Abstract
Objective
To screen the chitosan producing ability of endolichenic fungi and its antibacterial activity.
Methods
Lichen collected from mangroves was screened for endophytes and the chitosan producing ability of endolichenic fungi by submerged fermentation was also determined. Antibacterial activity was carried out against different pathogens.
Results
Totally 4 different groups of fungi were isolated from the lichen Roccella montagnei. Among the four genera, Aspergillus niger (A. niger) is potential to produce chitosan (1.3 g/L) on the twelfth day of incubation. Glucose plays an important role in the productivity of chitosan and the yield was maximum at 10% (1.93 g/L). Antibacterial activity revealed that Vibrio cholerae was sensitive to chitosan followed by Escherichia coli.
Conclusions
In conclusion, our findings suggest that A. niger is a potential candidate to produce more chitosan than the other strains and glucose plays an important role in the production of chitosan which proves to have a good antibacterial activity.
Keywords: Mangrove lichens, Roccella montagnei, Endolichenic fungi, Chitosan
1. Introduction
Mangroves are specialized ecosystems developed along estuarine sea coasts and river mouths in tropical and subtropical regions of the world, mainly in the intertidal zone. Hence, the ecosystem and its biological components are under the influence of both marine and freshwater conditions and have developed a set of physiological adaptations to overcome problems of anoxia, salinity and frequent tidal inundations. Mangrove ecosystems are of great ecological and economic significance in the tropical and sub-tropical coast[1]. Mangroves are tropical species which are rich in biodiversity where all kinds of relationship like mutualism, symbiotism and parasitism are present. The symbiotic association between an alga and a fungus has resulted in a new life form called lichen. Endolichenic fungi live in close association with algal photobionts inside asymptomatic lichen thalli and resemble fungal endophytes of plants in terms of taxonomy, diversity, transmission mode, and evolutionary history[2]. Approximately about eight percent of the terrestrial ecosystem is dominated by lichens[3]. Lichens, as the pioneer organisms, are widely distributed and dominated in the early developmental stages of a variety of bionetwork undergoing primary or secondary succession. Lichen thalli provide a mostly unexplored ecological niche for a wide variety of microorganisms including fungi. Fungi other than the obligate symbiotic mycobionts are frequently encountered during direct observation of lichen thalli, and their competitive presence often makes it difficult to isolate the mycobiont, although successful culture of certain mycobionts has been reported occasionally[4].
Chitosan which is a homopolymer of N-acetyl-D-glucosamine (Glc-NAc) residues linked by β-1-4 bonds, is the most widespread renewable natural resource following cellulose[5]. Mostly it is found in the cell walls and mycelia, stalks, and spores of Basidiomycetes, Ascomycetes, and Phycomycetes. Chitin is a natural substance found naturally in the exoskeletons of the insects, shells of crustaceans such as shrimps and in fungal cell walls. Chitosan is a partially deacetylated form of chitin by thermo-chemical deacetylation in concentrated sodium hydroxide[6]. Chitosan (poly-glucosamine) is a natural and biodegradable biopolymer. Chitosan has recently been used in many areas, for example, in cosmetics, pharmaceuticals, food additives and agriculture. Its uses are as a component of toothpaste, hand and body creams, shampoo, lowering of serum cholesterol, cell and enzyme immobilizer, as a drug carrier, material for production of contact lenses, or eye bandages, permeability control agent, as adhesive, chromatographic support, paper-strengthening agents, antimicrobial compounds, seed coats and flocculating and chelating agents in wastewater treatments[7].
In this study yield of chitosan production has been compared with different endolichenic fungal strains and its antibacterial activity was evaluated. Different concentrations of glucose were supplemented to enhance the mycelial growth for the chitosan production and the results were discussed briefly.
