Abstract
Objective
To assess the effect of quercetin (flavonoid) against lindane induced alterations in lipid profile of wistar rats.
Methods
Rats were administered orally with lindane (100 mg/kg body weight) and quercetin (10 mg/kg body weight) for 30 days. After the end of treatment period lipid profile was estimated in serum and tissue.
Results
Elevated levels of serum cholesterol, triglycerides, low density lipoprotein (LDL), very Low Density Lipoprotein (VLDL) and tissue triglycerides, cholesterol with concomitant decrease in serum HDL and tissue phospholipids were decreased in lindane treated rats were found to be significantly decreased in the quercetin and lindane co-treated rats.
Conclusions
Our study suggests that quercetin has hypolipidemic effect and offers protection against lindane induced toxicity in liver by restoring the altered levels of lipids. The quercetin cotreatment along with lindane for 30 days reversed these biochemical alterations in lipids induced by lindane.
Keywords: Lindane, Quercetin, Oxidative stress, Low density lipoprotein, Very low density lipoprotein, High density lipoprotein, Triglycerides
1. Introduction
India is an agriculture based country. In the process of development of agriculture, the use of pesticides as an agent to kill or control undesired pests, such as insects, weeds, rodents, fungi, bacteria or other organisms for boosting food production has become more prevalent[1]. Pesticides have a significant public health benefit by decreasing the food borne and vector borne diseases[2]. Widespread use of pesticides in agriculture has increased the number of intoxications in mammals, whereas many insects have reported to show resistance[3].
Lindane (γ-hexachlorocyclohexane) is a broad spectrum, environmentally persistent organochlorine pesticide. Organochlorine pesticides have very low water solubilities but are highly soluble in lipids and bioaccumulate in tissues[4]. Such organochlorine pesticides are slowly eliminated from soils, by evaporation, running water, soil adsorption and finally are dispersed by rain or distributed to phytophageous animals by plants[3].
Lindane is classified by the World Health Organization (WHO) as ‘moderately hazardous’. Most human exposure to lindane is from eating food contaminated with the pesticide. Another major source of human intake is drinking water. Lindane has been detected in drinking water, industrial effluent and sewage in Europe and U.S. and in rainwater in Tokyo. Acute exposure to lindane has been reported to cause irritation, dizziness, headaches, diarrhoea, nausea, vomiting and in some cases convolusions and death. Accordingly, exposure to excessive amounts of lindane through misuse or unintentional exposure causes seizures, convulsions and other signs of intoxication in humans[5],[6]. International Agency for Research in Cancer (IARC) has reported digestive tract inflammation, haemorrage, coma and death after lindane poisoning. Lindane exerts its toxicity mainly by stimulation of the central nervous system. Lindane's insecticidal activity is ascribed to neuroexcitation subsequent to inhibition of GABA channels[7]-[9]. Lindane inhibits uterine contractions by inhibiting myometrial gap junction permeability[10]. Lindane has been induces membrane perturbation, causes functional impairment in blood brain barrier, alters glutathione homeostasis and alteration in cytochrome P450 mono-oxygenase enzymes. Lindane enhances oxidative stress by interacting with the cell membrane, triggering the generation of Reactive Oxygen Species (ROS) and altering the level of antioxidant molecules. Thus causes severe physiological dysfunction in various organ systems[11]-[13].
Flavonoids belong to a group of natural substances with variable phenolic structures and are found in fruits, vegetables, grains, bark, roots, stems, flowers, tea, and wine. These natural products were known for their beneficial effects on health long before flavonoids were isolated as the effective compounds. More than 4000 varieties of flavonoids have been identified. The flavones are characterized by a planar structure because of a double bond in the central aromatic ring. One of the best described flavonoids, quercetin, is a member of flavones group. Flavonoids are a group of naturally occurring polyphenolic compounds widely distributed as secondary metabolites in plant kingdom[14]. Quercetin (3, 5, 7, 3, 4-pentahydroxy flavon), is one of the most prominent dietary antioxidants[15]. Quercetin occurs in glycosylated form in French beans, broccoli, apples and especially in onions[16]. Quercetin has been reported to increase the genomic stability in rats and enhance the antioxidative defense system by up regulating antioxidant enzymes[17]. Quercetin intake shows decreased incidence of cardiovascular and neoplastic diseases[18]-[20]. Quercetin has exhibited anticancer potential against a wide range of cancers such as prostate, cervical, lung, breast and colon by inhibiting cell proliferation by causing apoptosis and/or cell cycle arrest[21],[22]. The present study was designed to investigate the protective effect of quercetin against lindane induced alterations in lipid profile in rats.
