Abstract
Objective
To evaluate the aldose reductase inhibitory (ARI) activity of different fractions of Hybanthus enneaspermus for potential use in diabetic cataract.
Methods
Total phenol and flavonoid content of different fractions was determined. ARI activity of different fractions in rat lens was investigated in vitro.
Results
The results showed significant level of phenolic and flavonoid content in ethyl acetate fraction [total phenol (212.15±0.79 mg/g), total flavonoid (39.11±2.27 mg/g)] and aqueous fraction [total phenol (140.62±0.57 mg/g), total flavonoid (26.07±1.49 mg/g)] as compared with the chloroform fraction [total phenol (68.56±0.51 mg/g), total flavonoid (13.41±0.82 mg/g)] and petrolium ether fraction [total phenol (36.68±0.43 mg/g), total flavonoid (11.55±1.06 mg/g)]. There was a significant difference in the ARI activity of each fraction, and it was found to be the highest in ethyl acetate fraction [IC50 (49.26±1.76 µg/mL)] followed by aqueous extract [IC50 (70.83±2.82 µg/mL)] and it was least in the petroleum ether fraction [IC50 (118.89±0.71 µg/mL)]. Chloroform fraction showed moderate activity [IC50 (98.52±1.80 µg/mL)].
Conclusions
Different fractions showed significanct amount of ARI activity, where in ethyl acetate fraction it was found to be maximum which may be due to its high phenolic and flavonoid content. The extract after further evaluation may be used in the treatment of diabetic cataract.
Keywords: Hybanthus enneaspermus, Total phenol, Total flavonoid, Rat lens, Aldose reductase, Diabetic cataract, Different fractions
1. Introduction
Diabetes mellitus is a chronic disorder of carbohydrate, lipid and protein metabolism characterized by persistent elevation of blood glucose level in the body. It is a major risk factor of cataract which is the leading cause of blindness over the world. Various pharmacological strategies are used to prevent the cataract formation, among them aldose reductase inhibitors have received much attention because of its involvement in the pathophysiology of diabetic complications including cataract[1],[2]. The clinical consequences of these complications include lower limb amputation, end-stage renal failure, and loss of vision[3]. Prolonged exposure to chronic hyperglycemia in diabetes can lead to various complications, affecting the cardiovascular, renal, neurological and visual systems, such as lens, retina, nerves and kidney, which are insulin-insensitive, and are the target organs for complications such as cataracts, retinopathy, neuropathy and nephropathy[4]. Several mechanisms such as increased aldose reductase-related polyol pathway, increased advanced glycation end product formation, and excessive oxidative stress are involved in this process[5],[6]. Aldose reductase is found in almost all mammalian cells, but at high levels in organs such as the cornea, lens, retina, kidney, myelin sheath and sciatic nerves, which are affected by diabetic complications[7]. When polyol pathway activity is increased, it causes accumulation of polyol in lens fibers, influx of water and generation of osmotic stress, osmotic swelling, changes in membrane permeability, and oxidative stress culminating in tissue injury, that's why it has got special attention in the clinical treatment of secondary complications of diabetes[2]. Formation of sorbitol is a result of enzymatic degradation of glucose through polyol pathway which is a tissue poison whose accumulation increases osmotic pressure and may damage the tissues by causing them to swell. Since aldose reductase is localized primarily in lens epithelial cells, it is possible to prevent cataract via inhibition of the activity of aldose reductase[8].
According to World Health Organization (WHO) reports that about 80% of the world's population in 2001 used herbal medicine for health[7]. A number of studies have been undertaken to identify natural and synthetic compounds that inhibit aldose reductase and reduce oxidative stress, and the flavonoids are among the most potent aldose reductase inhibitors known. Moreover, of these quercetrin (a glycoside of quercetin), quercetin is believed to be among the strongest of the aldose reductase inhibitors, though their anticataractous activities in animal studies remain controversial. Due to the undesirable side effects of available drugs, therapeutic drugs against diabetic cataract from natural source are still required. Recently, some indigenous plants were reported to have potent aldose reductase inhibitory activities and their anticataract potentials were evaluated against galactose-induced biochemical changes in rat lens organ culture. Ocimum sanctum was the most effective aldose reductase inhibitor in vitro with an IC50 value of 20 µg/mL, which is comparable to that of Aralia extract[9].
