Abstract
Objective
To identify the available phytochemicals and carotenoids in the selected green algae and evaluate the potential genotoxic/antigenotoxic effect using lymphocytes.
Methods
Organic solvent extracts of Chlorococcum humicola (C. humicola) were used for the phytochemical analysis. The available carotenoids were assessed by HPLC, and LC-MS analysis. The genotoxicity was induced by the benzo(a)pyrene in the lymphocyte culture, the genotoxic and antigenotoxic effects of algal carotenoids with and without genotoxic inducer were evaluated by chromosomal aberration (CA), sister chromatid exchange (SCE) and micronucleus assay (MN).
Results
The results of the analysis showed that the algae were rich in carotenoids and fatty acids. In the total carotenoids lutein, β-carotene and α-carotene were found to be present in higher concentration. The frequency of CA and SCE increased by benzo(a)pyrene were significantly decreased by the carotenoids (P<0.05 for CA, P<0.001 for SCE). The MN frequencies of the cells were significantly decreased by the treatment with carotenoids when compared with the positive controls (P<0.05).
Conclusions
The findings of the present study demonstrate that, the green algae C. humicola is a rich source of bioactive compounds especially carotenoids which effectively fight against environmental genotoxic agents, the carotenoids itself is not a genotoxic substance and should be further considered for its beneficial effects.
Keywords: Chlorococcum humicola, Benzo(a)pyrene, Genotoxicity, Antigenotoxicity, Chromosomal aberration, Sister chromatid exchange, Micronucleus assay, Carotenoids, Green algae
1. Introduction
The cellular macromolecules of humans, such as DNA, proteins and lipids, are continuously at risk for endogenous and environmentally induced structural alterations. Several man-made chemicals find their way into the environment and pose health risks to the human population. Acute and chronic exposure to these environmental chemicals, such as pesticides, metals, polycyclic aromatic hydrocarbons, solvents, and alkylating agents, has been shown to produce marked toxicity at their target sites. Some of these chemicals are used as therapeutic agents by virtue of their antitumor, antibiotic activity[1].
Reactive oxygen species (ROS) are another important class of damage agents for cellular macromolecules. ROS, such as O2−, OH− and H2O2, are highly genotoxic/ mutagenic and harmful to cellular macromolecules such as DNA, proteins and lipids[2]. The adverse effect is represented by the oxidative stress that can arise from a lack of antioxidant defence or by an increase of oxidative processes in the body[3]. ROS produced in cells, largely as by-products of metabolic processes, constantly threaten the integrity and correct functioning of cellular DNA. Several oxidant species have the capacity to produce promutagenic lesions in DNA, which may play a significant role in the development of cancer[4].
It has been established that DNA damage induced by ROS is involved in aging and different human diseases such as atherosclerosis, cancer, neurodegenerative diseases and AIDS. The oxidative DNA damage arises from the attack of ROS on DNA bases or deoxyribose residues to produce damaged bases or strand breaks. In addition, ROS can attack protein or lipid molecules (process of lipid peroxidation) and generate intermediates that can react with DNA to form adducts[5].
Cancer chemoprevention is defined as the use of chemicals or dietary components to block, inhibit, or reverse the development of cancer in normal or preneoplastic tissue. A large number of potential chemopreventive agents have been identified, and they function by mechanisms directed at all major stages of carcinogenesis[6]. Because it is widely held that damage to DNA by reactive species is a significant contributor to carcinogenesis, it has been postulated that antioxidants in supplements or in foods containing them, which decrease oxidative DNA damage, should have a protective effect against cancer. It has been shown in many studies that DNA strand breaks can be reduced by antioxidant supplements or by various combinations of fruits and vegetables that are high in antioxidant nutrient content[7].
Therefore, as a strategy to prevent genotoxic event, antioxidant and radical scavenging properties of carotenoids, especially of β-carotene, are widely used. Carotenoids have been reported to have multiple biological activities such as anticarcinogen, antimutagen, antioxidant, anti-inflamatory, antiproliferative and antiatherogenic properties and chemopreventive agent against cancer diseases in various organs like lung, stomach, colon, breast and prostate. Also carotenoids play an important role in immune response, neoplastic transformation and control of growth and intracellular communication[8].
For the last couple of decades, natural products derived from plants, fruits, spices, herbs, etc have been the main focus of research to ameliorate the threat posed by harmful chemicals, toxins, etc from endogenous and exogenous sources. Overwhelming evidence from epidemiological studies indicates that diets rich in fruits and vegetables can be associated with a lower risk of numerous diseases. Natural products like fruits, vegetables, herbs, etc contain several promising chemopreventive compounds such as vitamins, minerals, carotenoids and an array of other phytochemicals[9].
Antioxidant activity is therefore considered to play an important role in the protective effects of fruits and vegetables against cancer. b-Cryptoxanthin, one of the six major carotenoids (b-carotene, lycopene, lutein, b-cryptoxanthin, zeaxanthin and a-carotene) routinely measured in human serum, is obtained primarily from citrus fruits, like other carotenoids. b-Cryptoxanthin is an antioxidant and may help prevent free radical damage to biomolecules including lipids, proteins and nucleic acids. Retinoids, the cleavage products of carotenoids, as well as being antioxidants, have in some cases (including b-cryptoxanthin) vitamin A activity and may play an important role in the prevention and treatment of certain cancers[10].
There are over 600 known carotenoids, including compounds such as lutein, alpha-carotene, beta-carotene and lycopene, and they are commonly found in many red, yellow and orange fruits and vegetables. Carotenoids are only synthesized in microorganisms and plants, where they are involved in photosynthesis. They are important dietary sources of vitamin A[11].
