Abstract
Objective
To prepare curcumin-piperine (Cu-Pi) nanoparticles by various methods and to study the effect of various manufacturing parameters on Cu-Pi nanoparticles and to identify a suitable method for the preparation of Cu-Pi nanoparticles to overcome oral bioavailability and cancer cell targeting limitations in the treatment of cancer.
Methods
Cu-Pi nanoparticles were prepared by thin film hydration method, solid dispersion method, emulsion polymerization method and Fessi method. Optimization was carried out to study the effect of various manufacturing parameter on the Cu-Pi nanoparticles.
Results
Out of four methods, Fessi method produced a minimum average particle size of 85.43 nm with a polydispersity index of 0.183 and zeta potential of 29.7 mV. Change of organic solvent (acetone or ethanol) did not have any significant effect on Cu-Pi nanoparticles. However, increase in sonication time, stirring speed, viscosity, use of 1:10:10 ratio of drug/polymer/surfactant, and use of anionic surfactant or combination of anionic surfactant with cationic polymer or combination of non-ionic surfactant with cationic polymer had a significant effect on Cu-Pi nanoparticles.
Conclusions
Cu-Pi nanoparticles coated with PEG containing copolymer produced by Fessi method had a minimum average particle size, excellent polydispersity index and optimal zeta potential which fall within the acceptable limits of the study. This dual nanoparticulate drug delivery system appears to be promising to overcome oral bioavailability and cancer cell targeting limitations in the treatment of cancer.
Keywords: Polymeric nanoparticles, Cancer treatment, Nanoparticles, Curcumin, Piperine
1. Introduction
One of the newest trends in food science and technology is functional food which is defined as foods that provide health benefits beyond basic nutrition. Among various functional food ingredients, polyphenols have attracted many researchers' attention because of their anti-oxidant, anti-inflammatory, and anti-cancer properties[1].
One such polyphenol is curcumin, which is isolated from the rhizome of the herb Curcuma longa Linn. Curcumin has been extensively studied and found to have diverse pharmacological activities[2]. Various animal and human studies have shown curcumin is extremely safe even at very high doses[3]. United States Food and Drug Administration has also declared curcumin as “generally regarded as safe”. In spite of its efficacy and safety, curcumin has not yet been approved as a therapeutic agent. The major barriers to the clinical usefulness of curcumin in the treatment of cancer are poor oral bioavailability and lack of cancer cell targeting. However, poor oral bioavailability of curcumin is mainly due to its poor aqueous solubility, intestinal metabolism, hepatic metabolism and rapid systemic clearance[4],[5]. These limitations can be overcome by nanoparticulate drug delivery system.
Hence, the primary aim of the study was to prepare curcumin-piperine (Cu-Pi) nanoparticles by various methods and to study the effect of various manufacturing parameter on the average particle size, polydispersity index and zeta potential of Cu-Pi nanoparticles and to identify a suitable method for the preparation of Cu-Pi nanoparticles to overcome oral bioavailability and cancer cell targeting limitations in the treatment of cancer.
2. Materials and methods
2.1. Materials
The following chemicals were obtained from commercial sources and used without any further purification. Curcumin (97%) was purchased from Himedia Laboratories (Mumbai, India). Piperine (97%) was purchased from Sigma-Aldrich (Bangalore, India). Ethanol (99.9%) was purchased from Brampton (Ontario, Canada). Acetone (analytical grade) was purchased from S.D Fine Chemicals (Mumbai, India). Poloxamer 188 (P 188) and Poloxamer 407 (P 407) were purchased from Sigma-Aldrich (Bangalore, India). Sodium lauryl sulfate (SLS) was purchased from S.D Fine Chemicals (Mumbai, India). Sodium alginate was purchased from Nice Chemical Pvt. Ltd. (Kerala, India). Eudragit E 100 was a generous gift from Degussa (India).
2.2. Methods
Cu-Pi nanoparticles were prepared by (a) thin film hydration method, (b) solid dispersion method, (c) emulsion polymerization method and (d) Fessi method.
2.3. Preparation of Cu-Pi nanoparticles by thin film hydration method
Cu-Pi nanoparticles were prepared by thin film hydration method as per the protocol of Wang et al with some modification[6]. Briefly, curcumin, piperine and surfactant were dissolved in organic solvent under sonication (40 kHz, Ultrasonic Cleaner, Lark, India) followed by removal of solvent under reduced pressure (Rotary evaporator, Lark, India) to form a thin film. Further 5 mL of ultra pure water (Milli-Q Academic, Millipore, Bangalore, India) was added to the thin film under sonication (40 kHz, Ultrasonic Cleaner, Lark, India) for 5 or 10 cycles (5 minutes per cycle). Nanoparticles were formed spontaneously and turned the solution slightly turbid. Free drugs were removed from the nanosuspension by centrifugation (Refrigerated Centrifuge, Remi, India) at 3 000 rpm for 10 minutes at 4 °C. The resultant nanosuspension was used for further characterization. Optimization was performed as per Table 1 to assess the impact of organic solvent, surfactant and sonication time on the average particle size, polydispersity index and zeta potential of Cu-Pi nanoparticles.