2. Materials and methods
2.1. Isolation and identification of endolichenic fungi
The lichen, Roccella montagnei (R. montagnei) was collected from Pichavaram mangroves (11°24′' N, 79°46′ E) situated in Southeast Coast of India. The samples were brought to the laboratory in sterile paper bags and processed within 6 h of collection. The lichen thalli was washed thoroughly and subjected to surface sterilization[8]. Briefly, lichen segments were subjected for serial washes and it was treated by immersing with 30% H2O2 solution for 90 sec followed by rinsing with 70% ethanol for 5 sec and 4% NaOCl for 90 sec and washed with sterile Milli-Q water. The segments were inoculated onto potato dextrose agar incorporated with chloramphenicol and were incubated in the light chamber for about 1 week at 25 °C. The colonies were isolated, subcultured and identified.
2.2. Submerged fermentation
Each strain was transferred separately into 1 000 mL conical flasks containing 500 mL of potato dextrose broth (PDB) and incubated at 25 °C for 12 days at 150 rpm. The wet biomass of mycelia was estimated from the aliquots withdrawn at every 2 days intervals. After incubation period, mycelia were harvested by vacuum filtration through Whatman No. 1 filter paper and washed with distilled water until a clear filtrate was obtained. The mycelia were lyophilized and ground to a powder and stored in desiccators at room temperature for further chitosan extraction.
2.3. Extraction and estimation of chitosan
Chitosan was extracted from the mycelia according to the method of Maghsoodi et al[9]. The harvested wet mycelia was added with 50 mL of 1 N NaOH solution and homogenized. The content was autoclaved, centrifuged at 6000 rpm for 20 min, and washed with distilled water to obtain neutral pH. The insoluble materials were dried at 40 °C for 24 h and treated with acetic acid 2% (v/v), as a chitosan solvent for 6 h at 95 °C (1:30 w/v). The acid insoluble fractions were centrifuged at 6 000 rpm for 15–20 min and the supernatant containing chitosan was collected and the pH was adjusted with 2 N NaOH in order to precipitate the fungal chitosan. The occulated chitosan was centrifuged at 6000 rpm, for 15 min and washed with distilled water to neutralize it. At the same time, ethanol (95%) and acetone were employed to rinse the chitosan and then it was dried in a vacuum oven dryer at 60 °C[10].
2.4. Effect of glucose concentration on chitosan extraction
PDB was supplemented with different concentrations of glucose ranging from 2% to 12%. Cultures were inoculated in PDB and incubated at 25°C for 16 days at 150 rpm in an incubator shaker[9].
2.5. Antibacterial activity of fungal chitosan
The human pathogenic bacteria such as Escherichia coli (E. coli), Vibrio cholerae (V. cholerae), Salmonella typhi (S. typhi), Staphylococcus aureus (S. aureus) were obtained from Department of Microbiology, Rajah Muthiah Medical College, Annamalai University. Pathogens were cultured on nutrient agar slants and antibacterial activity was assayed by disc diffusion method[11]. Then chitosan solution was prepared in 1% (v/v) acetic acid at 1% concentration (w/v) and stirred overnight at room temperature. Overnight culture was swabbed on mueller hinton agar (MHA) plates and discs were impregnated with the concentration of 50 µL, 100 µL, 150 µL of the chitosan solution, respectively. Discs were placed on MHA medium and were incubated at 37 °C for 24 h. The zone of inhibition was measured and the results were recorded.