Various studies, including those using short-term assays, have helped to identify a great number of antimutagenic properties found in some foods such as: β-carotene, ascorbic acid, linoleic acid, γ-tocopherol, vanillin, chlorophyllin, polyphenols and components found in black and green teas and mushrooms. For this reason, these substances in natural foods are extremely important not only due to their nutritional value but also as prophylactic agents against diseases such as cancer[12].
Various reports confirm the existence of bioactive carotenoids in green algae such as α-carotene, β-carotene, astaxanthin, lutein, zeaxanthin, cryptoxanthin, vialoxanthin, etc. Also, there are some reports on the biological activities of several seaweed species, specifically the antiproliferative activity of ethanolic and aqueous extracts from green algae[13] and Turbinaria orrata[14] against human cancer cells. Two previous in vitro studies, noted the anti-genotoxic activity of Ulva rigida and the DNA damage protecting activity and antioxidant potential of crude ethanolic extract of Codium tomentosum Stackhouse in human lymphocytes[15].
The present study, was aimed to investigate the presence of phytochemicals and carotenoids in the selected unicellular green algae Chlorococcum humicola (C. humicola) and, to evaluate the oxidative DNA damage-protecting activity of carotenoid extracts using human lymphocytes in vitro.
2. Materials and methods
2.1. Collection and culturing of algae
Fresh water, unicellular, nonmotile green algae C. humicola was obtained from the culture collected from the Department of Plant Biology and Plant Biotechnology, RKM Vivekanantha College, Chennai, India. Algal culturing was carried out with 100 mL Bold's basal medium supplemented with sterile compressed air and kept under fluorescent light (20 µmol/m/s) with 16 h light period and at (25±2) °C temperature. Algae samples were cleaned of epiphytes and necrotic parts were removed. Then the samples were rinsed with sterile water to remove any associated debris.
2.2. Preparation of algal crude extracts and phytochemical analysis
The algal samples were centrifuged at 2 500 rpm for 10 min to remove the water content. 25 g of fresh algae was extracted for 15 min with 50 mL of organic solvents, i.e. acetone, benzene, chloroform, diethyl ether, ethyl acetate, ethanol, hexane and methanol. All the crude extracts were used for the existence of available phytochemicals such as total carbohydrates, total amino acids, total proteins and total lipids. Saponins, tannins, glycosides, carotenoids, alkaloids, flavanoids and phenolic compounds were carried out by the standard methods[16].
2.3. Carotenoid extraction and estimation
Algal sample (1 g dry weight) was extracted with ethanol until all the pigments were removed, and filtered through a sintered glass filter (porosity 3; pore size 20–30 Q). An equal volume of diethyl ether was added to the combined ethanol extracts, followed by the addition of water droplets until two layers were formed. The ethereal epiphase, containing all the pigments, were washed free from ethanol with water, and the solvent was removed. The residue was then saponified with equal volume of 10% methanolic KOH and kept in overnight in the room temperature at dark, after which the carotenoid solution was washed with water to remove the alkali (pH: 7.0) dried over Na2SO4. The unsaponifiable residue was dissolved in a little ether and then in 10 mL of petroleum ether (b.p. 40–60 °C). This extraction was used for further analysis. The total caroteoids were estimated spectrophotometrically at 450 nm.
2.4. High performance liquid chromatography analysis (HPLC) of carotenoids
The carotenoid profile of C. humicola was analyzed by HPLC (LC-10A, Shimadzu) using a reversed phase (25 cm × 4.6 mm) column with an isocratic solvent system consisting of acetonitrile/methanol/dichloromethane (70:10:20) at a flow rate of 1.0 mL and detected at 467 nm[17].
2.5. Liquid chromatography-mass spectrometry (LC-MS) analysis of carotenoids
Identification of the different carotenoids was made using Hewlett Packard HB 5890 liquid chromatography (LC) coupled with 5989 B series mass spectrometer (MS). Mass spectra of carotenoids were acquired with an m/z detector and 400–600 scan range at 450 nm by a diode array detector and confirmed with respected standards. Identification of the individual components was performed by comparison of mass spectra with the profiles from the Wiley GC-MS 275 libraries[18].
2.6. Genotoxicity/antigenotoxicity assays
2.6.1. Leucocyte culture
Heparinised peripheral blood obtained from five healthy donors, who were non-smoking, non alcoholic, non users of drugs, antibiotics, analgesics were used in all experiments. Leucocyte cultures were set up for chromosomal aberration (CA) and sister chromatid exchange (SCE) analysis together along with control cultures for each. 5 mL of F-10 medium supplemented with 5 mL 10% AB serum and 0.3 mL of phytohemagglutinin were taken in sterile glass bottles. About 0.3 mL of blood was added to this and the bottles tightly corked and incubated at 37 °C for 24 h.
2.6.2. CA and SCE
At the end of 24 h, the total carotenoid extracts at a concentration of 100, 200 and 300 µg/mL were added to the cultures used for scoring CA. The cultures used for SCE were supplemented with BrdU at the concentration of 5 µg/mL and all the bottles were further incubated at 37 °C for 24 h in complete darkness. After 48 h of setting up the cultures used for CA analysis were terminated. While the cultures used for SCE analysis were treated with carotenoid extracts at a concentration of 100, 200 and 300 µg/mL and incubated at 37 °C for 24 h at the end they were terminated. For induction of oxidative and chromosomal damage, the lymphocytes were treated with benzo(a)pyrene [B(a)P] (25 µg/mL) as positive controls. The mutagenic solution was prepared immediately before use, to avoid degradation of the agents. As a negative control, distilled water was also used.