2. Materials and ethods
2.1. Chemicals
Lindane (γ-isomer) and quercetin (98.5%) were purchased from Sigma Aldrich Pvt. Ltd., Bangalore, India. All other analytical grade chemicals were purchased from HiMedia laboratories, Mumbai, India.
2.2. Animals
Female albino wistar rats weighing between 150 - 200 g each were used for this experiment. They were procured from Kerala Agricultural University Mannuthy, Trissur, Kerala. The rats were maintained in a controlled environment under standard conditions of temperature (28±2°C) and humidity with an alternating light and dark cycle. The animals were fed with commercially available pelleted rat chow (Sai Durga private limited, Bangalore) and water ad libitum. After a week of acclimatization, rats were divided into control and test group. Six rats were used in each treatment group. The study protocol was approved from the Institutional Animal Ethics Committee constituted in accordance with the rules and guidelines of the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments and Animals), India.
2.3. Treatment schedule
Lindane (100mg/kg) dissolved in 0.3 mL of olive oil and Quercetin (10 mg/kg) dissolved in 0.3 mL of 50% ethanol were administered to rats orally for 30 days. Where animals received co-treatment with lindane and quercetin, lindane was administered first followed by quercetin with about 15 minutes gap between the treatments.
2.4. Experimental procedure
The animals were divided into 6 groups with 6 rats in each group. Group I served as Control. Group II served as vehicle control-1, treated with 0.3 mL of Olive Oil (Vehicle for Lindane). Group III served as vehicle control-2, treated with 0.3 mL of 50% ethanol (Vehicle for Quercetin), Group IV received Lindane alone (100mg/kg body weight). Group V was treated with Quercetin alone (10 mg/kg body weight). Group VI received Lindane (100mg/kg body weight) and Quercetin (10 mg/kg body weight). After 30 days of treatment period, the animals were deprived of food overnight and anesthetized and then sacrificed by cervical decapitation. Blood was collected and serum was separated and used for lipid analysis. The liver tissue was dissected out, washed in ice-cold saline, patted dry and weighed. The liver tissue was used for the lipid extraction.
2.5. Lipid extraction from liver tissue
To 500mg of the liver tissue 150mL of chloroform methanol mixture in the ratio (2: 1) was added and the homogenate was prepared. This step was repeated three times to completely homogenize the residue and extract the lipid. The three extracts were pooled and the volume was measured. The contents were transferred to a separating funnel .The chloroform layer was then transferred to a flat bottom flask through anhydrous Na2SO4. The contents were flash evaporated to concentrate the extract. This concentrated mixture was then made to a known volume with chloroform. A volume of 1mL of the extract was transferred to a pre-weighed vial and aliquots were taken for the following estimations.
2.6. Lipid estimation
Cholesterol was estimated by the method of Parekh and Jung[23]. Phospholipids were estimated according to the method of Rouser et al[24]. Triglycerides were estimated according to the method of Rice[25].
2.7. Serum lipoproteins
Serum Lipoproteins were fractionated by a dual precipitation technique as described by Wilson et al(1973)[26].
2.8. Statistical analysis
Data were statistically evaluated using one-way analysis of variance, followed by Tukey's multiple comparison test. The values were expressed as mean±SD and were considered significant at P<0.001.
3. Results
3.1. The effects of lindane and quercetin supplementation on serum total cholesterol and triglycerides levels of adult wistar rats.