Hybanthus enneaspermus (H. enneaspermus) (L) F. Muell (Violaceae), is a herb distributed in different parts of India such as in Bundelkhand, Agra, Bengal, Tamilnadu, Gujarat, Karnataka, and it is also found in the other parts of the world such as Sri Lanka, tropical Asia, Africa, and Australia. This plant has been reported to contain phytoconstituents viz. dipeptide alkaloids, aurantiamide acetate, isoarborinol and β-sitosterol[10]. Traditionally H. enneaspermus is used as aphrodisiac, demulcent, tonic, and used to treat various diseases such as urinary infections, diarrhea, cholera, leucorrhoea, gonorrhea, dysuria, inflammation and sterility[11],[12]. Pharmacologically this plant has been screened for anti-inflammatory, antitussive, antiplasmodial, antimicrobial, and anticonvulsant activities[13].
Based upon the ethnopharmacological reports we have earlier reported that this plant showed significanct activity in the treatment of diabetes[14]. So the present study was aimed to evaluate the protective effects of different fractions of H. enneaspermus on diabetic complications such as aldose reductase inhibitory activity using rat lens[15]. Moreover, relationship between total phenol, flavonoid and its aldose reductase inhibitory potential was also investigated. To the best of our knowledge, this is the first report on the aldose reductase inhibitory effect of different fractions of H. enneaspermus.
2. Materials and methods
2.1. Chemicals and instrument
DL-glyceraldehyde, NADPH, quercetin, rutin were obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals and solvents used were of analytical grade. The absorbance measurements were recorded using the ultraviolet-visible spectrophotometer (Shimadzu, Pharmaspec-1700).
2.2. Collection of plant material
H. enneaspermus plant material was purchased from herbal vendors in Chennai. The plant was identified and authenticated by the chief botanist Tampcol Anna, Hospital Chennai. A voucher specimen (Cog/HE/01/08) was deposited in Department of Pharmaceutics, Institute of Technology Banaras Hindu University, Vanarasi, India for further reference.
2.3. Preparation of extract, and its fractions
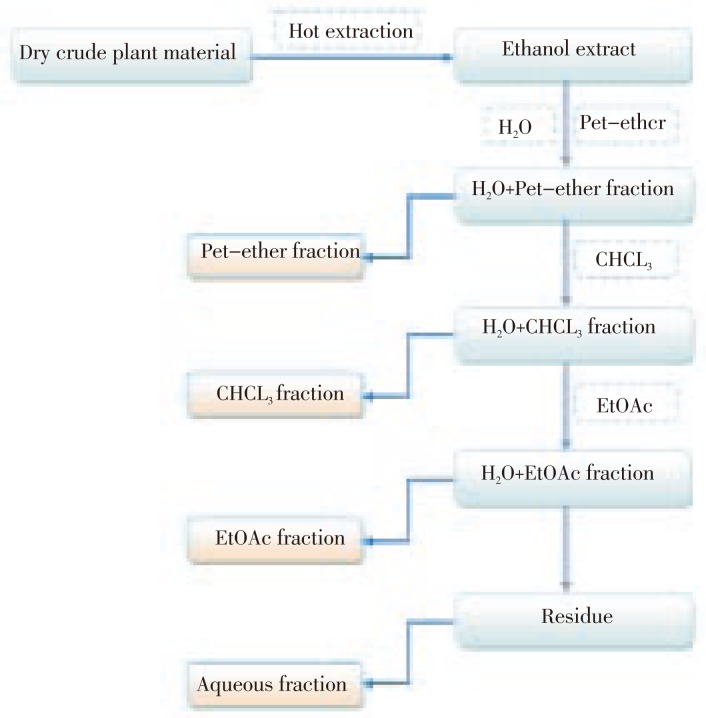
The ethanolic extract was prepared by Soxhlet extraction method by taking 1 kg of the powdered plant material extracting with ethanol. The extract was filtered, concentrated and finally dried in vacuo. The ethanol extract was then fractionated by the use of different solvents of varying polarity as shown in the flowchart (Figure 1).