2.6.3. Harvesting the cultures
Harvesting the cultures was carried out at the end of 48 h for CA and 72 h for SCE. Colchicine was added at a concentration of 0.2 µg/mL to the cultures 90 min prior to harvesting, to arrest the cells at metaphase. 90 min after colchicines addition, the cultures were centrifuged for 10 min at 1 000 rpm. The supernatant was discarded and pellet was mixed gently. The cells were then exposed to hypotonic KCl solution (0.075 M; pH 7.0) for 20 min at 37 °C. After 20 min, the cells were spun down and KCl was discarded, then the cells were fixed by slow addition of freshly prepared, chilled fixative solution (methanol: acetic acid in 3:1 ratio). The pellet was aspirated gently and mixed with the fixative evenly using a Pasteur pipette. The tubes were incubated at 4 °C for at least 30 min, after which the fixative was removed by centrifugation. The pellet was washed well 3–4 changes of fixative till a clear supernatant was obtained. A few drops of cell suspension were dropped on a clean, grease free slide which was stored under chilled distilled water prior to the slide preparation. The slide was dried at 60 °C on a hot plate.
2.6.4. Staining for CA
The slides were stained with BDH liquid Giemsa and were diluted 20 times with KH2PO4 solution (0.340 2%; pH 6.8) for 10 min. The slides were washed well with distilled water and air dried. Fifty to hundred complete well spread metaphases were scored for CA from each culture. The results were expressed as the number of CAs/metaphases.
2.6.5. Staining for SCE
The slides were aged for 3 days for at least 3 days prior to staining. The slides were stained in the dark with Hoechst 33258 stain dissolved in PBS to give a concentration of 10 µg/mL for 15 min. They were then rinsed with distilled water and arranged in a petridish. Distilled water was added onto the slides, so that a thin film of water was formed on the slides. The slides were then exposed to UV lamp (360 nm) at a height of 15–17 cm for 2 h. After UV exposure, the slides were treated with SSC solution maintained at 60 °C for 2 h. The slides were then washed thoroughly with distilled water, care being taken not to precipitate the salts. The slides were then counter stained with diluted Giemsa for 15 min. After staining, the slides were aged for 7 days. At least 20 well spread metaphases/cultures were scored for the occurrence of SCE. The results were expressed as the number of SCEs/metaphases[19].
2.6.6. Micronucleus assay (MN)
Two thousand binucleated cells for each experimental point were examined, following the scoring criteria adopted by the Human Micronucleus Project. We evaluated the binucleated micro nucleated lymphocyte (BNMN) frequency as the number of binucleated lymphocytes containing one or more MN per 1 000 binucleated cells. In the anti-clastogenicity experiments, the present reduction by treatment with carotenoids in the number of cells with micronuclei that showed protective activity was calculated according to Bonassi et al[20] using the following formula:
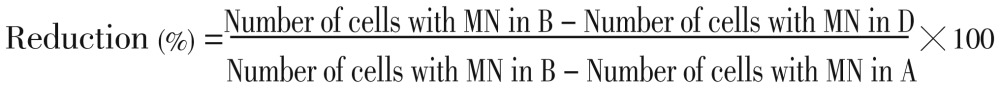 |
Where B was the group of cells treated with the corresponding positive control i.e. B(a)P, D was the group of cells treated with carotenoids plus the positive control and A was the negative control group.
2.6.7. Mitotic index (MI), proliferative index (PRI) and nuclear division index (NDI)
The MI was calculated from the number of metaphases in 2 000 cells, analyzed per culture for each dose group and donor in CA and SCE assays. In the SCE assay, PRI was calculated using the following formula:
PRI = (M1 + 2M2 + 3M3)/N where M1, M2 and M3 indicate those metaphases corresponding to first, second and third divisions, and N is the total number of metaphases scored. Moreover, in the micronuclei assay, 500 lymphocytes were scored to evaluate the percentage of binucleated cells, and NDI was calculated according to the following formula:
NDI = [MONO + 2BN + 3TRI + 4TETRA]/500 where MONO, BN, TRI and TETRA are mononucleated, binucleated, trinucleated and tetranucleated lymphocytes, respectively.
2.7. Statistical analysis
The different frequencies of DNA damage on lymphocyte cultures were compared by using two-way analysis of variance (ANOVA) with 95% confidence intervals. These analysis were carried out with commercial software programs SPSS 12.
3. Results
3.1. Phytochemical analysis
The existence of phytochemicals of C. humicola was shown in Table 1. Fatty acids and chief colouring pigment carotenoids were present at higher concentrations. Carbohydrates, proteins and aminoacids are also found to be available. Saponins and alkaloids were found at the major concentration. Flavanoids and phenolic compounds were identified. Tannins and glycosides were absent.
Table 1. Phytochemical analysis of various organic extracts of C. humicola.
| Identified compounds | Acetone | Benzene | Chloroform | Diethyl ether | Ethyl acetate | Ethanol | Hexane | Methanol |
| Carbohydrates | + | + | + | + | + | + | + | + |
| Fatty acids | + | + | + | + | + | + | + | + |
| Proteins | + | + | + | + | + | + | + | + |
| Aminoacids | + | + | + | + | + | + | + | + |
| Saponins | + | – | + | + | + | + | + | – |
| Tannins | – | – | – | – | – | – | – | – |
| Carotenoids | + | + | + | + | + | + | + | + |
| Flavonoids | + | + | + | + | + | + | + | + |
| Alkaloids | + | + | + | + | + | + | + | + |
| Phenolic compounds | + | + | + | + | + | + | + | + |
| Glycosides | – | – | – | – | – | – | – | – |
+: Present; –: Absent.