Table 1. Optimization of Cu-Pi nanoparticles prepared by thin film hydration method.
| Formulation | Curcumin (mg) | Piperine (mg) | Organic solvent (5 mL) | Surfactant (100 mg) | Sonication (Cycle) |
| F1 | 5 | 5 | Acetone | P 407 | 10 |
| F2 | 5 | 5 | Ethanol | P 407 | 10 |
| F3 | 5 | 5 | Ethanol | P 407 | 5 |
| F4 | 5 | 5 | Acetone | P 188 | 5 |
| F5 | 5 | 5 | Acetone | P 188 | 10 |
| F6 | 5 | 5 | Ethanol | P 188 | 10 |
2.4. Preparation of Cu-Pi nanoparticles by solid dispersion method
Cu-Pi nanoparticles were prepared by solid dispersion method as per the protocol of Kwon et al with some modification[7]. Briefly, Curcumin and piperine were dissolved in organic solvent under sonication (40 kHz, Ultrasonic Cleaner, Lark, India). Further this organic phase was added in a drop wise manner to 50 mL of ultra pure water (Milli-Q Academic, Millipore, Bangalore, India) containing surfactant and with or without sodium alginate as viscosity enhancer under sonication (40 kHz, Ultrasonic Cleaner, Lark, India) for 10 cycles (5 minutes per cycle). Nanoparticles were formed spontaneously and turned the solution turbid. Organic solvent was then removed by continuous overnight stirring (Magnetic Stirrer, Remi, India). Resultant nanoparticles were separated by ultracentrifugation (Refrigerated Centrifuge, Remi, India) at 19 000 rpm for 45 minutes at -20 °C. Obtained nano pellets were washed at least three times with ultra pure water (Milli-Q Academic, Millipore, Bangalore, India) and used for further characterization. Optimization was performed as per Table 2 to assess the impact of organic solvent, surfactant and viscosity on the average particle size, polydispersity index and zeta potential of Cu-Pi nanoparticles.
Table 2. Optimization of Cu-Pi nanoparticles prepared by solid dispersion method.
| Formulation | Curcumin (mg) | Piperine (mg) | Organic solvent (20 mL) | Surfactant (125 mg) | Viscosity enhancer (50 mg ) |
| F1 | 12.5 | 12.5 | Acetone | SLS | - |
| F2 | 12.5 | 12.5 | Ethanol | SLS | - |
| F3 | 12.5 | 12.5 | Ethanol | SLS | Sodium alginate |
| F4 | 12.5 | 12.5 | Acetone | P 188 | - |
| F5 | 12.5 | 12.5 | Ethanol | P 188 | - |
| F6 | 12.5 | 12.5 | Ethanol | P 188 | Sodium alginate |
2.5. Preparation of Cu-Pi nanoparticles by emulsion polymerization method
Cu-Pi nanoparticles were prepared by emulsion polymerization method. Briefly, surfactant (usually above the critical micelle concentration of the surfactant) was dissolved in 50 mL of ultra pure water (Milli-Q Academic, Millipore, Bangalore, India) under sonication (40 kHz, Ultrasonic Cleaner, Lark, India) and stored overnight in required temperature for the formation of micelles. Curcumin and piperine were dissolved in 5 mL of organic solvent under sonication (40 kHz, Ultrasonic Cleaner, Lark, India). Further this organic phase was added to 50 mL of aqueous phase containing micelles under stirring (Magnetic Stirrer, Remi, India) at 100 or 500 rpm for 1 hour at room temperature. Nanoparticles were formed spontaneously and turned the solution slightly turbid. Solvent was then removed by continuous overnight stirring (Magnetic Stirrer, Remi, India). Free drugs were removed by centrifugation (Refrigerated Centrifuge, Remi, India) at 3 000 rpm for 10 minutes at 4 °C. The resultant nanosuspension was used for further characterization. Optimization was performed as per Table 3 to assess the impact of organic solvent, surfactant and stirring speed on the average particle size, polydispersity index and zeta potential of Cu-Pi nanoparticles.