3. Results
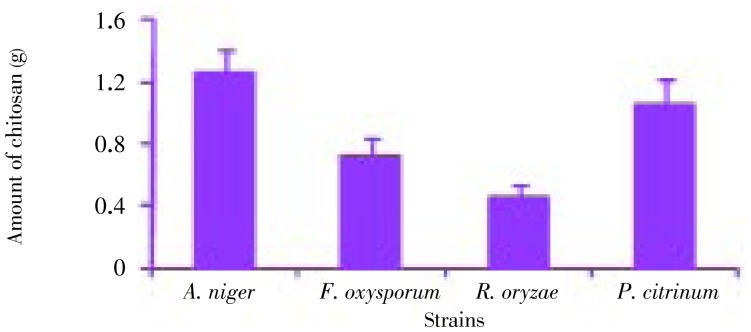
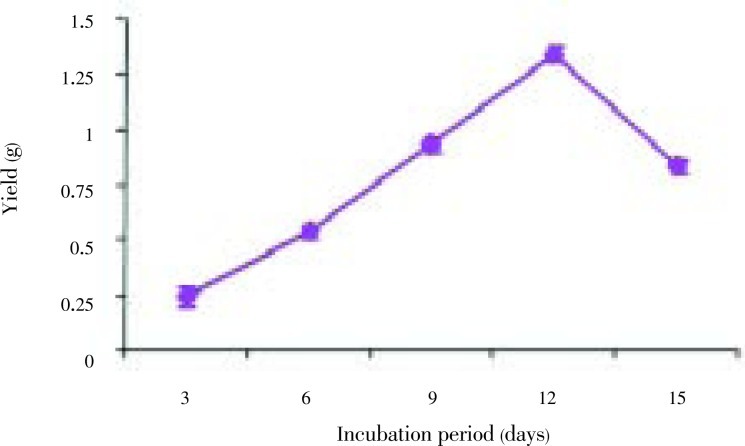
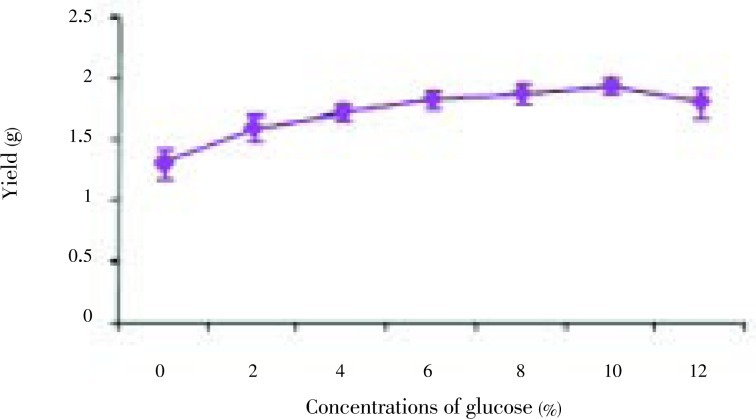
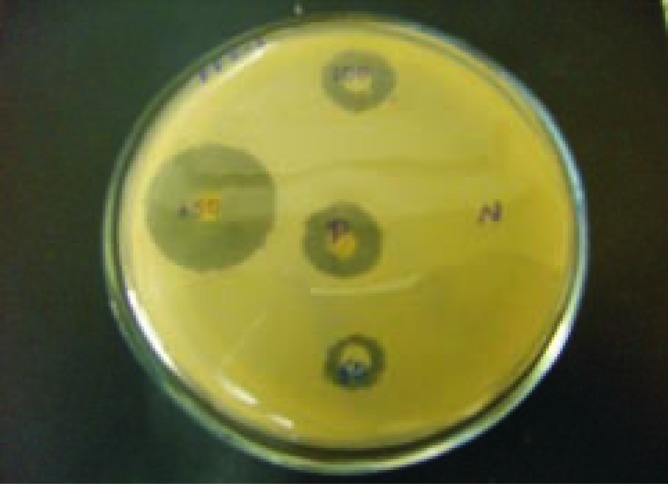
The fungal strains Aspergillus niger (A. niger), Penicillium citrinum (P. citrinum), Rhizopus oryzae (P. oryzae), Fusarium oxysporum (F. oxysporum) were isolated from the thallus of R. montagnei. Among the four strains tested for the production of chitosan, A. niger was found to be potential strain based on the yield. A. niger showed the highest yield of 1.3 g after 12 days of cultivation, while P. citrinum, R. oryzae and F. oxysporum grew very slowly compared with A. niger with yield of 1.05, 0.45 and 0.72 g on the 12th day of cultivation, respectively and it was shown in Figure 1. The chitosan production by each strain at different incubation time and cell growth was shown in Figure 2. A. niger was inoculated in PDB with different concentrations of glucose ranging from 2%-12%. Glucose concentrations were changed in the medium and the results showed that, on the 10th day of inoculation, the chitosan yield increased to 1.93 g/L in the medium containing 10% glucose. From Figure 3, growth of mycelial mass and chitosan production were increased with increasing glucose concentration from 2% to 10% and maximum attained at 10% concentration. Antibacterial activity revealed that V. cholerae was very sensitive to chitosan followed by E. coli and S. aureus. The zone of inhibition for V. cholerae by chitosan was 9 mm in 100 µL and 10 mm in 150 µL. The results for the antibacterial activity were expressed in Table 1 and Figure 1.
