Abstract
Burkholderia cepacia (B. cepacia) infection is rarely reported in an immunocompetent host. It is a well known occurence in patients with cystic fibrosis and chronic granulomatous disease where it increases both morbidity and mortality. It has also been included in the list of organisms causing nosocomial infections in an immunocompetent host, most of them transmitted from the immunocompromised patient in which this organism harbors. We report a rare case of isolation of B. cepacia from the bronchoalveolar lavage fluid of an immunocompetent agriculturist who presented with productive cough and fever associated with a pyopneumothorax. This is the first case of community acquired infection reported in an immunocompetent person in India.
Keywords: Burkholderia cepacia, Pyopneumothorax, Community acquired, Immunocompetent, Agriculturist
1. Introduction
Burkholderia cepacia (B. cepacia) is a widely known lung pathogen in patients with cystic fibrosis[1]–[3]. It is now an important emerging cause of multi-drug resistant nosocomial infections causing both morbidity and mortality[2]. However, infection in an immunocompetent person is extremely rare.
2. Case report
A 32-year-old male agriculturist with no premorbid illness presented with productive cough with minimal, foul smelling, yellowish blood tinged expectoration associated with right sided pleuritic chest pain and high grade fever (40 °C) of 15 days duration. The patient was a non-smoker and non-alcoholic with no history of drug addiction or high risk behavior.
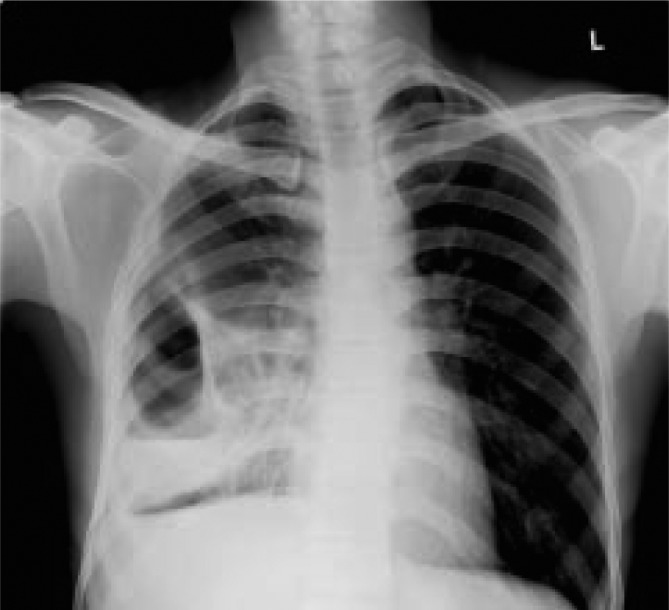
Examination revealed features suggestive of consolidation of the right middle & lower lobes of lung. Other systems were unremarkable. Initial lab investigations depicted a picture of anemia (Hb-10.8 mg/dL), high leucocyte count (18 500/cu.mm), raised ESR (117 mm/hr) and eosinophilia (12%). Chest roentogram revealed a loculated lesion in the right lower lung field with an air fluid level which was suggestive of a hydropneumothorax (Figure 1). Blood cultures sent on the day of admission, prior to the starting of antibiotics, were sterile. Sputum culture was also sent on day 1 of admission. The patient did not consent for CT thorax due to financial constraints. Ultrasound guided thoracocenthesis done showed purulent exudative fluid with a high cell count (neutrophilic predominant). He was then subjected to a fiberoptic bronchoscopy the next day, which showed purulent secretions in the right middle and lower lobes bronchial orifices with no endobronchial growth.
Figure 1. Erect postero-anterior view of chest roentogram showing loculated hydropneumothorax in the right lower lung field.
Aerobic cultures of sputum and bronchoalveolar lavage (BAL) fluid grew non-lactose fermenting, motile gram negative rods which were positive for catalase, oxidase and nitrate reduction. It utilized glucose, lactose, mannitol and maltose oxidatively. Lysine decarboxylation was positive while arginine hydrolysis and ornithine decarboxylation were negative (Figure 2). The isolate was identified as B. cepacia by phenotypic methods. The susceptibility testing was performed using Kirby-Bauer disk diffusion method as per the Clinical Laboratory Standards Institute (CLSI) guidelines. The pleural aspirate was however sterile. BAL PCR for mycobacterium tuberculosis and HIV serology were negative.
Figure 2. B. cepacia aminoacid decarboxylase test profile.

The patient was initially started on empirical broad-spectrum intravenous antibiotics (ceftiaxone and azithromycin) in view of his toxic condition. In vitro the organism was multidrug resistant except ceftazidime and meropenem. Thus the antibiotics were changed to ceftazidime. He responded clinically within 3 days of antibiotics with resolution of toxemia. Serial chest roentograms initially showed partial resolution of the lung lesion. Tube thoracostomy was deferred as the patient was improving. He received injectable antibiotics for a 2 week period. He made a complete clinical recovery at the time of discharge. After a month later's follow-up, chest roentogram showed evidence of radiological recovery.