Table 3. Optimization of Cu-Pi nanoparticles prepared by emulsion polymerization method.
| Formulation | Curcumin (mg) | Piperine (mg) | Organic solvent (5 mL) | Surfactant (100 mg) | Stirring speed (rpm) |
| F1 | 5 | 5 | Acetone | SLS | 100 |
| F2 | 5 | 5 | Ethanol | SLS | 500 |
| F3 | 5 | 5 | Acetone | SLS | 500 |
| F4 | 5 | 5 | Acetone | P 407 | 500 |
| F5 | 5 | 5 | Ethanol | P 407 | 500 |
| F6 | 5 | 5 | Ethanol | P 407 | 100 |
2.6. Preparation of Cu-Pi nanoparticles by Fessi method
Cu-Pi nanoparticles were prepared by a method developed by Fessi et al with some modifications[8]. Briefly, curcumin, piperine and Eudragit E 100 were dissolved in 20 mL of acetone under sonication (40 kHz, Ultrasonic Cleaner, Lark, India). Further this organic phase was added to 50 mL of ultra pure water (Milli-Q Academic, Millipore, Bangalore, India) containing surfactant under constant stirring (Mechanical Stirrer, Remi, India) at 500 or 1 000 rpm for at least 1 hour. Nanoparticles were formed spontaneously and turned the solution slightly turbid. Organic solvent was then removed by continuous overnight stirring (Magnetic Stirrer, Remi, India). Free drugs were removed by centrifugation (Refrigerated Centrifuges, Remi, India) at 3 000 rpm for 10 minutes at 4 °C. The resultant nanosuspension was used for further characterization. Optimization was performed as per Table 4 to assess the impact of surfactant, dose of surfactant, dose of Eudragit E 100 and stirring speed on the average particle size, polydispersity index and zeta potential of Cu-Pi nanoparticles.
Table 4. Optimization of Cu-Pi nanoparticles prepared by Fessi method.
| Formulation | Curcumin (mg) | Piperine (mg) | Eudragit E 100 (mg) | Surfactant (mg) | Stirring speed (rpm) |
| F1 | 12.5 | 12.5 | 250 | SLS (250) | 1 000 |
| F2 | 12.5 | 12.5 | 250 | SLS (250) | 500 |
| F3 | 12.5 | 12.5 | 100 | SLS (100) | 1 000 |
| F4 | 12.5 | 12.5 | 250 | P 188 (250) | 1 000 |
| F5 | 12.5 | 12.5 | 250 | P 188 (250) | 500 |
| F6 | 12.5 | 12.5 | 100 | P 188 (100) | 1 000 |
2.7. Particle size measurement
Particle size and polydispersity index were measured using Mastersizer (Mastersizer 2000, Malvern Instruments, UK) or Zetasizer (ZEN3600, Malvern Instrument, UK) based on the nature of nanosuspension. Average particles size below 100 nm and polydispersity index below 0.2 were considered acceptable.
2.8. Zeta potential measurement
Aggregation of nanoparticle in the formulation reduces the physical stability of the nanosuspension and aggregation of nanoparticles in the gut leads to decreased oral bioavailability. Charge on the nanoparticles plays a significant role in aggregation. Higher number of either positive or negative charge repels each other which inturn prevents the aggregation. Particle charge was quantified and expressed as zeta potential. As a rule of thumb, suspensions with zeta potential above ±30 mV were physically stable. Suspensions with a zeta potential above ±60 mV showed excellent stability. Suspensions below ±20 mV are of limited stability and below ±5 mV they undergo pronounced aggregation. Zeta potential was measured using Zetasizer (ZEN3600, Malvern Instrument, UK). Zeta potential around ±30 mV was considered acceptable.
2.9. Statistical analysis
Statistical analyses were performed using GraphPad Prism software (Version 5.04). Experiments were carried out in triplicate and student's t-test was used to assess the differences. The differences were considered significant at P<0.05.
3. Results
Cu-Pi nanoparticles were successfully prepared by four methods and the results of characterization of the best formulation in each method were tabulated in Table 5.
Table 5. Characterization of Cu-Pi nanoparticles prepared by various methods (Mean±SD).
| Method | Formulation code | Average particle size (nm) | Polydispersity index | Zeta potential (mV) |
| TFH | F5 | 321.80±21.03 | 0.54±0.01 | -09.20±1.25 |
| SD | F2 | 158.00±17.04 | 0.65±0.15 | -52.10±5.09 |
| EP | F2 | 705.60±44.40 | 0.75±0.07 | -49.80±3.48 |
| Fessi | F4 | 085.43±14.57 | 0.18±0.03 | 29.70±0.50 |
TFH: thin film hydration; SD: solid dispersion; EP: emulsion polymerization.