3.2. HPLC/GC-MS analysis of total carotenoids
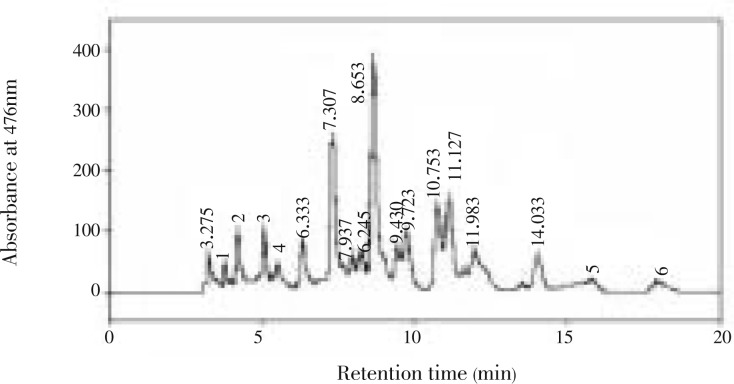
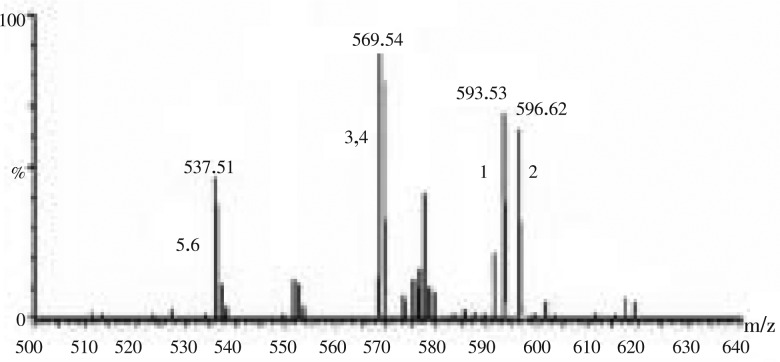
In C. humicola, six different carotenoid pigments were separated and detected. The carotenoid samples were eluted under isocratic conditions within 21 min. The carotenoid profile of C. humicola through C18 column was violaxanthin, astaxanthin, lutein, zeaxanthin, α-carotene and β-carotene (Table 2 and Figure 1). The carotenoids were analyzed using authentic standards. The carotenoids were identified by mass spectra showed in Figure 2. Spectra were generated from 10 µL injections of 50 ng/µL solutions. The carotenoid levels were as follows astaxanthin-17%, violaxanthin-4%, zeaxanthin-2%, lutein-41%, β-carotene-22%, and α-carotene-12%. Higher concentration of leutin and β-carotene was found in the selected green algae C. humicola.
Table 2. List of carotenoids, spectral data in the mobile phase and in different solvents.
| No | Pigment | Acetone | Benzene | Ethanol | Hexane | Eluent |
| 1 | Violaxanthin | – | 428 454 483 | 419 441 471 | 443 472 | 416 440 472 |
| 2 | Astaxanthin | – | 433 457 488 | 428 449 473 | 422 445 475 | 420 448 476 |
| 3 | Lutein | – | – | 420 445 475 | – | 424 448 472 |
| 4 | Zeaxanthin | 440 463 492 | – | – | 425 450 479 | 452 480 |
| 5 | α-carotene | – | – | – | 420 442 472 | 420 448 476 |
| 6 | β-carotene | – | – | – | 425 449 477 | 428 452 476 |
Figure 1. HPLC profile of carotenoids from C. Humicola extract.
1: violaxanthin; 2: astaxanthin; 3: lutein; 4: zeaxanthin; 5: α-carotene; 6: β-carotene.
Figure 2. LC-MS profile of carotenoids from C. humicola extract.
1: violaxanthin; 2: astaxanthin; 3: lutein; 4: zeaxanthin; 5: α-carotene; 6: β-carotene.
All these spectra were characterized by [M+H]+ ions. Additional ions corresponding to [M+H-H2O]+ were observed for astaxanthin, violaxanthin, lutein and zeaxanthin. Astaxanthin and zeaxanthin showed significant [M+H-H2O]+ ions in addition [M+H]+ ions. α-carotene and β-carotene compounds did not contain hydroxyl or epoxy functional group. These two compounds showed [M+H]+ ions. The presence of trace amount of violaxanthin, astaxanthin and zeaxanthin was found comparing with lutein and carotenes.
3.3. Chromosomal aberrations
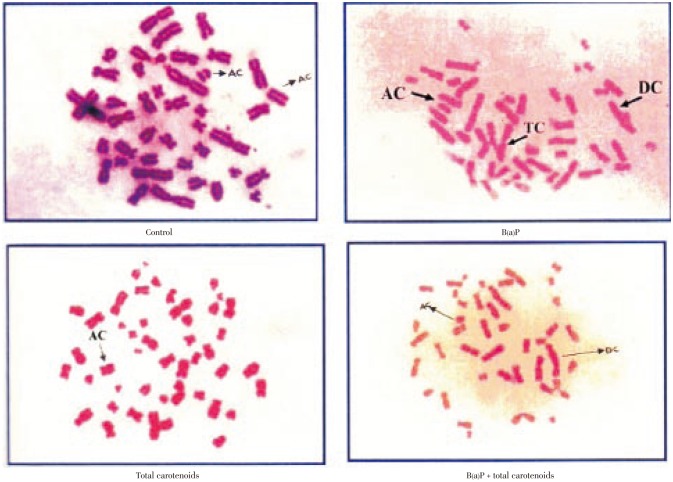
The structural chromosomal aberrations and their percentage in human lymphocytes on various treatments were also presented in Table 3 and Figure 3. Numbers of chromosomal aberrations of the cells treated with three different doses (100, 200, 300 µg/mL) of carotenoids were not statistically different from the negative control. The positive controls B(a)P at concentrations of 25 µg/mL were significantly increased in the number of abnormal metaphases and the total number of structural chromosomal aberrations, respectively, when compared with the negative control (P<0.001). The frequency of chromosomal aberrations was significantly decreased by the treatment of carotenoids plus B(a)P relative to the positive control of B(a)P alone (P<0.001) for the percentage of total carotenoids including deletions, acentric, dicentric and tricentric were increased significantly on B(a)P treatment which was effectively protected by total carotenoids with respective on different concentration.