3. Discussion
The B. cepacia complex is a group of phenotypically similar, genetically distinct, motile gram-negative aerobic bacilli with multi-trichous polar flagella found in both soil and water[1]–[6]. There are at least 15 species within this complex[5]. It has been widely documented as an important lung pathogen in patients with cystic fibrosis and chronic granulomatous disease associated with fatal outcomes[1]–[5]. B. cepacia was identified as a plant pathogen over 50 years ago[5]. However, recently it has been included in the list of organisms causing nosocomial infections[7],[8] and has emerged as an important cause of morbidity and mortality as it exhibits high intrinsic antibiotic resistance to most of the clinically available antibiotics[2],[5],[10]–[12], and is highly transmissible making management of diseased patients difficult.
The disease presentation can vary from asymptomatic carriage, superficial infections of the skin and soft tissue to deep-seated infections like pneumonia, especially in patients with cystic fibrosis[13]. It can also cause “cepacia syndrome” which is characterized by a confluent bronchopneumonia and septicemia, resulting in death[3],[4].
The virulence of this organism is what makes it so successful. It is known to produce siderophores, hemolysins and also resist neutrophilic killing. It produces lipopolysaccharide which has endotoxin activity and induces tumor necrosis factor-alpha (TNF-a) levels over nine times more than endotoxin extracted from Pseudomonas aeruginosa[13]. This is responsible for the strong pro-inflammatory response leading to a necrotizing response. They also have virulence factor genes which are clustered on distinct islands in bacterial chromosomes and on plasmids. These factors help in host cell attachment, invasion, intracellular survival and virulence regulation. The production of lipase also plays a role in invasion of lung epithelial cells[5],[6]. The multiresistance of B. cepacia bacteria appears to result from various efflux pumps that efficiently remove antibiotics from the cell. They also decrease contact of antibiotics with the bacterial cell surface due to their ability to form biofilms[15],[16]. Some B. cepacia strains have ability to invade and survive inside eukaryotic cells like human macrophages, and airway epithelial cells[17].
Although a common contaminant in hospital environment, with case reports of the pathogen having been recovered from sources such as nasal sprays, nebulizers and dialysis machines[18]–[23], B. cepacia is very rarely known to cause community-acquired infections in immunocompetent patients. There are a few case reports of nosocomial infection in India especially in ICU settings[24],[25]. However, to the best of our knowledge, this is the first report of B. cepacia isolation in a case of pyopneumothorax in an immunocompetent person in India. There was no evidence of cystic fibrosis or any other chronic lung disease in our patient. Being a farmer by profession, the likely source of infection in our patient could have been environmental as currently B. cepacia has been widely used for agricultural purposes due to its anti-nematodal and antifungal properties. However, since it can survive for several months together in water, the resulting contamination increases the risk for both increased human exposure and infection. This causes a conflict between its advantages in agricultural practices versus the potential harmful effects of acquiring severe infections from the contaminated environment[5],[12]. This case report illustrates that B. cepacia infection may also present outside the nosocomial setting. A high index of suspicion is required for a prompt diagnosis and appropriate antibiotic treatment should be initiated as per sensitivity pattern at the earliest.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Lynch JP. Burkholderia cepacia complex: impact on the cystic fibrosis lung lesion. Semin Respir Crit Care Med. 2009;30(5):596–610. doi: 10.1055/s-0029-1238918. [DOI] [PubMed] [Google Scholar]
- 2.Dizbay M, Tunccan OG, Sezer BE, Aktas F, Arman D. Nosocomial Burkholderia cepacia infections in a Turkish university hospital: a five-year surveillance. J Infect Dev Ctries. 2009;3(4):273–277. doi: 10.3855/jidc.124. [DOI] [PubMed] [Google Scholar]
- 3.Coutinho HD. Burkholderia cepacia complex: virulence characteristics, importance and relationship with cystic fibrosis. Indian J Med Sci. 2007;61(7):422–429. [PubMed] [Google Scholar]
- 4.Maschmeyer G. Mandell: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, Pennsylvania: Elsevier Churchill Livingstone; 2009. [Google Scholar]
- 5.McClean S, Callaghan M. Burkholderia cepacia complex: epithelial cell–pathogen confrontations and potential for therapeutic intervention. J Med Microbiol. 2009;58(1):1–12. doi: 10.1099/jmm.0.47788-0. [DOI] [PubMed] [Google Scholar]