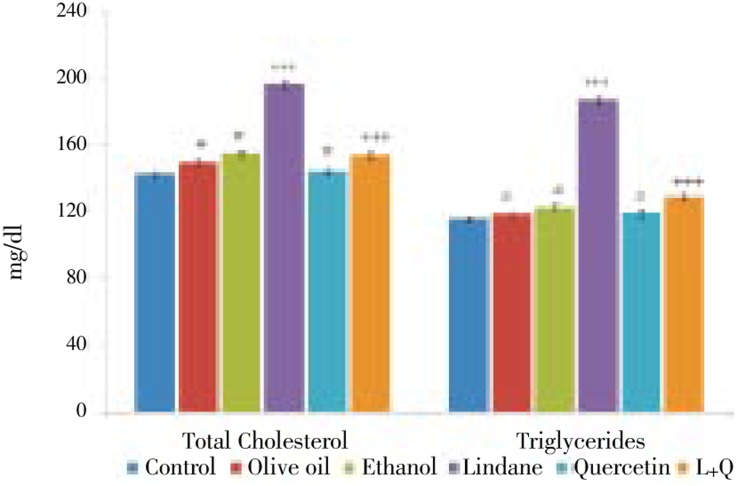
Serum total cholesterol and triglyceride levels were significantly increased (P<0.001) in lindane administered rats compared to that of control animals. In rats co-treated with quercetin along with lindane the serum cholesterol and triglyceride levels were significantly reduced (P<0.001) compared to rats treated with lindane alone. These levels were non significant in rats treated with olive oil or ethanol or quercetin alone when compared to the control (Figure 1).
Figure 1. The effects of Lindane and Quercetin supplementation on serum total cholesterol and triglycerides levels. Each bar represents, mean ± standard error of mean (SEM) of each group. ***P < 0.001, compared to control. +++P < 0.001, compared to lindane treated group. NS, not significant (#); compared to control (One way ANOVA followed by Tukey's multiple comparison. L+Q - Lindane + Quercetin.
3.2. The effect of lindane and quercetin supplementation on serum HDL, LDL and VLDL level of adult wistar rats
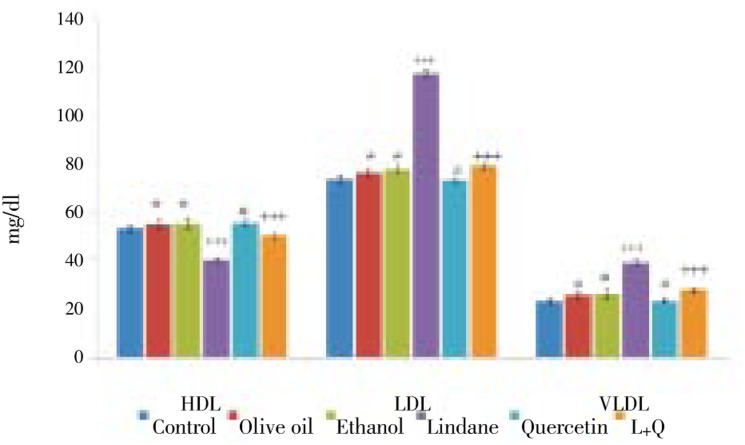
Serum HDL level was decreased significantly (P<0.001) and that of LDL, VLDL levels were found to be increased significantly (P<0.001) in lindane treated rats compared to control animals. These levels in Olive oil or ethanol or quercetin alone treated rats were non significant compared to control rats. In rats which received quercetin co-treatment along with lindane, the HDL fraction was increased significantly (P<0.001) with significant reduction in LDL, VLDL fraction compared to animals treated with lindane alone (Figure 2).
Figure 2. The effects of Lindane and Quercetin supplementation on serum HDL, LDL and VLDL levels. Each bar represents, mean ± standard error of mean (SEM) of each group. ***P < 0.001, compared to control. +++P < 0.001, compared to lindane treated group. NS, not significant (#); compared to control (One way ANOVA followed by Tukey's multiple comparison).
3.3. The effects of lindane and quercetin supplementation on tissue total cholesterol, phospholipid and triglyceride level of adult wistar rats.
The tissue phospholipid decreased significantly (P<0.001) whereas tissue total cholesterol and triglyceride levels were found to be significantly increased (P<0.001) in rats administered lindane alone. Rats co-treated with quercetin along with lindane registered a significant increase (P<0.001) in tissue phospholipid content whereas tissue cholesterol and triglyceride levels were decreased significantly (P<0.001) compared to rats treated with lindane alone. These levels in olive oil or ethanol or quercetin alone treated rats were non significant when compared to control animals (Table 1).