Table 3. Chromosomal aberration by total carotenoid treatment on lymphocyte culture.
| Treatment | Normal | Del | AC | DC | TC | No of aberrations | % Aberration | % Protection |
| Control | 49.00±4.32 | – | – | 1.00±0.05 | 0.00±0.00 | 1.00±0.04 | 2.00±0.17 | – |
| B(a)P | 20.30±1.22 | 11.30±0.91 | 6.40±0.52 | 6.60±0.52 | 5.00±0.42 | 29.30±2.00 | 58.60±4.71 | – |
| Carotenoids (100 µg/mL) | 45.40±3.20 | 1.30±0.09 | 0.00±0.00 | 1.20±0.06 | 0.00±0.00 | 2.50±0.28 | 5.00±0.51 | – |
| Carotenoids (200 µg/mL) | 47.20±3.71 | 0.00±0.00 | 1.60±0.08 | 0.00±0.00 | 0.00±0.00 | 1.60±0.09 | 3.20±0.27 | – |
| Carotenoids (300 µg/mL) | 48.50±3.86 | 0.00±0.00 | 0.00±0.00 | 1.00±0.03 | 0.00±0.00 | 1.00±0.07 | 2.00±0.18 | – |
| Carotenoids (100 µg/mL) + B(a)P | 36.60±2.11 | 6.20±0.66 | 2.10±0.24 | 2.60±0.06 | 2.50±0.10 | 13.40±1.14 | 26.80±1.18** | 31.80±2.01 |
| Carotenoids (200 µg/mL) + B(a)P | 37.90±2.31 | 5.20±0.52 | 2.60±0.18 | 2.50±0.07 | 1.80±0.09 | 12.10±1.04 | 24.20±1.20** | 32.60±2.17 |
| Carotenoids (300 µg/mL) + B(a)P | 39.70±2.16 | 3.20±0.25 | 2.60±0.19 | 2.20±0.05 | 2.30±0.17 | 10.30±1.01 | 20.60±1.10** | 38.00±2.18 |
Del: Deletion; AC: Acentric; DC: Dicentric; TC: Tricentric; **: P<0.001.
Figure 3. Effect of total carotenoids from C. humicola on CA in human lymphocytes.
AC: Acentric; DC: Dicentric; TC: Tricentric.
3.4. Sister chromatid exchange
The results of SCE were shown in Table 4 and Figure 4. The SCE frequencies of the cells treated with three different doses (100, 200 and 300 µg/mL) of total carotenoids were not statistically different from negative control values. The SCE frequencies per cell significantly increased in human lymphocyte cultures treated with B(a)P as positive controls relative to negative control (P<0.001). The SCE frequencies of the cells treated with B(a)P plus total carotenoids significantly decreased relative to the B(a)P positive control (P<0.001).
Table 4. Sister chromatid exchange by total carotenoid treatment on lymphocyte culture.
| Treatment | SCE | PRI | MI (%) |
| Control | 2.50±0.28 | 2.26±0.19 | 8.27±0.95 |
| B(a)P | 8.62±0.61 | 1.20±0.18 | 1.32±0.08 |
| Carotenoids (100 µg/mL) | 2.30±0.16 | 2.15±0.12 | 9.91±1.91 |
| Carotenoids (200 µg/mL) | 2.52±0.17 | 2.27±0.09 | 9.99±1.08 |
| Carotenoids (300 µg/mL) | 2.37±0.20 | 2.29±0.08 | 10.42±1.91 |
| Carotenoids (100 µg/mL) + B(a)P | 4.79±0.29** | 1.89±0.02* | 5.29±0.41** |
| Carotenoids (200 µg/mL) + B(a)P | 4.26±0.29** | 1.92±0.07* | 5.28±0.41** |
| Carotenoids (300 µg/mL) + B(a)P | 3.25±0.26** | 1.97±0.08* | 5.26±0.61** |
PRI: proliferative index; MI: mitotic index; **: P<0.001; *P<0.01.
Figure 4. SCE on treatment of total carotenoids from C. humicola in human lymphocute culture.

3.5. Micronucleus assay
The results of the micronucleus (MN) assays were shown in Table 5 and Figure 5. The MN frequencies of the cells treated with three different doses of total carotenoids were decreased relative to the negative controls, but not with statistical significance. The MN frequencies were significantly increased in human lymphocyte culture treated with B(a)P as positive controls compared with negative control. The MN frequencies of the cells were significantly decreased by the treatment with total carotenoids plus B(a)P when compared with the positive control B(a)P alone (P<0.05).
Table 5. Micronucleus frequency by total carotenoid treatment on lymphocyte culture (mean±SD).
| Treatment | MN | NDI | % Reduction |
| Control | 1.17±0.18 | 1.23±0.08 | – |
| B(a)P | 8.72±0.73 | 1.10±0.10 | – |
| Carotenoids (100 µg/mL) | 0.81±0.02 | 1.30±0.11 | – |
| Carotenoids (200 µg/mL) | 1.29±0.10 | 1.24±0.09 | – |
| Carotenoids (300 µg/mL) | 1.82±0.09 | 1.25±0.06 | – |
| Carotenoids (100 µg/mL) + B(a)P | 3.09±0.18* | 1.10±0.12 | 74.57 |
| Carotenoids (200 µg/mL) + B(a)P | 3.07±0.17* | 1.16±0.16 | 74.83 |
| Carotenoids (300 µg/mL) + B(a)P | 3.27±0.15* | 1.18±0.09 | 72.19 |
NDI: nuclear division index; *: P<0.05.