Figure 1. Chitosan extraction from different strains.
Figure 2. Chitosan yield on different incubation period.
Figure 3. Chitosan yield based on the glucose concentration.
Table 1. Antibacterial activity of chitosan isolated from endolichenic fungi.
| Treatment | Zone of inhibition (mm) |
||||
| V. cholerae | E. coli | S. typhi | S. aureus | ||
| Chitosan (µL) | 50 | – | – | – | – |
| 100 | 9 | 3 | – | – | |
| 150 | 10 | 7 | – | 6 | |
| Control | +ve | 5 | 4 | 6 | 7 |
| –ve | – | – | – | – | |
Figure 4. Antibacterial activity of the chitosan.
4. Discussion
The lichen was collected from the mangrove plant Rhizophora apiculata from Pichavaram mangroves. Lichens are very thin in nature, so mild sterilization was given to isolate the endolichenic fungi. Due to the spongy texture of lichen tissues, chemical surface sterilization is not needed. Also it should not be subjected for the prolonged sterilization with hydrogen peroxide or ethanol.
A. niger appeared to have a greater potential to be used for extraction of chitinous material due to a more abundant biomass production[12]. Maximum yield (0.8455 g/L) was obtained on the 6th day after inoculation under submerged fermentation by A. niger[9]. However, it has been previously reported that the late exponential phase produces the most extractable chitosan[13]. But in our present study, A. niger was shown to give a maximal yield of chitosan at 1.34 g/L dry weight. Chitosan yields from this study were lower than those of other fungal strains, e.g. Actinomucor sp., Mucor sp., Rhizopus sp. and Zygorhynchus sp.[14]. The chitosan yield of Lentinula edodes was reported to be 20–50 mg/g dry weight[15] and the chitosan yield of Rhizopus oryzae was 270–700 mg/L[16].
Similar to the present study, Maghsoodi et al[9] also studied the glucose concentration on chitosan production. They found that the maximum yield of chitosan was 0.9121 g/L when the medium containing 8% glucose concentrations on the twelfth day of inoculation and the yield was comparatively lower than the present study. Therefore, the cultivation method is also an important factor for fungal chitosan production.
The antimicrobial effect strengthened as the concentration of chitosan increased and one percent chitosan solution could inhibit both bacteria completely. It was also observed that the antimicrobial activity to S. aureus was much greater than to E. coli. 0.5% chitosan solution could inhibit the growth of S. aureus completely[17]. In addition, chitosan with a low molecular weight was reported to reduce the tensile strength and elongation of the chitosan membrane but to increase its permeability[18]. Even chitosan is considered as an antimicrobial agent, but it is not much effective as it is coupled with zinc. It was also reported that chitosan-zinc complex showed effectively wide spectrum antimicrobial activities against all of the microorganisms used in the test, although differences existed among different kinds of microorganisms[19]. Generally, the complexes had better antibacterial activity than antifungal activity. There were no obvious differences observed between the complexes' antibacterial activities against gram-positive bacteria with gram-negative bacteria[19]. As a kind of macromolecule polymer, chitosan seems to be unable to pass the outer membrane of bacteria, since this membrane functions as an efficient outer permeability barrier against macromolecules[15]. Because of the above reasons, chitosan has not shown any effect against S. typhi.
Hence from the present study, it was concluded that among the strains isolated from the lichen (R. montagnei), A. niger was considered as a potential candidate for the chitosan production and glucose played an important role in the production of chitosan. Chitosan has exhibited a good activity against bacterial strains; even it is very stronger if it has been coupled with zinc.
Acknowledgments
The authors are thankful to Professor Balasubramanian T, Dean of our centre for all the support and facilities rendered and Ministry of Environment and Forests, Govt. of India for the financial grant (No: 22-9/2008-CS-I) to carry out this work.