Table 1. Effect of quercetin on total cholesterol, phospholipids, triglycerides levels in liver tissue of experimental rats.
| Groups | total cholesterol (mg/g of tissue) | phospholipids (mg/g of tissue) | triglycerides (mg/g of tissue) |
| Control | 263.00±0.95 | 91.73±1.28 | 121.86±1.07 |
| Olive oil | 266.12±0.82NS | 92.61±1.45NS | 124.72±0.99NS |
| Ethanol | 267.79±0.95NS | 91.70±1.45NS | 127.04±0.92NS |
| Lindane | 524.90±0.89*** | 57.98±1.05*** | 152.51±1.19*** |
| Quercetin | 261.20±1.18NS | 95.17±0.57NS | 121.40±1.13NS |
| Lindane+Quercetin | 258.71±1.28+++ | 91.41±1.55+++ | 125.11±0.83+++ |
Values are means±standard (n=6) error of mean of each group. ***P<0.001, compared to control. +++P<0.001, compared to lindane treated group. NS, not significant, compared to control. (One way ANOVA followed by Tukey's multiple comparison).
4. Discussion
Lipids are precursors for hormones, and are used for energy storage, and they have a prominent role as messengers and regulators of inflammation[27]. Lipids are one of the most susceptible targets of free radicals[28]. This oxidative destruction is known as lipid peroxidation and may induce many pathological events. Diseases associated with high TG levels namell Diabetes mellitus, obesity, chronic renal disease, primary hyperlipoproteinemia carry high risk of cardiovascular disorder (CVD)[29]. Hypertriglyceridemia in combination with abnormally low concentrations of HDL cholesterol (high density lipoprotein cholesterol) is one the most common atherogenic profile of lipid metabolism of high prevalence seen in Indian population[30]. Hyperlipidemia and hypercholesterolemia are reported as the major risk factors in life style related diseases such atherosclerosis and related cardiovascular complications including cerebral paralysis and myocardial infarction[31]. Prevention or treatment of such disorders can be achieved through diet and/or drug administration[32],[33]. It is well documented that a low level of HDL-C is indicative of high risk for cardiovascular disease, an increase in HDL-C level could potentially contribute to antiatherogenicity[34],[35].
The liver is a metabolically versatile organ responsible for the regulation of internal chemical environment[36]. It is particularly important in the synthesis and regulation of circulating lipids, lipoproteins, triglycerides, cholesterol, cholesterol esters and in the degradation of cholesterol and steroids. The liver is the primary site for detoxification and facilitates clearance through excretion of water-soluble products and it is the major organ of antioxidant defense system[37].
Like other organochlorine pesticides, lindane persists in environment and bioaccumulates in human tissues[38]. Lindane dosage was selected from the previous report by Etim et al[39]. Lindane induces oxidative stress in blood and tissue of rats by decreasing the activities of antioxidant enzymes and increasing free radical generation[40]. It was reported that lindane accumulates in the adipose tissue. Adipose tissues are found surrounding the visceral organs which includes liver, muscle and heart. In recent years, the positive effects of flavonoids on human health have attracted more attention. Especially, quercetin, a flavonoid found in many plants, is widely distributed in fruits and vegetables. Most of the biological actions of quercetin seem to be associated with its potency as an antioxidant. The antioxidant effect of quercetin has been reported by different studies[41],[42]. Hence the protective effect of quercetin against lindane induced alterations in the liver lipid profile has been taken up for the present investigation.
In the present study there is a significant increase in the serum total cholesterol, LDL, VLDL and triglycerides followed by a significant decrease in the HDL cholesterol in the lindane treated rats compared to control animals. A significant increase in the cholesterol and triglyceride values followed by a significant decrease in the phospholipid level was observed in the liver tissue of lindane treated rats compared to control animals which is an indication of severe lindane induced hyperlipidemia. Lindane induced hepatotoxicty was reported earlier by Sharma et al[43]. Previous studies have reported elevated serum levels of triglycerides, cholesterol and phospholipids in rats and mice that were on diet contaminated with lindane[44]-[46]. The results of the present investigation are inline with the above mentioned reports.