3.1. Thin film hydration method
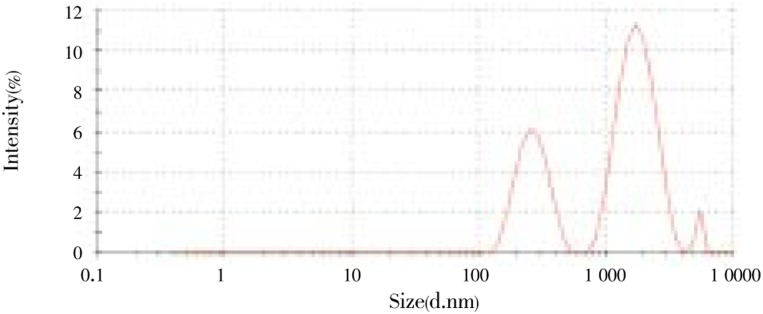
Six formulations (F1-F6) were prepared and characterized. Formulations F1, F2, F5 and F6 were used to assess the effect of solvent on the Cu-Pi nanoparticles and found use of either acetone or ethanol did not significantly (P>0.05) alter the average particle size, polydispersity index and zeta potential. Formulations F1, F2, F5 and F6 were used to assess the effect of surfactant on the Cu-Pi nanoparticles and found use of Poloxamer 407 significantly (P<0.05) increased the particles size and decreased the zeta potential than the use of Poloxamer 188 which may be due to higher ethylene oxide portion in Poloxamer 407[9]. However, change of surfactant did not significantly (P>0.05) alter the polydispersity index. Formulations F2, F3, F4 and F5 were used to assess the effect of sonication on the Cu-Pi nanoparticles and found increase in sonication time significantly (P<0.05) reduced the particles size which may be due to increased shear rate. However, increase in sonication time did not significantly (P>0.05) alter the polydispersity index and zeta potential. Out of six formulations, formulation F5 produced minimum average particle size of 321.8 nm (Figure 1) with polydispersity index of 0.537 and formulation F3 produced maximum average particle size of 410.3 nm with polydispersity index of 0.389. Similarly, formulation containing Poloxamer 188 produced maximum zeta potential of -9.84 mV (Figure 2). However, Cu-Pi nanoparticles produced by thin film hydration method did not satisfy the acceptance limit of average particle size, polydispersity index and zeta potential. Moreover, Figure 1 showed three different particle size peaks which may have increased the polydispersity index. To get more uniform particle size by this method, an additional step, filtration through 0.22 µm filter was required.
Figure 1. Particle size distribution of Cu-Pi nanoparticle (F5) by thin film hydration method.
Figure 2. Zeta potential of Cu-Pi nanoparticle (F5) by thin film hydration method.
3.2. Solid dispersion method
Six formulations (F1-F6) were prepared and characterized. Formulations F1, F2, F4 and F5 were used to assess the effect of solvent (acetone or ethanol) on the Cu-Pi nanoparticles and found change of solvent did not significantly (P>0.05) alter the average particle size, polydispersity index and zeta potential. Zeta potential of all six formulations in thin film hydration method was very low which may be due to non-ionic surfactants (Poloxamer). Hence we decided to study the effect of anionic (SLS) and non-ionic surfactant (Poloxamer 188) in this method. Formulations F1, F2, F4 and F5 were used to assess the effect of surfactant (SLS or Poloxamer 188) on the Cu-Pi nanoparticles and found use of SLS significantly (P<0.05) decreased the particles size and increased the zeta potential when compared to the use of Poloxamer 188. The possible mechanism for decreased average particle size in formulation (F1 and F2) containing SLS may be due to its anionic charge which provided high zeta potential of -51.2 mV and which inturn decreased the aggregation which may lead to significant size reduction. However, change of surfactant did not significantly (P>0.05) alter the polydispersity index. Formulations F2, F3, F5 and F6 were used to assess the effect of viscosity on the Cu-Pi nanoparticles and found increase in viscosity significantly (P<0.05) increased the average particles size which may be due to prevention of shear on the Cu-PI nanoparticle. However, increase in viscosity did not significantly (P>0.05) alter the polydispersity index and zeta potential. Out of six formulations, formulation F2 produced minimum average particle size of 158 nm (Figure 3) with polydispersity index of 0.646 and formulation F3 produced maximum average particle size of 510 nm with polydispersity index of 0.641. Similarly, formulation containing SLS produced maximum zeta potential of -52.1 mV (Figure 4). However, Cu-Pi nanoparticles produced by solid dispersion method did not satisfy the acceptance limit of average particle size, polydispersity index and zeta potential. Moreover, Figure 3 clearly indicated two particle size peaks which may have increased the polydispersity index. To get more uniform particle size by this method, an additional step, filtration through either 0.22 µm or 0.45 µm filter was required.
Figure 3. Particle size distribution of Cu-Pi nanoparticle (F2) by solid dispersion method.