Figure 1. Schematic diagram of fractionation of H. enneaspermus extract.
2.4. Phytochemical analysis
Total phenolic content of different fractions of H. enneaspermus was determined using Folin-Ciocalteu method. Absorbance of the final solution mixture was measured at 765 nm, gallic acid was used as a standard and results were expressed as mg of gallic acid equivalent per gram (mg GAE/g) of dried extract[16]. For the determination of the total flavonoid content the aluminium chloride method was incorporated using rutin as the standard. The absorption at 415 nm was read for determination of total flavonoid content. The amount of flavonoid in plant extracts was calculated using rutin as a standard[17].
2.5. Lens aldose reductase activity
2.5.1. Animals
Healthy adult Wistar albino rats (150–200 g) aged between 2 and 3 months were taken for the study. They were housed under standard environmental conditions [12 h light and 12 h dark cycle, (25±30) °C, (35–60)% relative humidity] in polypropylene cages with free access to pelleted food (Mona laboratoty animal feed) and water ad libitum throughout the experimental period. The experimental protocol has been approved by the Institutional Animal Ethics Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
2.5.2. Preparation of lens homogenate
Eyes of normal Wistar albino rats were removed immediately after sacrifice. The lenses were removed from the eye, washed with saline and fresh weights of lens were measured. Transparent lenses free from any disease were pooled and a 10% homogenate was prepared in 0.1 M phosphate buffer saline (pH 7.4). The homogenate was then centrifuged in a refrigerated centrifuge at 5 000 × g for 10 min, and then supernatant was collected and kept in ice. Protein content of the lens homogenate was determined[2].
2.5.3. Determination of aldose reductase activity
For the determination of the aldose reductase inhibitory activity of the different fractions, a sample cuvette was taken containing 0.7 mL of phosphate buffer (0.067 M), 0.1 mL of NADPH (25×10−5 M), 0.1 mL of lens supernatant, 0.1 mL of DL-glyceraldehyde (substrate) (5×10−4 M) and final volume was made to 1 mL. Absorbance was taken against a reference cuvette containing all components but not DL-glyceraldehyde. The final pH of the reaction mixture was adjusted to the pH 6.2. When substrate was added to the solution mixture, enzymatic reaction started, and absorbance (OD) was recorded at 340 nm for 3 min at 30 sec interval. Aldose reductase activity was calculated and expressed as ΔOD/min/mg protein[2].
2.5.4. Lens aldose reductase activity and plant extract
A stock solution of the different fractions was prepared by dissolving the extract into phosphate buffer saline (PBS). For determination of the aldose reductase inhibiting activity, 0.1 mL of each fraction from various stock solutions (final concentrations: 25, 50, 75, 100, 200 and 300 µg/mL) was added to both the reference and standard cuvettes. The reaction was initiated by the addition of 0.1 mL DL-glyceraldehyde and the rate of reaction was measured as mentioned above.
For each sample, ΔOD/min/mg protein was calculated. Percentage inhibitions of aldose reductase activity of the extract were calculated assuming normal rat lens having 100% activity. IC50 values were calculated for each sample by ploting a graph between log dose concentrations versus percent inhibition[2].
2.5.5. Kinetic studies of inhibitory activity against aldose reductase
The kinetic studies of inhibitory activity against aldose reductase of different fractions were analyzed using the Lineweaver-Burk plot.
2.6. Statistical analysis
Results were expressed as mean value ± standard error mean (SEM) of triplicate. Linear regression analysis was performed, quoting the correlation coefficient r2. Two-way ANOVA followed by Bonferroni post test was performed for evaluation of all data. GraphPad Prism (Version 5) software was used for all statistical analysis, and P<0.05 was considered as significance.