In the present study the rats co-treated with quercetin along with lindane showed significantly reduced levels of the serum total cholesterol, LDL, VLDL, triglycerides and tissue total cholesterol and triglyceride whereas the serum HDL cholesterol and tissue phospholipid levels were significantly increased compared to rats treated with lindane alone. This is in accordance with the previous study where quercetin presented the largest percentual reduction of cholesterol in the triton induced hyperlipidemic rats[47]. Quercetin is found to decrease the ceramide accumulation, lipid peroxidation[48] and restore the lipid profiles to normal[49].
Role of antioxidants like vitamin C, Vitamin E and curcumin was widely studied in xenobiotic induced oxidative stress and hepatoprotection[43]. Curcumin showed lipid lowering[50] and free radical scavenger activity[51]. Vitamin C reduced Cholesterol[50] prevented oxidation of LDL, reduced TG, raised HDL level and decreased oxidative damage to oxidized LDL-cholesterol by scavenging free radicals. Vitamin E reported to lower lipid peroxidation in brain of mice acutely intoxicated with lindane. All these antioxidants are free radicals scavengers and exhibits lipid peroxidation lowering effect by radical scavenging activity. The structure of quercetin plays an important role in its antioxidant effect. The o-dihydroxy-structure in the B-ring has been observed to confer higher stability to the radical form and to participate in electronic delocalization. It was suggested that the antioxidant activities were dictated both by their structural features and by their location in the membrane. Flavonoids are known to anchor on the polar head of the main phospholipids. Hence, quercetin distributed on the surface of the lipid bilayers as well as in the aqueous phase could scavenge free radicals. Quercetin also showed antioxidant and free radical scavenging actions[18]. Quercetin being a potent antioxidant exhibits scavenging effect on lindane induced free radicals and maintain serum and tissue lipid profile at near normal values.
From our study it is obvious that lindane induced alteration in the lipid profile can be ameliorated by quercetin co-treatment. Our study advocates the use of natural free radical scavenger quercetin in diet to reduce toxic effects of lindane.
Acknowledgments
The authors greatfully acknowledge the UGC SAP DRS (Under Graduate students research orientation programme) sponsored by the University Grants Commission, New Delhi which supported the present work.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Abhilash PC, Singh N. Pesticide use and application: An Indian scenario. J Hazardous Mater. 2009;165:1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Templeton DJ, Jamora N. Economic assessment of a change in pesticide regulatory policy in the Philippines. World Development. 2010;38:1519–1526. [Google Scholar]
- 3.Goudey-Perrière F, Lemonnier F, Bergougnoux V, Perrière C. Low doses of the pesticide lindane induce protein release by the fat body of female cockroach Blaberus craniifer (Dictyoptera) Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:492–501. doi: 10.1016/j.cbpc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Murphy S. Casarett & Doull's toxicology. New York: MacMillan Publishing; 1986. [Google Scholar]
- 5.Hrnčić D, Rašić-Marković A, Djuric D, Šušić V, Stanojlović O. The Role of nitric oxide in convulsions induced by lindane in rats. Food Chem Toxicol. 2011;49:947–954. doi: 10.1016/j.fct.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Zucchini-Pascal N, de Sousa G, Rahmani R. Lindane and cell death: At the crossroads between apoptosis, necrosis and autophagy. Toxicol. 2009;256:32–41. doi: 10.1016/j.tox.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Hfaiedh N, Murat J-C, Elfeki A. Protective effects of garlic (Allium sativum) extract upon lindane-induced oxidative stress and related damages in testes and brain of male rats. Pesticide Biochem Physiol. 2011;100:187–192. [Google Scholar]
