective effects of Commiphora mukul against isoprenaline induced cardiotoxicity: A biochemical and histopathological evaluation. J Environ Biol. 2011;32:731–738. [PubMed] [Google Scholar]
- 14.Ulbricht C, Basch E, Szapary P, Hammerness P, Axentsv S, Boon H, et al. Guggul for hypolipidemia: a review by the Natural Standard Research Collaboration. Complement Ther Med. 2005;13:279–290. doi: 10.1016/j.ctim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. The guggul for chronic diseases: Ancient medicine, modern targets. Anticancer Res. 2008;28:3647–3664. [PubMed] [Google Scholar]
- 16.Deng R, Yang D, Redke A, Yang J, Yan B. The hypolipedimic agent guggulsterone regulate the expression of human bile salt export pum: Dominance of trans activation over farsenoid X receptor mediated antagonism. J Pharmacol Exp Ther. 2007;320:1153–1162. doi: 10.1124/jpet.106.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kB ligand and by tumor cells by suppressing nuclear factor-kB activation. Clin Cancer Res. 2006;12:662–668. doi: 10.1158/1078-0432.CCR-05-1749. [DOI] [PubMed] [Google Scholar]
- 18.Kumari K, Augusti KT. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J Ethnopharmacol. 2007;109:367–371. doi: 10.1016/j.jep.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Greilberger J, Ledinski G, Kager G, Paigen B, J¨urgens G. The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis. 2004;172:239–246. doi: 10.1016/j.atherosclerosis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Ojha SK, Nandave M, Arora S, Narang R, Dinda AK, Arya DS. Chronic administration of Tribulus terrestris Linn extract improves cardiac function and attenuates myocardial infarction in rats. Int J Pharmacol. 2008;4:1–10. [Google Scholar]
- 21.Ramesh B, Saralakumari D. Antihyperglycemic, hypolipidemic and antioxidant activities of ethanolic extract of Commiphora mukul gum resin in fructose-fed male Wistar rats. J. physiol Biochem. 2012 doi: 10.1007/s13105-012-0175x. Article in press. [DOI] [PubMed] [Google Scholar]
- 22.Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118:29–32. [Google Scholar]
- 23.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL. Tissue sulphydryl. Arch Biochem Biophys. 1959;82:7077. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin's-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Soon YY, Tan BKH. Evaluation of the hypoglycemic and antioxidant activities of Morinda of.cinalis in streptozotocin-induced diabetic rats. Singapore Med J. 2002;43:77. [PubMed] [Google Scholar]
- 27.Beers RF, Sizer JW. Spectrophotometric method for measuring breakdown of hydrogen peroxide catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 28.Rotsruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Sci. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 29.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 30.Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitman S, Frankel S. Practical biochemistry in clinical medicine. Am J Clin Path. 1957;25:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Duncan DB. Multiple range and multiple tests. Biometrics. 1955;42:1–42. [Google Scholar]
- 33.Karuna R, Vijayabharathi G, Sreenivasa Reddy S, Ramesh B, Saralakumari D. Protective effects of Phyllanthus amarus aqueous extract ingestion on hyperglycemia and renal oxidative stress in streptozotocin induced diabetes rats. Indian J Pharmacol. 2011;43(3):414–418. doi: 10.4103/0253-7613.83112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoharan S, Gitanjali M, Linsa Mary A, Chellammal A, Vasudevan K, Balakrishnan S, et al. Antihyperglycemic and antilipidperoxdative potential of Ceiba pentandra in streptozotocin-induced diabetic rats. Cell Tissue Res. 2009;9:1731–1736. [Google Scholar]
- 35.Kedziora-Kornatowska K, Szewczyk-Golec K, Kozakiewicz M. Melatonin improves oxidative stress parameters measured in the blood of elderly type 2 diabetic patients. J Pineal Res. 2009;46:333–337. doi: 10.1111/j.1600-079X.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 36.Rajani Kanth V, Uma Maheswara Reddy P, Raju TN. Attenuation of streptozotocin-induced oxidative stress in hepatic and intestinal tissues of Wistar rat by methanolic-garlic extract. Acta Diabetol. 2008;45:243–251. doi: 10.1007/s00592-008-0051-x. [DOI] [PubMed] [Google Scholar]
- 37.Palanisamy A, Sorimuthu PS. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem Biol Interact. 2007;165:155–164. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Bavara JH, Narasimhacharya AVRI. Preliminary study on antihyperglycemic and antihyperlipaemic effects of Butea monosperma in NIDDM rats. Fitoterapia. 2008;79:328–331. doi: 10.1016/j.fitote.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Eidi A, Eidi M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab Syndr Clin Res Rev. 2009;3:40–44. [Google Scholar]
- 40.Badole SL, Bodhankar SL. Antidiabetic activity of cycloart-23- ene-β, 25-diol (B2) isolated from Pongamia pinnata (L.Pierre) in streptozotocin-nicotinamide induced diabetic mice. Eur J Pharmacol. 2010;632:103–109. doi: 10.1016/j.ejphar.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chem Biol Interact. 2009;182:67–72. doi: 10.1016/j.cbi.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Vlassara H, Palace MR. Diabetes and advanced glycation end products. J Inter Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 43.Gowrishankar NL, Manavalan R, Venkappayya D, David Raj C. Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl(4)-induced oxidative damage in rats. Food Chem Toxicol. 2008;46:3182–3185. doi: 10.1016/j.fct.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Barbora V, Norbert S, Robert LS, Aramesh S, Richard PE, Clifton B, et al. High alanine aminotransferase associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889–95. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- 45.Maiti R, Jana D, Das UK, Ghosh D. Antidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2004;92:85–91. doi: 10.1016/j.jep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh S, Suryawanshi SA. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rat. Indian J Exp Biol. 2001;39:748–759. [PubMed] [Google Scholar]