3. Results
3.1. Extraction yields
The yield in the ethanolic extract and its sub-fractions was given in Table 1. The yield percentages of different fractions in decreasing order were as follows: aquous fraction (5.1%) > chloroform (4.5%) > petrolium ether (3.6%) > ethyl acetate (2.8%).
Table 1. Phytochemical analysis and aldose reductase inhibitory activity of different fractions of H. enneaspermus (mean±SEM).
| Extract/fractions | Yield (%) | Total phenol (mg/g) | Total flavonoid (mg/g) | Aldose reductase inhibitory activity IC50 (µg/mL) |
| Petrolium ether | 3.6 | 36.68±0.43 | 11.55±1.06 | 118.89±0.71 |
| Chloroform | 4.5 | 68.56±0.51 | 13.41±0.82 | 98.52±1.80 |
| Ethyl acetate | 2.8 | 212.15±0.79 | 39.11±2.27 | 49.26±1.76 |
| Aqueous | 5.1 | 140.62±0.57 | 26.07±1.49 | 70.83±2.82 |
| Quercetin | NA | NA | NA | 2.74±0.04 |
NA: not analyzed.
3.2. Phytochemical analysis
The total amount of phenolic and flavonoid was determined in different fraction equivalent to gallic acid and rutin, respectively used as a standard (Table 1). Ethyl acetate fraction contained the maximum amount of phenol and flavonoid [total phenol (212.15±0.79 mg/kg) and total flavonoid (39.11±2.27 mg/kg)], whereas petrolium ether fraction contained the least amount [total phenol (36.68±0.43 mg/kg) and total flavonoid (11.55±1.06 mg/kg)].
3.3. Aldose reductase inhibitory activity
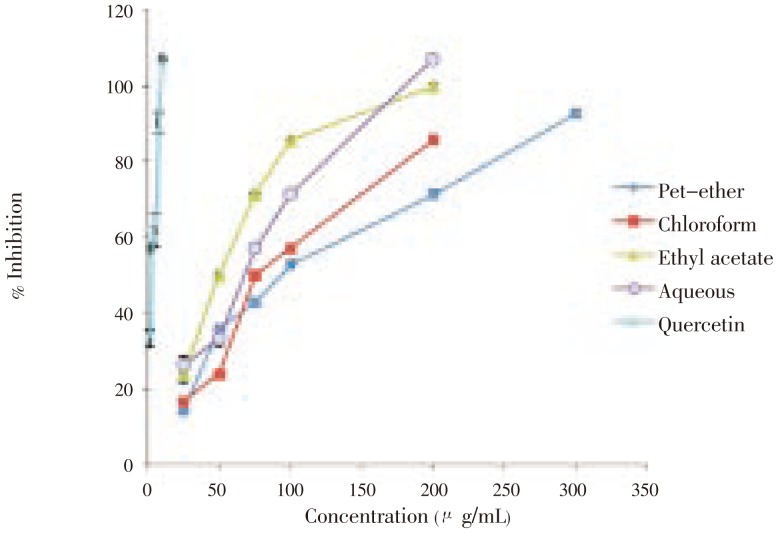
Different fractions were found to inhibit rat lens aldose reductase activity to various extents with IC50 values ranging from 2 µg/mL to >100 µg/mL (Table 1 and Figure 2). The aldose reductase activity in normal rat lens was found to be (0.014 4±0.000 7 µg/mL). Among the fractions, ethyl acetate fraction showed higher percentage of inhibition [IC50 (49.26±1.76 µg/mL)] followed by aqueous fraction [IC50 (70.83±2.82 µg/mL)]. Chloroform fraction [IC50 (98.52±1.80 µg/mL)] also showed significant inhibition but was less as compared with the ethyl acetate fraction. Petrolium ether fraction [IC50 (118.89±0.71 µg/mL)] was found to have the least inhibition potential against aldose reductase enzyme. The aldose reductase inhibitory activities of different fractions were presented in Table 1 and Figure 2. Quercetin was used as a standard in the experiments.