- 8.Bist R, Bhatt DK. The evaluation of effect of alpha-lipoic acid and vitamin E on the lipid peroxidation, gamma-amino butyric acid and serotonin level in the brain of mice (Mus musculus) acutely intoxicated with lindane. J Neurol Sci. 2009;276:99–102. doi: 10.1016/j.jns.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Ogata N, Vogel S, Narahashi T. Lindane but not deltamethrin blocks a component of GABA-activated chloride channels. FASEB J. 1988;2:2895–2900. doi: 10.1096/fasebj.2.13.2458984. [DOI] [PubMed] [Google Scholar]
- 10.Maranghi F, Rescia M, Macrì C, Di Consiglio E, De Angelis G, Testai E, et al. Lindane may modulate the female reproductive development through the interaction with ER-β: an in vivo-in vitro approach. Chem Biol Interact. 2007;169:1–14. doi: 10.1016/j.cbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Saravanan M, Prabhu Kumar K, Ramesh M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pesticide Biochem Physiol. 2011;100:206–211. [Google Scholar]
- 12.Vijaya Padma V, Sowmya P, Arun Felix T, Baskaran R, Poornima P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem Toxicol. 2011;49:991–998. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Bano M, Bhatt D. Neuroprotective role of a novel combination of certain antioxidants on lindane induced toxicity in cerebrum of mice. Res J Agri Bio Sci. 2007;3:664–669. [Google Scholar]
- 14.Vidhya A, Indira M. Protective effect of quercetin in the regression of ethanol-induced hepatotoxicity. Indian J Pharm Sci. 2009;71:527–532. doi: 10.4103/0250-474X.58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paolillo R, Romano Carratelli C, Rizzo A. Effect of resveratrol and quercetin in experimental infection by Salmonella enterica serovar Typhimurium. Int Immunopharmacol. 2011;11:149–156. doi: 10.1016/j.intimp.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Pandey KB, Rizvi SIbrahim. Current understanding of dietary polyphenols and their role in health and disease. Curr Nutr Food Sci. 2009;5:249–263. [Google Scholar]
- 17.Tieppo J, Vercelino R, Dias AS, Silva Vaz MF, Silveira TR, Marroni CA, et al. Evaluation of the protective effects of quercetin in the hepatopulmonary syndrome. Food Chem Toxicol. 2007;45:1140–1146. doi: 10.1016/j.fct.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med. 2011;51:1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem Pharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Pfeuffer M, Auinger A, Bley U, Kraus-Stojanowic I, Laue C, Winkler P, et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammatory parameters in men with different APOE isoforms. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Du G, Lin H, Yang Y, Zhang S, Wu X, Wang M, et al. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int Immunopharmacol. 2010;10:819–826. doi: 10.1016/j.intimp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Parekh AC, Jung DH. Cholesterol determination with ferric acetate-uranium acetate and sulfuric acid-ferrous sulfate reagents. Anal Chem. 1970;42:1423–1427. [Google Scholar]
- 24.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 25.Rice EW. Standard method of clinical chemistry. New York: Academic Press; 1970. [Google Scholar]
- 26.Wilson D, Spiger M, Done G. A dual precipitation method for quantitative plasma lipoprotein measurement without ultracentrifugation. J Lab Clin Med. 1973;82:473–482. [PubMed] [Google Scholar]
- 27.Watson AD. Thematic review series: Systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Rajani G, Purnima A. In vitro antioxidant and antihyperlipidemic activities of Bauhinia variegata Linn. Indian J Pharmacol. 2009;41:227–232. doi: 10.4103/0253-7613.58513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 30.Enas EA, Mehta J. Malignant coronary artery disease in young asian indians: Thoughts on pathogenesis, prevention, and therapy. Clin Cardiol. 1995;18:131–135. doi: 10.1002/clc.4960180305. [DOI] [PubMed] [Google Scholar]
- 31.Chovanèíková M, Simek V. Effects of high-fat and Chlorella vulgaris feeding on changes in lipid metabolism in mice. Biologia Bratislava. 2001;56:661–666. [Google Scholar]