Figure 4. Zeta potential of Cu-Pi nanoparticle (F2) by solid dispersion method.
3.3. Emulsion polymerization method
Six formulations (F1-F6) were prepared and characterized. Formulations F2, F3, F4 and F5 were used to assess the effect of solvent (acetone or ethanol) on the Cu-Pi nanoparticles and found change of solvent did not significantly (P>0.05) alter the average particle size, polydispersity index and zeta potential. In solid dispersion method we have studied the effect of anionic (SLS) and non-ionic surfactant (Poloxamer 188) and found significant change in Cu-Pi nanoparticles. Hence we have decided to study the effect of anionic (SLS) and non-ionic surfactant (Poloxamer 407) in this method. Formulations F2, F3, F4 and F5 were used to assess the effect of surfactant (SLS or Poloxamer 407) on the Cu-Pi nanoparticles and found use of SLS significantly (P<0.05) decreased the average particles size and increased the zeta potential than the use of Poloxamer 407. The possible mechanism for decreased average particle size in formulation (F1 and F2) containing SLS may be due to its anionic charge which provided high zeta potential of -49.8 mV and which inturn decreased the aggregation which may lead to significant size reduction. However, change of surfactant did not significantly (P>0.05) alter the polydispersity index. Formulations F1, F3, F5 and F6 were used to assess the effect of stirring speed on the Cu-Pi nanoparticles and found increase in stirring speed from 100 to 500 rpm significantly (P<0.05) decreased the average particles size which may be due to increased shear on the Cu-PI nanoparticle. However, increase in stirring speed did not significantly (P>0.05) alter the polydispersity index and zeta potential. Out of six formulations, formulation F2 produced minimum average particle size of 705.6 nm (Figure 5) with polydispersity index of 0.750 and formulation F1 produced maximum average particle size of 1 812.0 nm with polydispersity index of 1.023. Similarly, formulation containing SLS produced maximum zeta potential of -49.5 mV (Figure 6). However, Cu-Pi nanoparticles produced by emulsion polymerization method did not satisfy the acceptance limit of average particle size, polydispersity index and zeta potential. This method also required filtration step to get uniform particle size as the Figure 5 clearly indicated three peaks which had increased the polydispersity index.
Figure 5. Particle size distribution of Cu-Pi nanoparticle (F2) by emulsion polymerization method.
Figure 6. Zeta potential of Cu-Pi nanoparticle (F2) by emulsion polymerization method.
3.4. Fessi method
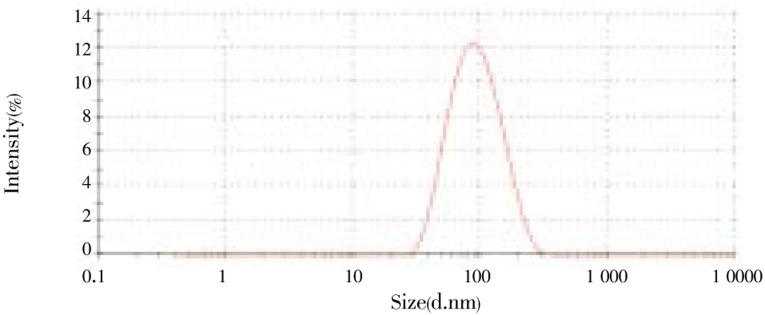
Six formulations (F1-F6) were prepared and characterized. In all three previous methods, study of effect of solvent on Cu-Pi nanoparticle did not produce significant change in Cu-Pi nanoparticles hence we have decided not to study the effect of solvent in this method. In solid dispersion method and emulsion polymerization method we have studied the effect of anionic and non-ionic surfactant and found anionic surfactant produced lesser average particle size and higher zeta potential. Non-ionic surfactant produced very low zeta potential and slightly increased average particle size than anionic surfactant. However, to prevent rapid elimination of drug by macrophages, coating with PEG containing copolymer is essential. Hence we have decided to use a cationic polymer Eudragit E 100 along with PEG containing copolymer (Poloxamer 188) intended to produce nanoparticles with high zeta potential and lesser average particle size. Formulations F1, F3, F4 and F6 were used to assess the effect of surfactant (SLS or Poloxamer 188) along with cationic polymer on the Cu-Pi nanoparticles and found no significant (P>0.05) change in average particles size and polydispersity index but use of SLS/Eudragit E 100 combination produced greater zeta potential than Poloxamer 188/Eudragit E 100 combination. We have also studied the effect of drug/polymer/copolymer on the Cu-Pi nanoparticles. Formulations F1, F3, F4 and F6 were used to assess the effect of drug/polymer/surfactant and found use of 1:10:10 ratio significantly (P<0.05) decreased the particles size than the use of 1:4:4 ratio. However, change of the ratio did not significantly (P>0.05) alter the polydispersity index and zeta potential. In emulsion polymerization method, we have studied the effect of stirring speed and found increase in stirring speed from 100 to 500 rpm significantly decreased the average particle size. Hence we have decided to study the effect of stirring (500 to 1 000 rpm) on the Cu-Pi nanoparticles. Formulations F1, F2, F4 and F5 were used to assess the effect of stirring speed and found increases in stirring speed from 500 to 1000 rpm did not significantly (P<0.05) alter the average particle size, polydispersity index and zeta potential. Out of six formulations, formulation F4 produced minimum average particle size of 85.43 nm (Figure 7) with polydispersity index of 0.183 and formulation F3 produced maximum average particle size of 239.0 nm with polydispersity index of 0.349. Formulation containing SLS/Eudragit E 100 combination produced maximum zeta potential of 53.1 mV (Figure 8) and formulation containing Poloxamer 188/Eudragit E 100 combination produced a zeta potential of 29.7 mV (Figure 9). However, formulation F4 satisfied the average particle size, polydispersity index and zeta potential acceptable limit. Moreover, Figure 7 clearly showed a single particle size peak.