Figure 5. Effect of total carotenoids from C. humicola on micronucleus in human lymphocyte.
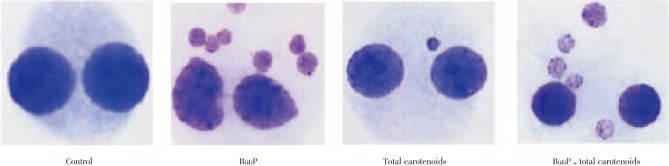
4. Discussion
Polycyclic aromatic hydrocarbons appear to be significant contributors to the genotoxicity and carcinogenicity of air pollution present in the environment for humans, and the most extensively studied member of this class of chemicals is B(a)P. Populations exposed to environmental air pollution show increased levels of PAH-DNA adducts and it has been postulated that another contributing cause of carcinogenicity by environmental air pollution may be the production of ROS following oxidative stress leading to oxidative DNA damage[21],[22]. B(a)P itself is unreactive, but it is converted to a highly reactive electrophile by enzymes involved in drug metabolism[23]. Therefore, they are routinely used for evaluating increased sensitivity to DNA cross-linking agents in studies using a variety of cytogenetic endpoints[24].
In the present study, we investigated potential bioactive compounds available in the selected green algae C. humicola. Primarily the phytochemical analysis of C. humicola extract was compared with spectral data in different types of solvents. All the absorption maxima of the spectral data coincide with the previous published data. It is generally agreed that all taxonomic groups of algae have specific sets of pigments, which vary in concentration from one species to another. It is reported that there is a fixed relationship, in green algae, and total carotenoids (70%-90%)[25]. The α-carotene and β-carotene were found to be lower level in C. humicola that may be related to the conversion of these compounds to lutein. This may be the reason for the higher concentration of lutein existence among total carotenoids in C. humicola. The results were correlated with Ranga Rao et al who reported that those carotenoids were from Botryococcus braunii[17]. The present results confirmed that C. humicola is rich in carotenoid pigments, and the variation of their concentration values depends on a multiple factors.
The genotoxic/antigenotoxic, cytotoxicity and chemo-protective effect of total carotenoids were analyzed against B(a)P by CA, SCE, and MN. Because CA, SCE and MN values were significantly increased in culture treated with only B(a)P, our results agree with available data. For the determination of cytotoxicity, the MI, PRI and NDI ratios were also evaluated. Only B(a)P treatment decreased MI, PRI and NDI ratios relative to control, as expected. Moreover, in these tests total carotenoids did not show any genotoxic effect as the sole treatment for human lymphocytes in all these genotoxicity assays. MI, PRI and NDI ratios were decreased with high dose total carotenoid treatment, especially at a dose of 300 µg/mL. Consequently, we suggest that carotenoids had a relatively low cytotoxic effect on human lymphocytes in vitro, even in high doses. Selected carotenoid doses (100, 200 and 300 µg/mL) significantly decreased B(a)P induced DNA damage compared with positive control in all three tests (P<0.001, P<0.01 and P<0.05). While carotenoids decreased DNA damage induced by B(a)P in the CA and MN tests. The increase of SCE frequency in the treatment of B(a)P plus total carotenoids may have been a synergistic effect of total carotenoids and B(a)P. Consequently, the results of CA, SCE and MN tests show that carotenoids have a strong antigenotoxic and chemoprotective effect against B(a)P induced DNA damage and a moderate antigenotoxic and chemo-protective effect against B(a)P induced DNA damage. In our opinion, the antigenotoxic and chemo-protective effect may have originated from the anti-oxidative capacity of carotenoids in vitro and in vivo which was reported earlier.
Since micronuclei are the result of chromosome breaks or disturbances of the mitotic spindle and chromosomal aberrations result from clastogenic events with and without chromosomal rearrangements, these parameters are usually considered to be clear evidence for mutagenicity. On the other hand, SCE may not represent actual damage to chromosomes, but could instead be considered as a result of damage repair. Thus, the different dose-response effects obtained for the different end-points tested indicate that B(a)P primarily induce clastogenic events while the SCE-inducing activity becomes significant at high concentrations. This observation could eventually be linked to the shapes of the dose-response curves for chromosomal aberrations and micronuclei, and could indicate the generation of differently acting metabolites[26].
Schabath and his co-workers carried out a case-control study looking at levels of carotenoids and endogenous DNA damage in lymphocytes of bladder cancer patients and healthy controls. They found an increase in DNA damage in cases, with high DNA damage combined with low carotenoid intake being associated with the highest risk[27]. Zhao and colleagues, also used the alkaline comet assay to look at endogenous DNA damage in their intervention study, involving 37 healthy post-menopausal women supplemented with carotenoids (lutein, lycopene and beta-carotene). This study showed a decrease in DNA damage with carotenoid supplementation[28]. Watters and his team found an inverse association between DNA damage in the lymphocytes of 164 healthy subjects and the carotenoid lycopene, using the alkaline comet assay[29].
Carotenoids, the most potent biological quenchers of singlet O2, act as chain-breaking antioxidants, and flavonoids inhibit the enzymes responsible for O2−-production. β-Carotene and flavonoids have been reported to prevent DNA damage against H2O2 in human lymphocytes assessed by the comet assay[30].