- 32.Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 33.LaRosa J, Hunninghake D, Bush D, Criqui MH, Getz GS, Gotto AM, Jr, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81:1721–1733. doi: 10.1161/01.cir.81.5.1721. [DOI] [PubMed] [Google Scholar]
- 34.Wilson P, Abbott R, Castelli W. High density lipoprotein cholesterol and mortality. The framingham heart study. Arteriosclerosis. 1988;8:737–741. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 35.Assmann G, Nofer J-R. Atheroprotective effects of high-density lipoproteins. Ann Rev Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 36.Behl M, Nyska A, Chhabra RS, Travlos GS, Fomby LM, Sparrow BR, et al. Liver toxicity and carcinogenicity in F344/N rats and B6C3F1 mice exposed to Kava Kava. Food Chem Toxicol. 2011;49:2820–2829. doi: 10.1016/j.fct.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arulmozhi V, Krishnaveni M, Karthishwaran K, Dhamodharan G, Mirulnalini S. Antioxidant and antihyperlipidemic effect of Solanum nigrum fruit extract on the experimental model against chronic ethanol toxicity. Phcog Mag. 2010;6:42–50. doi: 10.4103/0973-1296.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Etim OE, Farombi EO, Usoh IF, Akpan EJ. The protective effect of aloe vera juice on lindane induced hepatotoxicity and genotoxicity. Pak J Pharm Sci. 2006;19:333–337. [PubMed] [Google Scholar]
- 40.Fidan A, Cigerci I, Baysu-Sozbilir N, Kucukkurt I, Yuksel H, Keles H. The effect of dose dependent gamma hexachlorocyclohexane (lindane) on blood and tissue antioxidant defense system, lipid peroxidation and histopathological changes in rats. J Anim Vet Adv. 2008;7:1480–1488. [Google Scholar]
- 41.Kalender Y, Kaya S, Durak D, Uzun FG, Demir F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ Toxicol Pharmacol. 2012;33:141–148. doi: 10.1016/j.etap.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez-Gallego JI, López-Revuelta A, Hernández-Hernández AHernández-Hernández A, Sardina JL, López-Ruano G, et al. Comparative antioxidant capacities of quercetin and butylated hydroxyanisole in cholesterol-modified erythrocytes damaged by tert-butylhydroperoxide. Food Chem Toxicol. 2011;49:2212–2221. doi: 10.1016/j.fct.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Sharma P, Shankar S, Agarwal A, Singh R. Variation in serum lipids and liver function markers in lindane exposed female wistar rats: attenuating effect of curcumin, vitamin C and vitamin E. Asian J Exp Biol Sci. 2010;1:440–444. [Google Scholar]
- 44.Ravinder P, Srinivasan K, Radhakrishnamurty R. Dietary hexachlorocyclohexane induced changes in blood and liver lipids in albino mice. Indian J Exp Biol. 1990;28:155–157. [PubMed] [Google Scholar]
- 45.Boll M, Weber L, Stampfl A. The effect of α-hexachlorocyclohexane (lindane) on the activities of liver lipogenic enzymes and on serum lipids in rats. Z Naturforsch. 1995;50c:135–142. doi: 10.1515/znc-1995-1-220. [DOI] [PubMed] [Google Scholar]
- 46.Boll M, Weber L, Stampfl A. The response of rat serum lipids to diets of varying composition or contaminated with organochlorine pesticides. Z Naturforsch. 1996;51:91–100. doi: 10.1515/znc-1996-1-216. [DOI] [PubMed] [Google Scholar]
- 47.Ricardo KFS, Oliveira TTd, Nagem TJ, et al. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Brazilian Arch Biol Technol. 2001;44:263–267. [Google Scholar]
- 48.Babenko N, Shakhova E. Effects of flavonoids on sphingolipid turnover in the toxin-damaged liver and liver cells. Lipids Health Dis. 2008;7:1. doi: 10.1186/1476-511X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnaveni M, Mirunalini S, Karthishwaran K, Dhamodharan G. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn. (Euphorbiaceae) on streptozotocin induced diabetic rats. Pak J Nutr. 2010;9:43–51. [Google Scholar]
- 50.Manjunatha H, Srinivasan K. Hypolipidemic and antioxidant effects of dietary curcumin and capsaicin in induced hypercholesterolemic rats. Lipids. 2007;42:1133–1142. doi: 10.1007/s11745-007-3120-y. [DOI] [PubMed] [Google Scholar]
- 51.K.Indira P Free radical reactions of curcumin in membrane models. Free Radical Biol Med. 1997;23:838–843. doi: 10.1016/s0891-5849(97)00026-9. [DOI] [PubMed] [Google Scholar]