- 6.Leitão JH, Sousa SA, Ferreira AS, Ramos CG, Silva IN, Moreira LM. Pathogenicity, virulence factors, and strategies to fight against Burkholderia cepacia complex pathogens and related species. Appl Microbiol Biotechnol. 2010;87(1):31–40. doi: 10.1007/s00253-010-2528-0. [DOI] [PubMed] [Google Scholar]
- 7.Bressler AM, Kaye KS, LiPuma JJ, Alexander BD, Moore CM, Reller LB, et al. Risk factors for Burkholderia cepacia complex bacteremia among intensive care unit patients without cystic fibrosis: a case-control study. Infect Control Hosp Epidemiol. 2007;28:951–958. doi: 10.1086/519177. [DOI] [PubMed] [Google Scholar]
- 8.Lee JKF. Two outbreaks of Burkholderia cepacia nosocomial infection in a neonatal intensive care unit. J Pediatr Child Health. 2008;44(1–2):62–66. doi: 10.1111/j.1440-1754.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 9.Bontemps C, Elliott GN. Burkholderia species are ancient symbionts of legumes. Mol Ecol. 2010;19(1):44–52. doi: 10.1111/j.1365-294X.2009.04458.x. [DOI] [PubMed] [Google Scholar]
- 10.Araque-Calderon Y, Miranda-Contreras L, Rodriguez-Lemoine V, Palacios-Pru EL. Antibiotic resistance patterns and SDS-PAGE protein profiles of Burkholderia cepacia complex isolates from nosocomial and environmental sources in Venezuela. Med Sci Monit. 2008;14:49–55. [PubMed] [Google Scholar]
- 11.Leitão JH, Sousa SA, Cunha MV, Salgado MJ, Melo-Cristino J, Barreto MC, et al. Variation of the antimicrobial susceptibility profiles of Burkholderia cepacia complex clonal isolates obtained from chronically infected cystic fibrosis patients: a five-year survey in the major Portuguese treatment center. Eur J Clin Microbiol Infect Dis. 2008;27(11):1101–1111. doi: 10.1007/s10096-008-0552-0. [DOI] [PubMed] [Google Scholar]
- 12.Slama TG. Gram-negative antibiotic resistance: there is a price to pay. Crit Care Med. 2008;12:4. doi: 10.1186/cc6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa SA, Ramos CG, Leitão JH. Burkholderia cepacia complex: emerging multihost pathogens equipped with a wide range of virulence factors and determinants. Int J Microbiol. 2011;2010:607575. doi: 10.1155/2011/607575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw D, Poxton IR, Govan JRW. Biological activity of Burkholderia (Pseudomonas) cepacia lipopolysaccharide. FEMS Immunol Med Microbiol. 1995;11:99–106. doi: 10.1111/j.1574-695X.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 15.George AM, Jones PM, Middleton PG. Cystic fibrosis infections: treatment strategies and prospects. FEMS Microbiol Lett. 2009;300(2):153–164. doi: 10.1111/j.1574-6968.2009.01704.x. [DOI] [PubMed] [Google Scholar]
- 16.Dales L, Ferris W, Vandemheen K, Aaron SD. Combination antibiotic susceptibility of biofilm-grown Burkholderia cepacia and Pseudomonas aeruginosa isolated from patients with pulmonary exacerbations of cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2009;28(10):1275–1279. doi: 10.1007/s10096-009-0774-9. [DOI] [PubMed] [Google Scholar]
- 17.Saldías MS, Valvano MA. Interactions of Burkholderia cenocepacia and other Burkholderia cepacia complex bacteria with epithelial and phagocytic cells. Microbiology. 2009;155(9):2809–2817. doi: 10.1099/mic.0.031344-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghazal SS, Al-Mudaimeegh K, Al Fakihi EM, Asery AT. Outbreak of Burkholderia cepacia bacteremia in immunocompetent children caused by contaminated nebulized sulbutamol in Saudi Arabia. Am J Infect Control. 2006;34:394–398. doi: 10.1016/j.ajic.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Dolan SA, Dowell E, Lipuma JJ, Valdez S, Chan K, James JF. An outbreak of Burkholderia cepacia complex associated with intrinsically contaminated nasal spray. Infect Control Hosp Epidemiol. 2011;32(8):804–810. doi: 10.1086/660876. [DOI] [PubMed] [Google Scholar]
- 20.Mann T, Ben-David D, Zlotkin A, Shachar D, Keller N, Toren A, et al. An outbreak of Burkholderia cenocepacia bacteremia in immunocompromised oncology patients. Infection. 2010;38(3):187–194. doi: 10.1007/s15010-010-0017-0. [DOI] [PubMed] [Google Scholar]
- 21.Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51(12):4217–4224. doi: 10.1128/AAC.00138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aygencel G, Dizbay M, Sahin G. Burkholderia cepacia as a cause of ecthyma gangrenosum-like lesion. Infection. 2008;36:271–273. doi: 10.1007/s15010-007-6357-8. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Manufacturer's recall of nasal spray contaminated with Burkholderia cepacia complex. Morb Mortal Wkly Rep. 2004;53:246. [Google Scholar]
- 24.Gautam V, Ray P, Puri GD, Sharma K, Vandamme P, Madhup SK, et al. Investigation of Burkholderia cepacia complex in septicaemic patients in a tertiary care hospital, India. Nepal Med Coll J. 2009;11(4):222–224. [PubMed] [Google Scholar]
- 25.Mukhopadhyay C, Bhargava A, Ayyagari A. Two novel clinical presentations of Burkholderia cepacia infection. J Clin Microbiol. 2004;42(8):3904–3905. doi: 10.1128/JCM.42.8.3904-3905.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