Figure 7. Particle size distribution of Cu-Pi nanoparticle (F4) by Fessi method.
Figure 8. Zeta potential of Cu-Pi nanoparticle (F1) by Fessi method.
Figure 9. Zeta potential of Cu-Pi nanoparticle (F4) by Fessi method.
4. Discussion
Cu-Pi nanoparticles were successfully prepared by (a) thin film hydration method, (b) solid dispersion method, (c) emulsion polymerization method and (d) Fessi method. In all these methods, nanoparticles were formed spontaneously which were identified by turbidness. Instantaneous formation of nanoparticles occurs as a result of the polymer deposition on the interface between the organic phase and aqueous phase when aqueous miscible organic solvent diffused out quickly into the aqueous phase from each transient particle intermediate. According to the “Marangoni effect”, the transient particle intermediate causes a size reduction to the nano range. Formation of colloidal nanosuspension was confirmed by Tyndall effect (scattering of light by dispersed colloidal particle)[10]. In each method, we have used constant weight of curcumin and piperine as these weights were produced minimum average particle size in initial trials.
We have used either acetone or ethanol as an organic phase in all four methods as both the drugs and polymer are soluble in both solvents. Acetone and ethanol belong to class III solvents which are regarded as less toxic and lower risk to human health with daily exposure of 50 mg or less per day which would be acceptable without justification. Higher amounts may also be acceptable provided they are realistic in relation to manufacturing capability and good manufacturing practice[11]. The solubility of organic solvents in water was an important parameter affecting the mean size of nanoparticles[12]. Hence we have decided to study the effect of organic solvent on Cu-Pi nanoparticles and found use of either acetone or ethanol has no significant effect on Cu-Pi nanoparticles.
Surfactant helps in reducing the particle size due to the adsorption of surfactants on the surface of nanoparticles and prevents the aggregation due to the static repulsion and special hindrance. However, studies suggest that addition of anionic surfactant is the best choice to reduce the aggregation than the cationic and non-ionic surfactant[13]. But to prevent rapid clearance of drug from the systemic circulation by macrophages, a coating with PEG containing non-ionic surfactants are essential[14]-[16]. Hence we have decided to study the effect of non-ionic surfactant (Poloxamer 188 and Poloxamer 407), anionic surfactant (SLS) and combination of either non-ionic surfactant or anionic surfactant with cationic polymer (Eudragit E 100) on Cu-Pi nanoparticles and found use of SLS has produced a maximum zeta potential around -50 mV with average particle size above 150 nm and use of non-ionic surfactant has produced a maximum zeta potential below -10 mV with average particle size above 250 nm. However, use of Poloxamer 188/Eudragit E 100 combination has produced a maximum zeta potential around 30 mV with average particle size less than 100 nm. Similarly use of SLS/Eudragit E 100 combination has produced a maximum zeta potential 53.1 mV with the average particle size above 100 nm.
Sonication has been long used to produce nanosize particles. The principal effect of sonication is cavitation events (bubble formation). Bubbles, whose size is near the resonant size for the applied frequency begins to oscillate nonlinearly and eventually collapse. As a result of such collapse, a violent implosion occurs that produces extremely high temperatures, high pressures, and shock waves which randomly and uniformly shatter large particles into smaller discoid sections[17]. Sonication at various powers and frequencies (43-480 kHz) was studied and found small number of cavitation events with stronger physical disturbance can reduce the size more efficiently than the large number of cavitation events with weaker disturbance[18]. Hence we have decided to study the sonication (40 kHz) effect (5 and 10 cycle) on the Cu-Pi nanoparticles and found sonication for 10 cycles (5 minutes per cycle) has produced as significant size reduction than sonication for 5 cycles.