Evaluation on antigenotoxic/antimiutagenic activity of the crude ethanolic extracts of Codium tomentosum stock house (Chlorophyceae), in human lymphocyte cultures shows the strong evidence on antigenotoxic effect[31]. There are several prospective studies investigating the possible effect of b-cryptoxanthin (and other carotenoids) on risk of various cancers. Toniolo found that levels of b-cryptoxanthin and lutein in serum were linked with reduced risk of breast cancer in a dose dependent fashion[32]. Beta carotene, lycopene, b-cryptoxanthin, zeaxanthin and lutein are reduced in colorectal adenomas, suggesting that mucosal carotenoids could serve as biomarkers for predisposition to colorectal cancer[33]. Women with high circulating concentrations of b-cryptoxanthin and tocopherol may be at a reduced risk of cervical atypical squamous cells of undetermined significance[34].
In vitro studies looking at the effects of carotenoids on DNA repair have examined a range of human cell types including leukaemia cells where beta-carotene decreased peroxynitrous acid-induced damage, hepatoma cells where lycopene decreased oxidative lesions, melanocytes where lycopene caused a reduction in UVA-induced damage and neuroblastoma cells where lutein and zeaxanthin decreased UVA-induced DNA damage[35].
The present study has clearly demonstrated that total carotenoids have significant antigenotoxic activities. In addition, the chemo-protective and antigenotoxic potential of total carotenoids was observed which is of great significance in radioprotection, and may be useful for human pathological condition. Furthermore, the different carotenoids should be isolated, purified and characterized in order to understand the mechanisms underlying its chemo-protective effect and antigenotoxic effects. Therefore, algal species C. humicola, as alternative sources of natural antigenotoxic agents, have attracted much attention from biomedical scientists.
Acknowledgments
The authors wish to acknowledge Dr. Sivasubramonium V, Head, Post Graduate and Research Department of Plant Biology & Plant Biotechnology, RKM Vivekanada College, Chennai, India for providing the algal cultures throughout the study and Indian Institute of Technology, Chennai, India for carrying out the spectral analysis.
Footnotes
Foundation Project: This work was financially supported by Bharathiar University, Tamilnadu, India.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Lee SH, Kohn H. Nucleophilic activation of a tetra-substituted mitomycin cyclic bis-disulfide. Chem Pharm Bull. 2009;57(2):149–157. doi: 10.1248/cpb.57.149. [DOI] [PubMed] [Google Scholar]
- 2.Mantena RKR, Wijburg OLC, Vindurampulle C, Bennett-Wood VR, Walduck A, Drummond GR, et al. Reactive oxygen species are the major antibacterials against Salmonella typhimurium purine auxotrophs in the phagosome of RAW 264, 7 cells. Cell Microbiol. 2008;10(5):1058–1073. doi: 10.1111/j.1462-5822.2007.01105.x. [DOI] [PubMed] [Google Scholar]
- 3.Cornelli U. Antioxidant use in nutraceuticals. Clin Dermatol. 2009;27:175–194. doi: 10.1016/j.clindermatol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 4.WCRF/AICR . Food, nutrition, physical activity, and the prevention of cancer. Washington DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 5.Nikoli B, Stanojevi J, Ga BV, Simi D, Vukevi JK. Effects of vitamin C on DNA damage. Food Technol Biotechnol. 2006;44(4):449–456. [Google Scholar]
- 6.Samarth RM, Panwar M, Kumar M, Kumar A. Protective effects of Mentha piperita Linn on benzo[a]pyrene-induced lung carcinogenicity and mutagenicity in Swiss albino mice. Mutagenesis. 2006;21(1):61–66. doi: 10.1093/mutage/gei075. [DOI] [PubMed] [Google Scholar]
- 7.Russell M. The multifunctional carotenoids: insights into their behavior. J Nutr. 2006;136:2690S–2692S. doi: 10.1093/jn/136.10.2690S. [DOI] [PubMed] [Google Scholar]
- 8.Nishino H, Murakoshi M, Tokuda H, Satomi Y. Cancer prevention by carotenoids. Arch Biochem Biophys. 2009;483:165–168. doi: 10.1016/j.abb.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 9.El-Baky A, El Baz FK, El-Baroty GS. Production of carotenoids from marine microalgae and its evaluation as safe food colorant and lowering cholesterol agents. Am Eurasian J Agric Environ Sci. 2007;2:792–800. [Google Scholar]
- 10.Steven G, Thomas D, Haffner J, Kroetsch T, Davidson SR, James WE, et al. Effect of short-term lycopene supplementation and postprandial dyslipidemia on plasma antioxidants and biomarkers of endothelial health in young, healthy individuals. Vasc Health Risk Manag. 2008;4(1):213–222. doi: 10.2147/vhrm.2008.04.01.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Guterre ZDR, Mantovan MS, da Eir AF, Ribeir LR, Jorda BQ. Genotoxic and antigenotoxic effects of organic extracts of mushroom Agaricus blazei Murrill on V79 cells. Genet Mol Biol. 2005;28(3):458–463. [Google Scholar]
- 13.Wu LC, Ho JA, Shieh MC, Lu IW. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agric Food Chem. 2005;53:4207–4212. doi: 10.1021/jf0479517. [DOI] [PubMed] [Google Scholar]
- 14.Deslandes E, Pondaven P, Auperin T, Roussakis C, Guezennec J, Stiger V, et al. Preliminary study of the in vitro antiproliferative effect of a hydroethanolic extract from the subtropical seaweed Turbinaria ornata (Turner J. Argadh) on a human nonsmall-cell bronchopulmonary carcinoma cell line (NSCLC-N6) J Appl Phycol. 2000;12:257–262. [Google Scholar]