Figure 2. Effect of different fractions of H. enneaspermus and quercetin on aldose reductase activity.
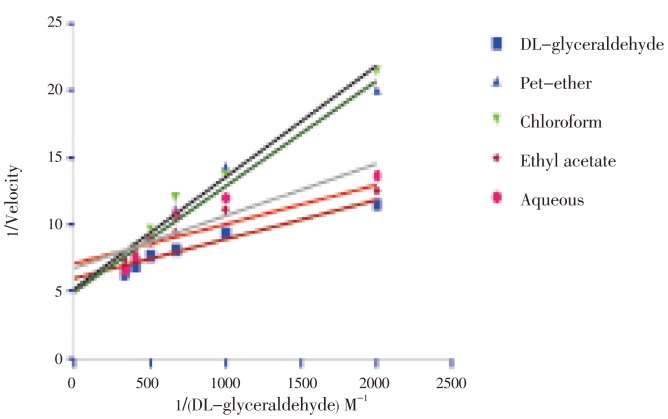
Kinetic study was performed for the entire fraction to understand exactly the type of inhibition, and based upon the data it is clear that ethyl acetate fraction showed non compititive inhibition, because the value of Vmax (0.1544±0.00029] and km (0.5849±0.0017×10−3 mM) did not differ as compared with the substrate DL-glyceraldehyde (0.1746±0.0017, 0.6089±0.0012×10−3 mM). In all other fractions viz. petrolium ether, aqueous and chloroform fraction the value of the Vmax and Km differs significantly as compared with DL-glyceraldehyde which reveled that these fraction showed competitive inhibition. The values of Vmax and Km for all fractions were given in Table 2 and Figure 3.
Table 2. Kinetic parameters of rat lens aldose reductase (mean±SEM).
| Group | Aldose reductase activity | Vmax | Km×10−3 mM | Ki |
| 1 | DL-glyceraldehyde | 0.1746±0.0017 | 0.6089±0.0012 | 0.0000±0.0000 |
| 2 | DL-glyceraldehyde + petrolium ether | 0.3840±0.0004a | 4.5916±0.0035a | 1.8670±0.0060a |
| 3 | DL-glyceraldehyde + chloroform | 0.3315±0.0004ab | 3.9620±0.0210b | 1.3349±0.0043ab |
| 4 | DL-glyceraldehyde + ethyl acetate | 0.1544±0.00029abc | 0.5849±0.0017abc | 0.0985±0.0003abc |
| 5 | DL-glyceraldehyde + aqueous | 0.1978±0.0004abcd | 1.2600±0.0021abcd | 0.3052±0.0010abcd |
a: comparing with the DL-glyceraldehyde; b: comparing with the DL-glyceraldehyde + petrolium ether; c: comparing with DL-glyceraldehyde + chloroform; d: comparing with the DL-glyceraldehyde + ethyl acetate fraction.
Figure 3. Effect of different fractions of H. enneaspermus on the Lineweaver-Burk plot of aldose reductase activity with DL-glyceraldehyde as substrate.
4. Discussion
H. enneaspermus is used to treat various types of ailments in the traditional system of medicine including the diabetes. It contains good amount of phenol and flavonoid contents. It is used in the treatment of diabetes as well as an antioxidant, so on the basis of this data, the present study was carried out to determine the protective effects of different fractions of H. enneaspermus on aldose reductase enzyme system of rat lens to determine the potential anticataract effect of this plant[14],[15].
The phytochemical value reported in this paper might be used as an analytical tool for the standardization of H. enneaspermus, if any adulterants or contaminants are there in the material it can be easily identified by the use of these parameters. This data can also be used for the comparative analysis of the sample which is collected in different place as well as time. The result reveals that ethyl acetate was the most effective solvent for extracting phenolics from H. enneaspermus. The total phenolic and flavonoid content of the ethyl acetate fraction was higher than those of the petroleum ether, chloroform and aqueous fraction. This could be explained by the possible formation of complexes by certain part of the phenolic compounds with other components, which are more extractable in ethyl acetate than those of other fractions[18],[19]. Different fractions showed significanct aldose reductase inhibitory activity however, it was maximum in the case of ethyl acetate fraction followed by aqueous fraction (quercetin used as standard). The aqueous fraction also showed comparatively significant inhibitory activity. In case of petroleum ether and chloroform fractions the activity was found to be less.