Mixing speed plays an important role in controlling the size and size distribution of the resulting emulsion particles. Below a critical stirring speed, spherical particles could not be formed and the inversion process resulted in macroscopically non-homogeneous multi shape structures. Fully spherical particles were formed above critical stirring speed. Further increase in the rotational speed of the mixer, significantly reduced the size of the spherical particles with a wide and random size distributions controlled and considerably narrowed by the stirring speed[19]. The result of the emulsion polymerization method has confirmed that increase in stirring speed from 100 to 500 rpm has significantly decreased the average particle size to around 700 nm and further increase in stirring speed in Fessi method from 500 to 1 000 rpm has reduced the average particle size below 100 nm which is mainly due to high shear rate[20].
Use of binder with medium or high viscosity leads to marked reduction of heterogeneities of average particle size[21]. However, increase in viscosity results in agglomerates[22]. Harris et al has shown that nasal formulations containing 0.00%, 0.25%, and 0.50% methylcellulose produced a dose-related increase in average particle size to 51, 81 and 200 µm, respectively[23]. However, the current study has also confirmed that by increasing the viscosity using sodium alginate has decreased the heterogeneity of particle size significantly but increased the average particle size of Cu-Pi nanoparticles.
Use of Poloxamer (188 or 407) has produced very low zeta potential less than -10 mV which may lead to aggregation on prolonged standing during storage and in the gut which may decrease the bioavailability. Studies have shown that use of mixed copolymer leads to average particle size reduction, produces optimal zeta potential and high entrapment efficiency, increases drug encapsulation, sustains release, prolongs circulation time of the drug, significantly enhances the bioavailability and also increases stability[24]-[28]. Hence we have decided to use the mixed copolymer approach and the present study has shown a marked decrease in average particle size and produced optimal zeta potential when a combination of Poloxamer 188 with Eudragit E 100 was used.
Based on the literature survey, acceptable limit for average particles size, polydispersity index and zeta potential was set at 100 nm, 0.2 and ±30 mV, respectively[2],[29].
In conclusion, we intended to prepare Cu-Pi nanoparticles to overcome curcumin limitations in the treatment of cancer. Cu-Pi nanoparticles were prepared by thin film hydration method, solid dispersion method, emulsion polymerization method and Fessi method as these methods are easy, highly reproducible and can be produced without any sophisticated instruments[30],[31]. Out of four methods, Fessi method has produced minimum average particle size with excellent polydispersity index and optimal zeta potential which fall within the acceptable limits of the study. This dual nanoparticulate drug delivery system appears to be promising to overcome oral bioavailability and cancer cell targeting limitations of curcumin in the treatment of cancer.
Acknowledgments
The authors are thankful to Mr. Mohanaram Pillai, Technical Writer (Freelance) for his editing service.
Footnotes
Foundation Project: This work is financially supported by UGC Sanction order No.F.4-1/2006 (BSR)/7-269/2009 (BSR).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Yu HL, Huang QR. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010;119:669–674. [Google Scholar]
- 2.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol. 2011;2(1):1–14. doi: 10.4291/wjgp.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YZ, Li YJ, Zhang LJ, Fang XL. Pharmacokinetics and biodistribution of Paclitaxel-loaded Pluronic P105 polymeric micelles. Arch Pharm Res. 2008;31(4):530–538. doi: 10.1007/s12272-001-1189-2. [DOI] [PubMed] [Google Scholar]
- 7.Kwon SH, Kim SY, Ha KW, Kang MJ, Huh JS, Im TJ, et al. Pharmaceutical evaluation of genistein-loaded Pluronic micelles for oral delivery. Arch Pharm Res. 2007;30(9):1138–1143. doi: 10.1007/BF02980249. [DOI] [PubMed] [Google Scholar]
- 8.Fessi H, Puisieux F, Devissaguet JP. Process for the preparation of dispersible colloidal systems of a substance in the form of nanoparticles. 1992. US5118528.