- 15.Celikler S, Yildiz G, Vatan O, Bilaloglu R. In vitro antigenotoxicity of Ulva rigida C. Agardh (Chlorophyceae) extract against induction of chromosome aberration, sister chromatid exchange and micronuclei by mutagenic agent MMC. Biomed Environ Sci. 2008;21:492–498. doi: 10.1016/S0895-3988(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 16.Scholtz B, Liebezeit G. Chemical screening for bioactive substances in culture media of microalgae and cyanobacteria from marine and brackish water habitats-first results. Pharm Biol. 2006;4497:544–549. [Google Scholar]
- 17.Rao RA, Sarada R, Baskar V, Ravisankar GA. Antioxidant activity of Botryococcus braicnii extract in in vitro models. J Agric Food Chem. 2006;54:4593–599. doi: 10.1021/jf060799j. [DOI] [PubMed] [Google Scholar]
- 18.Rao RA, Sarada R, Baskar V, Ravisankar GA. Identification of carotenoids from green algae Haematococcus pluvialis by HPLC and LCMS and their antioxidant properties. J Microbiol Biotechnol. 2009;19:1333–1341. [PubMed] [Google Scholar]
- 19.Kulkarni PS, Ambani LM, Vaidya AB. Modified FPG technique for demonstrating sister chromatid exchange (SCE) J Postgrad Med. 1982;28:9–12. [PubMed] [Google Scholar]
- 20.Bonassi S, Fenech M, Lando C, Lin YP, Ceppi M, Chang WP, et al. Micronucleus project: international database comparison for results with the cytokinesis block micronucleus assay in human lymphocytes: effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ Mol Mutagen. 2001;37:31–45. [PubMed] [Google Scholar]
- 21.Singh R, Sram RJ, Binkova B, Kalina I, Popov TA, Georgieva T, et al. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat Res. 2007;620(1–2):83–92. doi: 10.1016/j.mrfmmm.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Tarantini A, Maitre A, Lefebvre E, Marques M, Rajhi A, Douki T. Polycyclic aromatic hydrocarbons in binary mixtures modulate the efficiency of benzo[a]pyrene to form DNA adducts in human cells. Toxicology. 2011;279(1–3):36–44. doi: 10.1016/j.tox.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Landvik NE, Gorria M, Arlt VM, Asare N, Solhaug A, Lagadic-Gossmann D, et al. Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: possible implications for their mutagenic and carcinogenic effects. Toxicology. 2007;231:159–174. doi: 10.1016/j.tox.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Sram RJ, Binkova B, Kalina I, Popov TA, Georgieva T, et al. The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat Res. 2007;620(1–2):83–92. doi: 10.1016/j.mrfmmm.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Hegazi MMI. Separation, identification and quantification of photosynthetic pigments from three red sea seaweeds using reversed-phase high-performance liquid chromatography. Egypt J Biol. 2002;4:1–6. [Google Scholar]
- 26.Canales-Aguirre A, Padilla-Camberos E, Gómez-Pinedo U, Salado-Ponce H, Feria-Velasco A, De Celis R. Genotoxic effect of chronic exposure to DDT on lymphocytes, oral mucosa and breast cancer of female rats. Int J Environ Res Public Health. 2011;8:540–553. doi: 10.3390/ijerph8020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schabath MB, Grossman HB, Delclos GL, Hernandez LM, Day RS, Davis BR, et al. Dietary carotenoids and genetic instability modify bladder cancer risk. J Nutr. 2004;134:3362–3369. doi: 10.1093/jn/134.12.3362. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Tian H, Ma X, Li L. Epigallocatechin gallate, the main ingredient of green tea induces apoptosis in breast cancer cells. Front Biosci. 2006;11:2428–2433. doi: 10.2741/1980. [DOI] [PubMed] [Google Scholar]
- 29.Watters JL, Satia JA, Kupper LL, Swenberg JA, Schroeder JC, Switzer BR. Associations of antioxidant nutrients and oxidative DNA damage in healthy African-American and white adults. Cancer Epidemiol Biomarkers Prev. 2007;16:1428–1436. doi: 10.1158/1055-9965.EPI-06-1030. [DOI] [PubMed] [Google Scholar]
- 30.Astley SB, Hughes DA, Wright RM, Elliott RM, Southon S. DNA damage and susceptibility to oxidative damage in lymphocytes: effects of carotenoids in vitro and in vivo. Br J Nutr. 2004;91:53–61. doi: 10.1079/bjn20031028. [DOI] [PubMed] [Google Scholar]
- 31.Celikler S, Vatan O, Yildiz G, Bilaloglu R. Evaluation of anti-oxidative, genotoxic and antigenotoxic potency of Codium tomentosum Stackhouse ethanolic extract in human lymphocytes in vitro. Food Chem Toxicol. 2009;47(4):796–801. doi: 10.1016/j.fct.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Toniolo P. Serum carotenoids and breast cancer. Am J Epidemiol. 2001;153:1142–1147. doi: 10.1093/aje/153.12.1142. [DOI] [PubMed] [Google Scholar]
- 33.Muhlhofer A. Carotenoids are decreased in biopsies from colorectal adenomas. Clin Nutr. 2003;22:65–70. doi: 10.1054/clnu.2002.0598. [DOI] [PubMed] [Google Scholar]
- 34.Goodman MT. The association of plasma micronutrients with the risk of cervical atypical squamous cells of undetermined significance (ASCUS) Asian Pac J Cancer Prev. 2000;1:227–235. [PubMed] [Google Scholar]
- 35.Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J Photochem Photobiol. 2006;85:205–215. doi: 10.1016/j.jphotobiol.2006.07.009. [DOI] [PubMed] [Google Scholar]