Aldose reductase reduces glucose to sorbitol and then metabolized to fructose by sorbitol dehydrogenase. Normally this accounts for less than 3% of glucose consumption. However, in the presence of high glucose, the activity of this pathway is substantially increased and could represent up to 30% of total glucose consumption. So aldose reductase inhibition in the early onset of the secondary complication in the diabetic mellitus will be beneficial, as ethyl acetate fraction showed good inhibitory activity against aldose reductase so it can be potentially used to treat diabetic complication in the early stage. The inhibitory effects of plant phytochemicals, including polyphenols which are currently regarded as natural antioxidants, and their antioxidant activities are important for human health, against carbohydrate hydrolyzing enzymes which contribute to the lowering of blood glucose in diabetics and are capable of reducing oxidative stress by scavenging reactive oxygen species and preventing cell damage[20],[21]. In addition, some flavonoids and polyphenols as well as sugar derivatives are found to be effective in inhibiting α-glucosidase and aldose reductase enzyme[22]. Previous study shows this plant is safe and has no toxic effect in animal at a dose level of 5 g/kg b.w., and has good amount of phenol content as well[14].
Kinetic studies suggest that these compounds can interact and inhibit enzyme in both compititive and uncompititve manner, appearing to interact with the enzyme at a site independent of either substrate or enzyme. Based upon the data obtained, it was found that ethyl acetate fraction had significant activity against aldose reductase enzyme, this may be due to the presence of the phenol and flavonoid contents. Moreover, from the pioneering studies on sorbinil and alrestatin to recent investigation on zopolrestat and zenarestat, several compounds in clinical trials or in the market for the treatment of the diabetic complications have been developed but were subsequently withdrawn, suggesting that no universally potent inhibitor currently exists[3].
Kinetic study was performed for all the fraction to understand the type of inhibition. The ethyl acetate fraction shows non comptitive inhibition whereas petrolium ether and chloroform and aqueous fraction shows competitive inhibition. Effect of different fractions on rat lens aldose reductase activity in Lineweaver-Burk plot using DL-glyceraldehyde as a substrate was established. The Lineweaver-Burk plot was made in between 1/velocity vs 1/DL-glyceraldehyde. From the value of Vmax and Ki it was concluded that ethyl acetate fraction shows the maximum inhibitory potential whereas petrolium ether fraction shows the least inhibition in the entire tested fraction. Moreover, aqueous and chloroform fraction also shows significant inhibition but less as compared with the other fractions. From km value it was concluded that affinity towards substrate for enzyme in case of ethyl acetate fraction was maximum and for petrolium ether fraction it was found to be less.
There are reports of aldose reductase inhibiting activity of few natural products such as root of Salacia oblonga and Salviae multiorrhizae, Glycerrhiza uralensis, Radix astragali and puerarin. These plants are rich in bioflavonoids, which are reported to reduce the aldose reductase activity. It further strengthens our study because H. enneaspermus also contains flavonoid and other phytochemicals[2]. Aldose reductase inhibitors including quercetin are currently the most commonly used oral agents for their good penetrations through cellular membranes and fast metabolism of sorbitol by sorbitol dehydrogenase considered for treatment of diabetic complications. Further it was concluded that flavonol and flavanone having the 7-hydroxy and/or catechol moiety at the B ring exhibit the strong activity in inhibition of aldose reductase. In vitro inhibitory activity of quercitirin (a glycoside of quercetin) was reported and also compared with cinnamaldehyde from Cinnamomum that showed higher activity[8]. Similarly, flavanone and flavonol glucosides isolated from a plant popularly known as ‘plant insulin’ (Myrcia multiflora) have been reported to possess aldose reductase inhibitory activity[23].