- 9.Hillery Anya M., Florence Alexander T. The effect of adsorbed poloxamer 188 and 407 surfactants on the intestinal uptake of 60-nm polystyrene particles after oral administration in the rat. Int J Pharm. 1996;132:123–130. [Google Scholar]
- 10.Mandal B, Alexander KS, Riga AT. Sulfacetamide loaded Eudragit RL100 nanosuspension with potential for ocular delivery. J Pharm Pharm Sci. 2010;13(4):510–523. [PubMed] [Google Scholar]
- 11.Bari SB, Kadam BR, Jaiswal YS, Shirkhedkar AA. Impurity profile: significance in active pharmaceutical ingredient. Eurasian J Anal Chem. 2007;2(1):32–53. [Google Scholar]
- 12.Song KC, Lee HS, Choung IY, Cho KI, Ahn YK, Choi EJ. The effect of type of organic phase solvents on the particle size of poly(d,l-lactide-co-glycolide) nanoparticles. Colloids Surfaces Physicochem Eng Asp. 2006;276:162–167. [Google Scholar]
- 13.Chai LY, Yu YF, Zhang G, Peng B, Wei SW. Effect of surfactants on preparation of nanometer TiO2 by pyrohydrolysis. Trans Nonferrous Met Soc China. 2007;17:176–180. [Google Scholar]
- 14.Cryan SA. Carrier-based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 2005;7(1):20–41. doi: 10.1208/aapsj070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 16.Mohanraj VJ, Chen Y. Nanoparticles-A review. Trop J Pharm Res. 2006;5(1):561–573. [Google Scholar]
- 17.Essa EA. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J Pharm. 2010;4:227–233. [Google Scholar]
- 18.Yamaguchi T, Nomura M, Matsuoka T, Koda S. Effects of frequency and power of ultrasound on the size reduction of liposome. Chem Phys Lipids. 2009;160(1):58–62. doi: 10.1016/j.chemphyslip.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Aravand MA, Semsarzadeh MA. Particle formation by emulsion inversion method: effect of the stirring speed on inversion and formation of spherical particles. Macromol Symp. 2008;274:141–147. [Google Scholar]
- 20.Mazumder B, Sarkar MK, Dey S, Roy N. Effect of formulation and process variables on the characteristics of microspheres of antiviral drug (stavudine) prepared by oil in oil solvent evaporation technique. Int J Pharm Pharm Sci. 2010;2(2):52–59. [Google Scholar]
- 21.Schaefer T, Johnsen D, Johansen A. Effects of powder particle size and binder viscosity on intergranular and intragranular particle size heterogeneity during high shear granulation. Eur J Pharm Sci. 2004;21(4):525–531. doi: 10.1016/j.ejps.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Johansen A, Schaefer T. Effects of interactions between powder particle size and binder viscosity on agglomerate growth mechanisms in a high shear mixer. Eur J Pharm Sci. 2001;2(3):297–309. doi: 10.1016/s0928-0987(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 23.Harris AS, Svensson E, Wagner ZG, Lethagen S, Nilsson IM. Effect of viscosity on particle size, deposition, and clearance of nasal delivery systems containing desmopressin. J Pharm Sci. 1988;77:405–408. doi: 10.1002/jps.2600770510. [DOI] [PubMed] [Google Scholar]
- 24.Kulthe SS, Inamdar NN, Choudhari YM, Shirolikar SM, Borde LC, Mourya VK. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: Implications for controlled and targeted drug delivery. Colloids Surf B Biointerfaces. 2011;88:691–696. doi: 10.1016/j.colsurfb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Mu CF, Balakrishnan P, Cui FD, Yin YM, Lee YB, Choi HG, et al. The effects of mixed MPEG-PLA/Pluronic copolymer micelles on the bioavailability and multidrug resistance of docetaxel. Biomaterials. 2010;31:2371–2379. doi: 10.1016/j.biomaterials.2009.11.102. [DOI] [PubMed] [Google Scholar]
- 26.Chang YC, Chu IM. Methoxy poly(ethylene glycol)-b poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur Polym J. 2008;44:3922–3930. [Google Scholar]
- 27.Laroiya I, Pankaja SS, Mittal S, Kate V. A study of Helicobacter pylori infection, dietary pattern and habits in patients with gastric cancer in South India. Asian Pac J Trop Dis. 2012;2(1):24–26. [Google Scholar]
- 28.Yusuf N, Ahmed Ali M, Islam MF, Khanam JA. Screening of cervical cancer by VIA among women in Rajshahi Medical College Hospital. Asian Pac J Trop Dis. 2012;2(1):70–72. [Google Scholar]
- 29.Wang JP, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly (ethylene glycol)-lipid conjugate and positively charged lipids. J Drug Target. 2005;13(1):73–80. doi: 10.1080/10611860400011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan RL, Sun X, Liu J, Gong T, Zhang ZR. Mixed micelles loaded with silybin-polyene phosphatidylcholine complex improve drug solubility. Acta Pharmacol Sin. 2011;32:108–115. doi: 10.1038/aps.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller BG, Leuenberger H, Kissel T. Albumin nanospheres as carriers for passive drug targeting: an optimized manufacturing technique. Pharm Res. 1996;13(1):32–37. doi: 10.1023/a:1016064930502. [DOI] [PubMed] [Google Scholar]