Footnotes
Foundation Project: This work was financially supported by Ministry of Environment and Forests, Govt. of India (grant No. 22-9/2008-CS-I).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Chowdhury MSN, Hossain MS, Mitra A, Barua P. Environmental functions of the Teknaf Peninsula mangroves of Bangladesh to communicate the values of goods and services. Mesopot J Mar Sci. 2011;26(1):79–97. [Google Scholar]
- 2.U'Ren JM, Lutzoni F, Miadlikowska J, Arnold AE. Community analysis reveals close affinities between endophytic and endolichenic fungi in mosses and lichens. Microb Ecol. 2010;60:340–353. doi: 10.1007/s00248-010-9698-2. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SR, Thajuddin N, Upreti DK. Diversity of lichens in Kollihills of Tamil Nadu, India. Int J Biodivers Conserv. 2001;3(2):36–39. [Google Scholar]
- 4.Li WC, Zhou J, Guo SY, Guo LD. Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers. 2007;25:69–80. [Google Scholar]
- 5.Limam Z, Selmi S, Sadok S, El Abed A. Extraction and characterization of chitin and chitosan from crustacean by-products: biological and physicochemical properties. Afr J Biotechnol. 2011;10(4):640–647. [Google Scholar]
- 6.Rajat K, Jyoti A. Chitosan as classic biopolymer: a review. Int J Pharm Life Sci. 2011;1(7):369–372. [Google Scholar]
- 7.Kannan M, Nesakumari M, Rajarathinam K, Singh AJAR. Production and characterization of mushroom chitosan under solid-state fermentation conditions. Adv Biol Res. 2010;4(1):10–13. [Google Scholar]
- 8.Bates ST, Cropsey GWG, Caporaso JG, Knight R, Fierer N. Bacterial communities associated with the lichen symbiosis. Appl Environ Microbiol. 2011;77(4):1309–1314. doi: 10.1128/AEM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maghsoodi V, Razavi J, Yaghmaei S. Production of chitosan by submerged fermentation from Aspergillus niger. Chem Chem Eng. 2009;16(2):145–148. [Google Scholar]
- 10.George TS, Guru KSS, Vasanthi NS, Kannan KP. Extraction, purification and characterization of chitosan from endophytic fungi isolated from medicinal plants. World J Sci Technol. 2011;1(4):43–48. [Google Scholar]
- 11.Kumar R, Kumar SS, Chakraborti A. Comparison of broth dilution and disc diffusion method for the antifungal susceptibility testing of Aspergillus flavus. Am J Biomed Sci. 2010;2(3):202–208. [Google Scholar]
- 12.Romanazzi G, Gabler FM, Margosan D, Mackey BE, Smilanick JL. Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopathology. 2009;99(9):1028–1036. doi: 10.1094/PHYTO-99-9-1028. [DOI] [PubMed] [Google Scholar]
- 13.Maghsoodi V, Yaghmaei S. Comparison of solid substrate and submerged fermentation for chitosan production by Aspergillus niger. Chem Chem Eng. 2010;17(2):153–157. [Google Scholar]
- 14.Kim WJ, Lee WG, Theodore K, Chang HN. Optimization of culture conditions and continuous production of chitosan by the fungi, Absidia coerulea. Biotechnol Bioprocess Eng. 2001;6:6–10. [Google Scholar]
- 15.Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol. 2001;71:235–244. doi: 10.1016/s0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 16.Streit F, Koch F, Laranjeira MCM, Ninow JL. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz J Microbiol. 2009;40:20–25. doi: 10.1590/S1517-83822009000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tajik H, Moradi M, Rohani SMR, Erfani AM, Jalali FSS. Preparation of chitosan from brine shrimp (Artemia urmiana) cyst shells and effects of different chemical processing sequences on the physicochemical and functional properties of the product study on antimicrobial activity of chitosan with different molecular weights. Molecules. 2008;13:1263–1274. doi: 10.3390/molecules13061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohy Eldin MS, Soliman EA, Hashem AI, Tamer TM. Antibacterial activity of chitosan chemically modified with new technique. Trends Biomater Artif Organs. 2008;22(3):125–137. [Google Scholar]
- 19.Badawy MEI, Rabea EI. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int J Carbohydr Chem. 2011;2011:29. [Google Scholar]