Recent studies show that majority of the plasma antioxidants are depleted in type 2 diabetes patients[24]. It has been suggested from the ESR studies that patients with diabetes mellitus are susceptible to higher levels of oxidative stress[25]. This oxidative stress causes imbalance between the oxidant and antioxidant defense mechanisms resulting in lipid peroxidation, DNA damage, and enzyme inactivation, including free radical scavenger enzymes contributing to tissue damage[26]–[28]. Because H. enneaspermus extract had significant antioxidant activity so it may be used to treat the imbalance of antioxidant and cure different diabetic complications[29].
In conclusion, the present study was carried out to determine the inhibitory potential of different fractions of H. enneaspermus on aldose reductase enzyme. The relationship between phenolic, flavonoid and aldose reductase inhibition was also investigated. The ethanolic extract has been chosen for fractionation due to its expected flavonoid contents that were reported to have antidiabetic activity. To evaluate the aldose reductase inhibitory activity, activity guided fractionation of H. enneaspermus was carried out on rat lens aldose reductase enzyme. The ethyl acetate fraction of the ethanol extract was found to exihibit maximum rat lens aldose reductase inhibitory activity as compared to the other fractions.
Acknowledgments
The financial assistance from University Grants Commission, New Delhi, for Dinesh Kumar Patel (Senior Research Fellowship) is greatly acknowledged.
Footnotes
Foundation Project: This work was financially supported by University Grants Commission, New Delhi (grant No. IT/DEV/08-09/3252/L).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Osadebe PO, Omeje EO, Nworu SC, Esimone CO, Uzor PF, David EK, et al. Antidiabetic principles of Loranthus micranthus Linn. parasitic on Persea americana. Asian Pac J Trop Med. 2010;3:619–623. [Google Scholar]
- 2.Halder N, Joshi S, Gupta SK. Lens aldose reductase inhibiting potential of some indigenous plants. J Ethnopharmacol. 2003;86:113–116. doi: 10.1016/s0378-8741(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 3.Soni LK, Gupta AK, Kaskhedikar SG. QSAR study of 5-arylidene-2, 4-thiazolidinediones as aldose reductase inhibitors. Med Chem Res. 2008;17:258–266. doi: 10.1016/j.bmcl.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 4.Ueda H, Kuroiwa E, Tachibana Y, Kawanishi K, Ayala F, Moriyasu M. Aldose reductase inhibitors from the leaves of Myrciaria dubia (H. B. & K.) McVaugh. Phytomedicine. 2004;11:652–656. doi: 10.1016/j.phymed.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 5.El-Kabbani O, Ruiz F, Darmanin C, Chung RP. Aldose reductase structures: implications for mechanism and inhibition. Cell Mol Life Sci. 2004;61:750–762. doi: 10.1007/s00018-003-3403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung HA, Islam MD, Kwon YS, Jin SE, Son YK, Park JJ, et al. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem Toxicol. 2011;49:376–384. doi: 10.1016/j.fct.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kim HM, Mok S, Lee JM, Cho EJ, Choi CK, Ku J, et al. Inhibition of aldose reductase from rat lenses by methanol extracts from Korean folk plants. Nat Prod Sci. 2010;16:285–290. [Google Scholar]
- 8.Goodarzi MT, Zal F, Malakooti M, Safari MR, Sadeghian S. Inhibitory activity of flavonoids on the lens aldose reductase of healthy and diabetic rats. Acta Med Iran. 2006;44:41–45. [Google Scholar]
- 9.Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, et al. Water extract of Aralia elata prevents cataractogenesis in vitro and in vivo. J Ethnopharmacol. 2005;101:49–54. doi: 10.1016/j.jep.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Narayanswamy VB, Setty MM, Malini S, Shirwaikar A. Preliminary aphrodisiac activity of Hybanthus enneaspermus in rats. Pharmacologyonline. 2007;1:152–161. [Google Scholar]
- 11.Weniger B, Lagnika L, Vonthron-Senecheau C, Adjobimey T, Gbenou J, Moudachirou M, et al. Evaluation of ethno botanically selected Benin medicinal plants for their in vitro antiplasmodial activity. J Ethnopharmacol. 2004;90:279–284. doi: 10.1016/j.jep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Hemalatha S, Wahi AK, Singh PN, Chansouria JPN. Anticonvulsant and free radical scavenging activity of Hybanthus enneaspermus: a preliminary screening. Indian J Tradit Knowl. 2003;2:383–388. [Google Scholar]
- 13.Tripathy S, Sahoo SP, Pradhan D, Sahoo S, Satapathy DK. Evaluation of anti arthritic potential of Hybanthus enneaspermus. Afr J Pharm Pharacol. 2009;3:611–614. [Google Scholar]
- 14.Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:316–322. doi: 10.1016/S2221-1691(11)60051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das S, Dash SK, Padhy SN. Ethno botanical information from Orissa state, India: a review. J Hum Ecol. 2004;14:165–227. [Google Scholar]
- 16.Zovko Končić M, Kremer D, Gruz J, Strnad M, Biševac G, Kosalec I, et al. Antioxidant and antimicrobial properties of Moltkia petraea (Tratt.) Griseb. flower, leaf and stem infusions. Food Chem Toxicol. 2010;48:1537–1542. doi: 10.1016/j.fct.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Kumaran A, Karunakaran RJ. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006;97:109–114. [Google Scholar]
- 18.Zhu KX, Lian CX, Guo XN, Peng W, Zhou HM. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–1126. [Google Scholar]
- 19.Zhao B, Hall CA. Composition and antioxidant activity of raisin extracts obtained from various solvents. Food Chem. 2008;108:511–518. doi: 10.1016/j.foodchem.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Viswanatha GLS, Vaidya SK, Ramesh C, Krishnadas N, Rangappa S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna. Asian Pac J Trop Med. 2010;3:965–970. [Google Scholar]
- 21.Girija K, Lakshman K, Chandrika U, Ghosh SS, Divya T. Anti-diabetic and anti-cholesterolemic activity of methanol extracts of three species of Amaranthus. Asian Pac J Trop Biomed. 2011;1:133–138. doi: 10.1016/S2221-1691(11)60011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh P, Huang G, Ho Y, Lin Y, Huang S, Chiang Y, et al. Activities of antioxidants, α-glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in Taiwan. Bot Stud. 2010;51:293–302. [Google Scholar]
- 23.Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002;83:30–38. [Google Scholar]
- 24.Dewanjee S, Maiti A, Sahu R, Dua TK, Mandal V. Effective control of type 2 diabetes through antioxidant defense by edible fruits of Diospyros peregrina. Evid Based Complement Alternat Med. 2011;2011:1–7. doi: 10.1093/ecam/nep080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai. 2010;93:682–693. [PubMed] [Google Scholar]
- 26.Patel DK, Kumar R, Prasad SK, Hemalatha S. Pedalium murex Linn (Pedaliaceae) fruits: a comparative antioxidant activity of its different fractions. Asian Pac J Trop Biomed. 2011;1:395–400. doi: 10.1016/S2221-1691(11)60087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel DK, Kumar R, Laloo D, Hemalatha S. Evaluation of phytochemical and antioxidant activities of the different fractions of Hybanthus enneaspermus (Linn.) F. Muell. (Violaceae) Asian Pac J Trop Med. 2011;4:391–396. doi: 10.1016/S1995-7645(11)60110-7. [DOI] [PubMed] [Google Scholar]
- 28.Oyedemi SO, Adewusi EA, Aiyegoro OA, Akinpelu DA. Antidiabetic and haematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian Pac J Trop Biomed. 2011;1:353–358. doi: 10.1016/S2221-1691(11)60079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasad SK, Kumar R, Patel DK, Hemalatha S. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm Biol. 2010;48:1397–1404. doi: 10.3109/13880209.2010.486837. [DOI] [PubMed] [Google Scholar]